Abstract
Gastric cancer remains one of the leading causes of global cancer mortality. Multipotent gastric stem cells have been identified in both mouse and human stomachs, and they play an essential role in the self-renewal and homeostasis of gastric mucosa. There are several environmental and genetic factors known to promote gastric cancer. In recent years, numerous in vitro and in vivo studies suggest that gastric cancer may originate from normal stem cells or bone marrow–derived mesenchymal cells, and that gastric tumors contain cancer stem cells. Cancer stem cells are believed to share a common microenvironment with normal niche, which play an important role in gastric cancer and tumor growth. This mini-review presents a brief overview of the recent developments in gastric cancer stem cell research. The knowledge gained by studying cancer stem cells in gastric mucosa will support the development of novel therapeutic strategies for gastric cancer.
Keywords: Stomach, gastric epithelial cells, gastric cancer, stem cells, cancer stem cells
1. Introduction
Although the number of cases and the mortality associated with gastric cancer (GC) has recently been declining, it remains the fourth most common cancer and the second leading cause of global cancer mortality [1]. Every year about one million new patients are diagnosed and 800,000 GC-related deaths occur in the world [2]. Of all the cases reported, two-thirds occurred in developing countries, with high-risk areas including China, Japan, and Central and South America. The American Cancer Society estimates that in the United States about 21,320 cases of GC will be diagnosed and about 10,540 people will die from GC in 2012 [3]. The detailed mechanisms that regulate GC are not yet fully understood; however, several factors (environmental and genetic) are reported to play an important role in promoting GC [4–11]. The environmental factors include Helicobacter pylori (H. pylori) infection, foods high in salt, nitrites, smoking, low fiber intake, and a diet low in fruits and vegetables [4,5,7–9,12–17]. In addition to environmental factors, genetic factors such as sex, hereditary diffuse GC (mutation in the E-cadherin/CDH1 gene), hereditary non-polyposis colorectal cancer (Lynch syndrome), and familial adenomatous polyposis (FAP) also play crucial roles in GC [4,5,7–9,12–17].
Among GC patients, 90% develop adenocarcinoma and only 10% develop lymphoma or a gastrointestinal stromal tumor. Gastric adenocarcinomas include the intestinal (50% differentiated) type, diffuse (33% undifferentiated) type, and mixed (17%) type [9, 18, 19]. The invasive nature of GC is linked to mutations in several oncogenes, tumor suppressor genes, changes in several growth factors and their receptors, epigenetic alterations, altered expression of microRNAs, inflammatory cytokines, and angiogenesis [9–11]. It is estimated that about $1.82 billion each year is spent in the United States on GC treatment [20]. Regardless of traditional treatments such as surgery, radiotherapy, chemotherapy, and targeted therapy, the overall five-year survival rate in GC patients is very low [21].
Recent studies suggest that like other tumors, GC is a heterogeneous disease [22]. However, the mechanisms that control GC invasion and metastasis remain to be clarified. It is postulated that tumors develop because of a rare subpopulation of cells (known as cancer stem cells [CSCs]) within a tumor. CSCs have been identified in many solid tumors, and they are promising for the development of anticancer drugs that can target all CSC subsets within a tumor to prevent recurrence [23]. Because of the failure of traditional treatments, CSCs, which already received great attention in the field of cancer research, are potential novel therapeutic targets in the treatment of GC. This mini-review presents a brief overview of the recent developments in gastric stem cell research and the role of CSCs in GC.
2. Gastric epithelial stem cells
2.1. The stomach epithelium
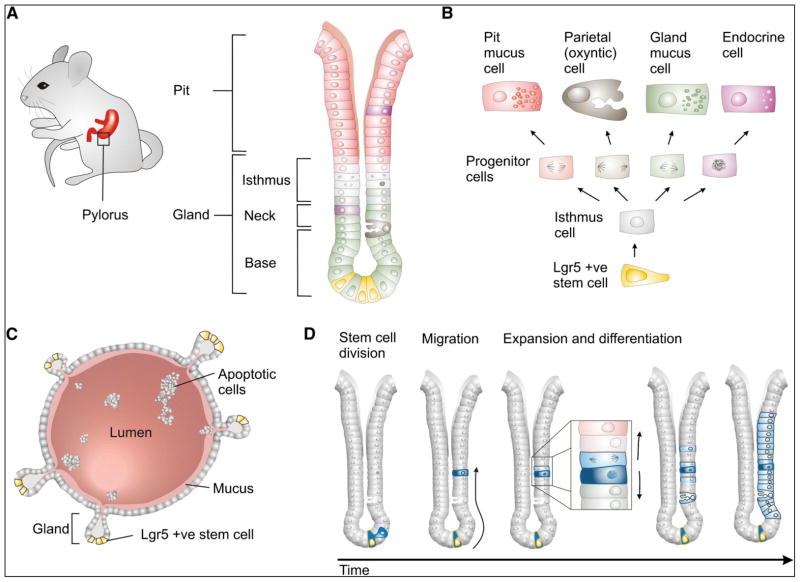
The mammalian gastrointestinal (GI) tract is involved in digestion, nutrient absorption, and homeostasis. It also shows the highest cellular turnover [24–25]. Although human and mouse stomachs may be functionally similar, they are different anatomically [25]. Histologically, the human gastric mucosa, which is of endodermal origin, is divided into three zones: cardiac, fundus/corpus, and antral/pyloric [25,26]. Gastric mucosa is also surrounded by subepithelial myofibroblasts (SMFs) [27]. In each gastric zone, the gastric unit is composed of a short pit and a long tubular gland that subdivides into the isthmus, neck, and base regions [24]. The corpus and pyloric zones of the stomach are different in morphology, cell type, and cellular turnover. The proximal corpus zone contains long glands with small pits and several epithelial cell types, such as surface mucous cells (pit), acid-producing parietal cells (oxyntic), mucous neck cells, chief cells (zymogenic), and hormone-secreting endocrine cells (gastrin and somatostatin) [25,26,28]. The distal pyloric zone is simple, contains short glands with a single long pit, and is composed of mucous-producing cells, endocrine cells, and rare parietal cells [25,26,28–30].
2.2. Stem cells in the gastric gland: location, identification, and markers
Gastric stem cells (GSCs) play essential roles in the self-renewal and homeostasis of the gastric gland, and they are crucial in epithelial repair after injury [24,31,32]. The self-renewal of the human stomach differs from that of the mouse [25]. In the gastric gland, based on circumstantial morphological and cell kinetic evidence, and in combination with 3H-thymidine-labeling, multipotent stem cells (undifferentiated granular-free progenitors) have been characterized at the isthmus region of both the fundic and antral zones [24,25,29,30,33–35]. The multipotent GSCs first differentiated into three progenitor cells: pre-pit, pre-neck, and pre-parietal cells. The granule-free pre-pit cells migrate up towards the lumen to become terminally differentiated mucous-secreting pit cells. The granule-free pre-neck cells migrate downwards and differentiate into zymogenic/chief cells. In contrast to pit and zymogenic lineages, parietal cells and endocrine cells differentiate within the isthmus from pre-parietal cells and migrate up either towards the lumen or down to the gland [24,25,33,34,36–39]. Furthermore, it has been reported that gastric fundic units are composed of many types of stem cells [40].
Some of the stem cell markers were detected to label the GSC populations [41–45, Table 1]. However, in vivo lineage tracing to identify such populations was not identified until recently [26,45,46, Table 1]. Qiao et al. [45] found a rare population of quiescent Villin+ cell resides in the isthmus region of the pyloric zone of the stomach; however, these cells are not involved in normal gastric gland homeostasis, but are active only in response to damage. Recently, Lgr5 (leucine-rich repeat-containing G-protein-coupled receptor 5), which is considered as a stem cell marker in intestine, colon, and hair follicle and have been reported to express at the base of the antrum zone of the gastric gland [26]. Furthermore, by lineage tracing, Lgr5+ cells have been functionally characterized as self-renewing, multipotent stem cells, located at the base of the glands. They are responsible for the long-term renewal of the gastric epithelium and can also generate self-renewing gastric organoids in vitro [Fig. 1; 26,46]. Using lineage tracing with trefoil factor 2 (TFF2) transgenic lines, Quante et al. [47] demonstrated that TFF2 is restricted to the isthmus region and can produce only mucous neck, parietal, and chief cells. Furthermore, keratin, type I cytoskeletal 19 (Krt19)+ cells have been shown through lineage-tracing experiments to label gastric progenitor cells [48]. Mist1, a basic helix-loop-helix transcription factor, is another marker identified in the gastric unit, which is expressed in mature chief cells. Lineage-tracing experiments suggest that Mist1+ cells can produce spasmolytic polypeptide-expressing metaplasia (SPEM) [49, 50]. Using Sox2-CreER; ROSA26-lsl-EYFP mice, Arnold et al. [51] performed lineage tracing and found that a small population of Sox2 (sex determining region Y)-box 2)+ cells can populate the entire glands of both the corpus and pylorus zones of the stomach, suggesting that Sox2-expressing cells can self-renew and give rise to the mature cell types of the glandular stomach. Recently, it was found that the two zones (fundic and antral) of the gastric gland vary because of differences in proliferation and differentiation, as well as in expression profiles [52].
Table 1.
Summary of putative gastric stem/progenitor cells and cancer stem cell markers
| Markers | Location and function | References | |
|---|---|---|---|
| Normal stomach | Gastric cancer | ||
| Villin promoter | Gland base, antrum of mouse stomach and give rise to all cell types (lineage tracing). Normally quiescent, multiply in response to interferon gamma |
n/t | Qiao et al. [45] |
| Lgr5 | Base of pyloric gland, give rise to all cell types (lineage tracing) | Luminal surface, tumor center and invasion front (Immunostaining, 100 GC patients analyzed) | Barker et al. [26] |
| Simon et al. [83] | |||
| Mist1 | Mature chief cells of corpus gland base in mice and human | Give rise to SPEM (lineage tracing using 3 mouse models of oxyntic atrophy (induced by DMP-777 treatment, L-635 treatment, or H felis infection) Present in the preneoplastic states (normal & gastritis) and lost in neoplasia in human | Ramsey et al. [50] |
| Nam et al. [49] | |||
| Lennerz et al. [111] | |||
| TFF2 | Isthmus region of corpus, gland base (mRNA expression) in mice, give rise to neck, chief and parietal cells only (lineage tracing) | Expressed in SPEM following DMP-777 treatment, due to trans-differentiation of chief cells progressive loss of expression in H pylori-positive gastritis, IM and GC in human and mice |
Quante et al. [47] |
| Peterson et al. [112] | |||
| DCKL1/DCAM KL1 | Isthmus region of corpus (immunostaining) | Expression expanded in murine models of acute gastritis, chronic ulcer and in Kras environment | Giannakis et al. [44] |
| Zhang and Huang [97] | |||
| Kikuchi et al. [98] | |||
| Okumura et al. [99] | |||
| Musashi-1 | Isthmus/neck region of antrum in human stomach (Immunohistochemistry) | Weakly expressed Elevated in gastric lesions and invasive GCs |
Akasaka et al. [43] |
| Wang et al. [110] | |||
| Sox2 | 1 and 2 cells above the base of corpus and antrum in mice stomach, give rise to cells in both the corpus and pylorus (lineage tracing) | Expression associated with invasion and lymph node metastasis in GC (Immunostaining, 290 GC patients analyzed) | Arnold et al. [51] |
| Matsuoka et al. [100] | |||
| Sox9 | Weak expression in neck/isthmus of corpus, moderate expression in neck/isthmus of pylorus (immunostaining) | strong expression observed in GC (46 patients analyzed by immunostaining) | Sashikawa Kimura et al. [101] |
| pSmad2/3L-Thr | Small number of cells in the corpus and antrum, isthmus region (immunostaining) | Significantly increased in the corpus and antrum of mice with Helicobacter-associated gastritis | Fukui et al. [102] |
| Agr2 (anterior gradient 2) | Corpus neck and base of antrum gland (immunstaining) | Involve in the development of neoplasia | Gupta et al. [103] |
| Bmi-1 | Weak to moderate expression in pits and isthmus of corpus and neck region of antrum | Strong expression in GC (immunostaining) | Reinisch et al. [104] |
| Oct4 | Isthmus region of pit gland, pyloric antrum of human stomach (immunostaining) | Increased expression in GC | Al-Marzoqee et al. [105] |
| CD44 | Strong expression in lower glandular cells of the gastric antrum and rare expression in corpus | Strong expression GC (isolated cells from cancer cell lines and mouse and human gastric samples produce spheroid colonies, and show tumorigenicity) | Takaishi et al. [86] |
| Wang et al. [110] | |||
| CD71− cell | n/t | Show higher tumorigenicity and multipotency, highly invasive and to exist in the invasive fronts of cancer foci | Ohkuma et al. [94] |
| CD133 | Base of gastric glands, antrum | Strong expression in GC, expressed on the luminal surface membrane of gland- forming cells (patient samples) | Zhao et al. [106] |
| Qiao and Gumucio [31] | |||
| Fukamachi et al. [107] | |||
| Jiang et al. [92] | |||
| Aldh1 | Cytoplasm of parietal cells (patient samples-immunostaining) | Highly expressed in GC, basal lesion of the metaplastic gland | Zhi et al. [149] |
| Wakamatsu et al. [108] | |||
| Nishikawa et al. [109] | |||
| EpCAM+/CD44+ | n/t | Isolated cells produce spheroid colonies, and show tumorigenicity | Han et al. [90] |
| CD44+CD24+ | n/t | Isolated cells from gastric tissues of patients produce spheroid colonies, and show tumorigenicity | Zhang et al. [87] |
| CD44+CD54+ | n/t | Isolated cells from human gastric tumor tissues and peripheral blood of patients produce spheroid colonies, and show tumorigenicity | Chen et al. [89] |
| CD90 | n/t | Isolated cells from gastric primary tumors produce spheroid colonies, and show tumorigenicity | Jiang et al. [92] |
| ABCB1 | n/t | Highly expressed in poorly differentiated GC | Jiang et al. [92] |
| ABCG2 | n/t | Highly expressed in poorly differentiated GC | Jiang et al. [92] |
Figure 1.
Adult stem cell-driven epithelial renewal in the pyloric stomach. (A) The location and general architecture of pyloric gastric units, (B) schematic diagram showing generation of functional epithelial cells from LGR5+ pyloric stem cells, (C) Cartoon of a self-renewing gastric organoid grown from a single LGR5+ pyloric stem cell. (D) A model for LGR5+ stem cell-driven epithelial renewal [Adapted from Barker et al. 2010, Cell Stem Cell, 7: 656–670, with permission from Elsevier].
Based on the above experiments, it is clear that gastric mucosa and glands are maintained by bidirectional self-renewal of gastric stem and progenitor cells [25]. Recent studies have suggested that the balance between self-renewal and differentiation in gastric mucosa is regulated by several signaling pathways or molecule such as wnt, notch, hedgehog, and runt-related transcription factor 3 (Runx3) [25,26,28,32,44,46,53–56].
3. Gastric cancer stem cells
3.1. Stem cells and cancer stem cells
Stem cells are functional units of growth that regenerate tissues and organs and play a role in tissue homeostasis and repair after damage or loss [57,58]. Stem cells have the unlimited ability to self-renew and the capacity to differentiate into several specialized cell types. Stem cells reside in a microenvironment called a niche that protects them from depletion and overproliferation [57,58]. Recent studies have shown that tumors contain phenotypically and functionally heterogeneous cancer cells. Tumors may originate from a small subpopulation of CSCs that are able to maintain long-term tumor growth, tumor recurrence, and apoptosis and chemotherapy resistance [23,59–62]. CSCs display characteristics that are similar to normal stem cells, including unlimited self-renewal, proliferation, and multi-lineage differentiation. It is also postulated that CSCs occupy the same niche, called the CSC niche, as normal stem cells [63]. The existence of CSCs was first demonstrated by Bonnet and Dick [64] from human acute myeloid leukemia (AML) using cell surface markers CD34+/CD38−. Cell surface markers were used because leukemic stem cells can reproduce the tumor after serial xenografting into immunodeficient mice. In recent years, accumulating evidence indicates the presence of CSCs in many solid tumors, including those in brain cancer [65], breast cancer [66], head and neck cancer [67 and references therein], renal cancer [68], colon cancer [69 and references therein], pancreatic cancer [70], liver cancer [71], melanoma [72], and lung cancer [73]. Although many uncertainties have developed in the last few years about the existence of CSCs, recent in vivo evidence suggests that tumors originate from CSCs [74–76].
3.2. Origin, identification, and regulation of gastric cancer stem cells (GCSCs)
3.2.1. Lineage-tracing and expression analysis
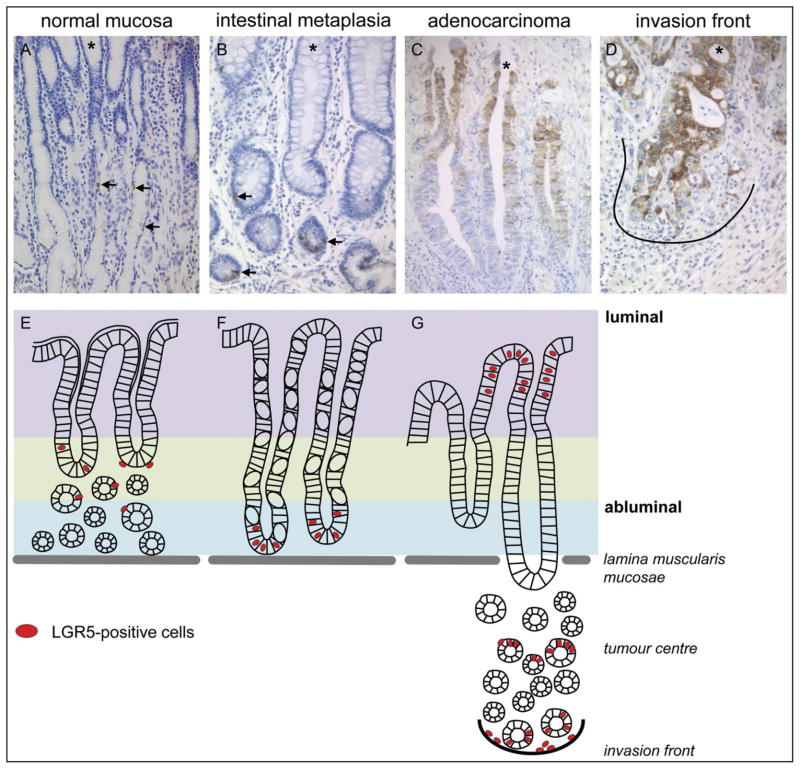
Gastric mucosa is histologically complex and is maintained by multiple stem cells located in different regions of the fundic and antral zones. It is suggested that GC may originate from normal resident stem cells or bone marrow–derived cells (BMDCs) [26,28,46,77–82]. McDonand et al. [40] provided evidence of intestinal metaplasia to dysplasia in human gastric units and found that intestinal dysplasia is clonal, contains multiple stem cells, and spreads by gland fission. However, the first study to trace the origin of GC from stem cells came from the experiments demonstrated by Barker et al. [26]. In this experiment, they tested the tumorigenic potential of the Lgr5+ pyloric stem cells by injecting a single dose of tamoxifen into Lgr5-EGFP-CreERT2/APCflox/flox mice to activate the Lgr5-driven Cre in the pyloric stem cells. Their lineage-tracing results suggest that APC loss in Lgr5+ stem cells efficiently drives the rapid appearance of proliferating adenomas in the pylorus zone of the stomach [26]. Simon et al. [83] further tested the prevalence, distribution, and tumor biological significance of Lgr5 cells in the human stomach by studying the differential expression of Lgr5 at the transcriptional and translational levels (Fig. 2A–G). They used malignant and non-malignant tissues from different primary tumor sites in 127 patients and tested the clinico-pathological significance of Lgr5 expression in 100 patients with GC. Simon et al. [83] found that Lgr5+ cell expression was higher in malignant compared to non-malignant tissues. Furthermore, they found the relocation of Lgr5+ cells in different stages of GC [83]. They showed that in non-neoplastic stomach mucosa, Lgr5+ cells were located mainly in the mucous neck region; in intestinal metaplasia, the cells were located in the crypt base; and in GC, the cells were located at the luminal surface, tumor centre, and invasion front (Fig. 2D). Furthermore, Simon et al. found that the tumor centre and invasion front of GC are significantly correlated with local tumor growth and nodal spread. Nevertheless, they also found that patients with Lgr5+ cells have a shorter median survival rate than patients with Lgr5-negative cells GCs. This study suggests that Lgr5 could be a general marker of stemness in the GI tract [26,83]. It has been demonstrated that targeted deletion of Klf4 (Kruppel-like factor 4) in Villin+ quiescent gastric progenitor cells at the antral mucosa induces transformation of the gastric mucosa and tumorigenesis in the antrum in mice [84]. Recently, Quante et al. [85] have shown the presence of Lgr5-labeled cells in the gastric cardia of Lgr5-Cre-ERT mice crossed with Rosa-LacZ reporter mice shortly after tamoxifen induction that within 7 days produces lineage-traced cardia epithelium. Furthermore, when they crossed L2-IL-1β mice with Lgr5-Cre-ERT/Rosa-LacZ mice, they observed labeled cells in Barrett esophagus (BE) metaplasia within four months of tamoxifen induction, which were treated with bile acid at the ages of 6–8 weeks. The above findings suggest that Lgr5+ cells in the cardia possibly function as progenitor cells and serve as the cells of origin for BE and dysplasia. In addition, Quante et al. also found an accumulation of doublecortin-like kinase 1 (Dclk1) + cells near metaplastic mucous-secreting cells in BE tissues, since Dclk1+ cells are highly expressed in gastric cardia. Furthermore, they also found that Lgr5 and Dclk1 expression was significantly elevated in the gastric cardia of BE patients. The above experiments using lineage-tracing and expression analyses suggest that BE metaplasia in mice and humans may originate from gastric cardia lineage [85].
Figure 2.
Staining patterns of LGR5 in gastric cancer tissues. (A, E) healthy gastric mucosa, (B, F) intestinal metaplasia, (C, G) gastric adenocarcinoma, with invasion front (D). (A–D) show representative immunohistochemical staining with an anti-LGR5 antibody on the whole mount sections of intestinal types gastric cancers (GC). (E–G) schematic model of the distributional changes in different stages of gastric tumorigenesis. Arrows mark the LGR5+ cells, astrick (*) marks the luminal site and the black line highlights the tumor host interface (invasion front). Original magnification x200. [Adapted from Simon E, et al. 2012, PLoS ONE 7(4): e35486, with permission from Dr. Christoph Rocken].
3.2.2. Cell surface markers
The concept of CSCs may provide a novel approach in GC therapies. Recently, gastric cancer stem cells (GCSCs) have been reported in many GC cell lines as well as in primary tumors using several candidate cell surface markers [22,86–112, Table 1] and a side population (SP) assay [113–118, but see 119].
3.2.2.1. GC cell lines
Takaishi et al. [86] examined several human GC cell lines (NCI-N87, AGS, MKN-28, MKN-45, and MKN-74) and found that CD44 can be used as a potential GCSC marker. They also found that a CD44+ cellular fraction isolated from these cell lines had a sphere-forming capacity, and they found xenograft tumors in the stomachs of immunodeficient mice [86]. Furthermore, Zhang et al. [87] examined the expression of cell surface markers CD44 and CD24 in gastric cell lines and in AGS and GC tissues from five patients using fluorescence-activated cell sorting. They identified the tumorigenic properties, the self-renewal, and the differentiation between CD44+/CD24+ and CD44−/CD24− cell populations by in vivo serial transplantation and in vitro culture. They found that these cells have the capacity to both self-renew and form tumors, which suggests that CD44+/CD24+ can be used as a GCSC marker. Yang et al. [88] isolated and characterized GCSCs from GC cell line SGC7901, which has the capacity for in vitro invasion and in vivo metastasis. Furthermore, they found that decreased expression of E-cadherin and increased expression of MMP-2 (matrix metalloproteinase-2) may be associated with invasion and metastasis in GCSCs [88]. Recently, aldehyde dehydrogenase 1 (ALDH1) was identified as an additional marker for GCSCs by Katsuno et al. [93] using human GC cell lines. Recently, Liu et al. [95] found that non-adherent spheroid body-forming cells from GC cell line MKN-45, cultured in a stem cell–conditioned medium, contained GCSC characteristics of sustained self-renewal, high proliferation, resistance to drugs, and high expression of CSC markers such as Oct4 (octamer-binding transcription factor 4), Nanog, Sox2, and CD44, compared to parental cells [95].
3.2.2.2. Human GC tissues
Chen et al. [89] isolated CSCs from human GC tissues and the peripheral blood of GC patients using CD44 and CD54 surface markers. These CD44− and CD54+ cells have the capacity to generate tumors both in vivo and in vitro. The results from Chen et al. suggest that CD44 and CD54 are potential biomarkers for GCSCs. In another report, using human GC tissues, the epithelial cell adhesion molecule (EpCAM) and CD44 were identified as putative GCSC markers [90]. Furthermore, CD90 was identified as a potential CSC marker in human gastric primary tumors [91]. Ohkuma et al. [94] further identified CD71 as a potential marker for CSCs in human GC tissues. Recently, aldehyde dehydrogenase 1 (ALDH1) was identified as an additional marker for GCSCs by Katsuno et al. [93] using human GC cell lines. Jiang et al. [92] demonstrated the expression of CSC markers ATP-binding cassette sub-family B member 1 (ABCB1), ATP-binding cassette sub-family G member 2 (ABCG2), and CD133 in 90 human GC tissue samples and 3 human GC cell lines and concluded that the expression of these markers varied in GC with various degrees of differentiation; poorly differentiated GC can express a high level of CSC markers [92].
3.2.3. Side population (SP) assay
In addition to the surface markers named above, studies demonstrated the presence of CSCs in side population (a subset of stem cells) cells isolated from human GC tissues and cell lines. Using flow cytometry and the DNA-binding dye, Hoechst 33342, Haraguchi et al. [113] isolated SP cells from many human GC cell lines and found that the cell lines contained 0.3–2.2% SP cells. Furthermore, Fukuda et al. [114] isolated SP cell populations (ranging from 0.02 to 1.93) from human GC cell lines (MKN45, KATOIII, MKN74, MKN28, and MKN1) using flow cytometry. They found that the MKN45 cell line harbored the highest percentage of SP cells because tumorigenesis was shown in vivo. SP cells were isolated by Nishii et al. [115] using GC cell lines OCUM-2M, OCUM-2D, and OCUM-2MD3. Nishii et al. found that these SP cells from GC cell lines can self-renew and produce non-SP cells and peritoneal metastasis. In addition, they found increased expression of adhesion molecules α2, α5, β3-integrins, and β5-integrins, and CD44 in SP cells compared to parent cells [115]. Furthermore, Schmuck et al. [116] characterized the SP cells from two GC cell lines, AGS and MKN45. They found that SP cells were smaller and expressed CD133 and MSI-1, which produce SP and non-SP cells in recultivation experiments. Their transcriptional analyses showed that SP cells expressed genes that encoded for stem cell properties such as FZD7, HEY1, SMO, and ADAM17 [116]. Ehata et al. [117] characterized the SP cells within human diffuse-type GC cells isolated from patients and found the amount of SP cells between 1 and 4% of the total cells. The SP cells showed greater tumorigenicity than non-SP cells in vivo. Recently, She et al. [118] analyzed SP cells and non-SP cells in human GC cell lines KATO III, HS-746T, and AGS. They found that KATO III and HS-746T had high tumorigenic capacities. KATO III contained 0.57% of SP cells out of the total cell population, and HS-746T contained 1.04% SP cells out of the total cell population. Only 0.02% SP cells were reported in the AGS cell line. However, She et al. did not find any clear difference between SP and non-SP cells when they injected these cells into nude mice [118]. Of note, studies suggest that not all SP cells contain CSC characteristics [119]; therefore, the precise identification of CSCs from GC cell lines needs to follow the functional assay such as sphere formation and xenotransplantation in immunodeficient mice [62,77].
3.2.4. Regulation of GCSCs
Recent studies suggest that several signaling pathways including hedgehog and wnt/beta-catenin are essential for maintaining GCSCs in human GC [87, 120–122]; therefore, understanding the regulation of these pathways represents a justified therapeutic approach to target GCSCs.
3.3. H. pylori, BMDC, and gastric cancer stem cells (GCSCs)
H. pylori is a spiral-shaped, gram-negative microaerophilic bacterium that colonizes in the stomachs of almost half of all humans [123]. It is known that H. pylori infection of the stomach results in stomach inflammation, which leads to step-wise changes such as chronic gastritis, intestinal metaplasia, and ultimately GC [123–125]. H. pylori was isolated by Marshall and Warren in 1984, and it is classified as a class 1 carcinogen [12]. There are four major virulence factors reported from H. pylori: cytotoxin-associated antigen A (CagA), cag-pathogenicity island (cagPAI), vacuolating cytotoxin (VacA), and outer membrane proteins (OMPs). It was demonstrated that H. pylori carrying the major protein virulence factor, CagA, is closely associated with the development of GC [126]. In the last few years, several studies demonstrated the possible interaction between H. pylori, stem cells, and GC [78,82,123,125,127–131]. Recent studies suggest that CSCs exist in GC and that these cells possibly originated from resident stem cells, differentiated epithelial cells [26], or stem cells derived from BMDCs [78]. The existence of CSCs from BMDCs and the interaction between these two types of cells were first demonstrated by Houghton et al. [78] using models of Helicobacter-induced cancer. They found that chronic infection of C57BL/6 mice with H. felis results in chronic inflammation and injury in gastric mucosa, which results in the loss of resident GSCs and induces BMDC repopulation in the stomach, followed by hyperplasia, metaplasia, dysplasia, and finally GC [78]. These results from Houghton et al. suggest that Helicobacter plays an important role in the progression of GC by modulating stem cells. These results were further confirmed by Varon et al. [82] using a similar mouse model. Furthermore, Giannakis et al. [128] used a genetically engineered gnotobiotic mouse model of chronic atrophic gastritis (ChAG) and found that H. pylori could attach and invade GSCs and that this residency results in GC initiation. Ferrand et al. [129] demonstrated that GI epithelial cells infected by different strains of H. pylori can influence the migration of mesenchymal stem cell (MSC) due to the secretion of a combination of cytokines by infected epithelial cells, which are NF- B (nuclear factor kappa-light-chain-enhancer of activated B cells) dependent. Recently, Uehara et al. [125] demonstrated the relationship between H. pylori colonization, GC, and DNA damage within Lgr5+ epithelial stem cells in the stomachs of patients with GC. They found that Lgr5+ cells expanded in the presence of H. pylori in the antrum of patients with GC. Furthermore, they found that Lgr5+ cells were more susceptible to DNA damage compared to Lgr5-negative cells, which suggests that H. pylori infection directly affects epithelial stem cells in the stomach, resulting in GC [125]. Similarly, Noto et al. [131] recently found that H. pylori induced the expansion of a KLF5+ cell population that was also positive for stem cell marker, Lrig1 (leucine-rich repeat and Ig-like domain-containing-1). They also found that the degree of KLF5 expression increased in parallel with the advancing stages of GC compared to normal gastric tissue. Recently, Tsugawa et al. [7] used CD44v9-expressing GC cell lines to study the ability of intracellular CagA to escape from autophagy, and they found a molecular link between H. pylori–derived CagA and GC stem-like cells. The studies described above suggest that chronic inflammation because of H. pylori infection plays an important role in transforming resident stem cells into tumor cells.
3.4. GCSC niche and novel therapeutic strategies in GC
Like normal stem cells, CSCs have the capacity for self-renewal and multi-lineage differentiation. Accumulating evidence suggests that the behavior of stem cells and CSCs is controlled by a tissue-specific niche microenvironment [23,58,60–63,132–136]. The balance between self-renewal and differentiation of a stem cell is essential for normal tissue homeostasis, which is missing in the CSC niche [137]. There are many components of the niche that have been suggested to regulate normal stem cell and CSC properties in vivo, and these components are involved in tumor growth, including extracellular matrix, stromal cells, vascular and endothelial molecules, secreted modifier proteins, growth factors, bone marrow–derived myofibroblasts, and hypoxia. In the stomach, GSCs are surrounded by a sheet of subepithelial myofibroblasts (SMFs) that acts as a niche and secretes different types of growth and differentiation factors, including bone morphogenetic proteins (BMPs), transforming growth factor beta 1 (TGF-β1), Wnt ligands, the chemokine stromal-derived factor 1 (SDF), and matrix metalloproteinases (MMPs) [25,27]. Recently, bone marrow–derived myofibroblasts, an important niche component, have been shown to be crucial in GC [80,81,138]. Guo et al. [138] have shown that gastric tumor cells activate stromal fibroblasts (SFs) and become myofibroblasts, which express vascular endothelial growth factor A (VEGFA) and other angiogenic factors. They suggested that suppressing fibroblast activation by inhibiting tumor cell–derived factors would be an effective strategy for the chemoprevention of GC, in combination with the eradication of H. pylori [138]. Shibata et al. [81] showed that overexpression of stromal cell-derived factor-1 (SDF-1/CXCL12), a ligand for CXCR4 (C-X-C chemokine receptor type 4), induces GC recruitment of BMDCs and modulation of the progenitor niche. In addition to myofibroblasts, vascular endothelial growth factor (VEGF), TGF-β, vasculogenic mimicry (VM), and hypoxia-inducible factors (HIFs) are critical components of the CSC niche that regulates GC [139–146]. Understanding the origin of CSCs and their interaction with niches would be helpful for precisely targeting CSCs.
For many years, conventional anticancer therapies such as chemotherapy, radiation, and immunotherapy have been used, which can kill only differentiated tumor cells, resulting in tumor size reduction; however, the tumors relapse after some time, possibly because of the presence of quiescent CSCs, as per the CSC hypothesis. In the gastric mucosa, quiescent and active stem cells have been reported, which suggests that there is dire need to design drugs that specifically target CSCs, including stem cell–targeting drugs, stemness-inhibitor drugs, stemness-promoting drugs, and microenvironment-modulating drugs [23, 62, 74–76, 147]. For targeting GCSCs, several novel strategies have been suggested, including a combination of chemotherapy-associated apoptosis, targeted imaging, and tumor stem cell differentiation induction; inducing apoptosis in the tumor; therapies targeting GCSC cell surface molecules; monoclonal antibody development; targeting the GCSC microenvironment; and inhibiting GCSC pathways [77,81,93,118,122,142,148–160]. Jiang et al. [91] treated gastric tumor cells, which express CD90, with trastuzumab (humanized anti-ERBB2 antibody) and found that trastuzumab can reduce the CD90+ population in tumor size and growth when it is combined with traditional chemotherapy. Zhi et al. [149] screened ALDH activities using several GC cell lines and divided them based on ALDH (high) and ALDH (low) GC groups. They found that ALDH (high) cancer cells displayed high CSC properties because they express higher levels of Sox2, Nanog, and Nestin; they express more floating spheroid bodies and more colony formation; and they exhibit more resistance to traditional chemotherapeutic drugs, 5-Fu and cisplatin (CDDP), compared to those properties found in ALDH (low) cancer cells. In addition, Zhi et al. found that ALDH (high) cancer cells were very sensitive to salinomycin, compared to ALDH (low) cancer cells. This suggests that ALDH could be a potential marker of the CSC population in GC and that salinomycin could be used as selective therapy for the CSC fraction that is resistant to traditional anticancer drugs, 5-Fu and CDDP [149]. Recently, Akagi [150] examined the roles of myeloid cell leukemia-1 (Mcl-1), an anti-apoptotic protein, in chemotherapy-associated apoptosis using seven GC cell lines, as well as whether Mcl-1 plays any role in apoptosis resistance in CSC-like populations in GC. Akagi found that six out of the seven GC cell lines overexpressed the Mcl-1 protein and showed resistance to 5-FU and CDDP. Depleting the Mcl-1 protein by RNAi results in effectively sensitizing these cells to anticancer drug-induced mitochondrial cytochrome c release, caspase activation, and apoptosis. Furthermore, Akagi found the expression of Mcl-1 mRNA in CD44+ CSC-like cells. Reduced Mcl-1 expression enhances apoptosis in CD44+ cells, similar to CD44-negative cells. Akagi’s results suggest that Mcl-1 mediates chemotherapy resistance in CSC-like populations.
Park et al. [154] discussed a near-infrared-sensitive molecular imaging probe based on hydrogel complexes, which can target GCSC marker, CD44. It has been shown that genetically engineered stem cells (GESTECs) expressing the cytosine deaminase (CD), a suicide enzyme, and human interferon β (IFN-β) fusion gene has a synergic antitumor effect on gastric cancer cells [159]. It is known that MSCs have the capacity to migrate into tumors; therefore, they can be utilized as potential vehicles cancer therapy. Zhu et al. [160] engineered umblical cord blood mesenchymal stem cells (UCB-MSCs) to deliver a secretable form of LIGHT, a member of tumor necrosis factor (TNF) superfamily. They found that MSC-LIGHT had a strong suppressive effect on gastric cancer growth, which suggest that this system have the potential to be used as efficient delivery vehicles in the treatment of GCs Zhu et al. [160]. Zhan et al. [157] demonstrated that the expression of orphan receptor TR3, a regulator of cell proliferation and apoptosis, is elevated in gastric tumorsphere cells, which display CSC properties. Loss of TR3 results in reduced stem cell properties in GC cells and tumorsphere cells, as well as reduced expression of Oct-4 and Nanog and the invasion-related gene MMP-9. Zhan et al. identified Nanog as a novel target of TR3. Their data suggest that TR3 is essential for CSC maintenance in human GC cells; therefore, TR3 can be used as a new therapeutic target for GC [157]. Although the strategies described above would be helpful in developing anti-CSC drugs to cure GC, not all pathways/markers should be active in each CSC in tumor tissues. Therefore, early diagnosis and drugs having multiple targets are crucial to CSCs for treating GC and other types of cancer [60].
4. Conclusion
In summary, accumulative evidence supports the existence of CSCs that have the capacity to generate tumors, are resistant to chemotherapy, and can produce more differentiated non-tumorigenic cells within gastric tumors. In the last few years, using lineage tracing and molecular marker labeling, several markers identified multiple pools of quiescent or active stem cells in gastric units. Some of these markers are upregulated in GCSCs. In addition, several GCSC populations have been defined, showing that heterogeneity exists in GC, which suggest that we need to use a combination of biomarkers to target different populations of GCSCs. Studies also suggest that CSC behavior is regulated by the niche microenvironment. Furthermore, chronic inflammation because of H. pylori infection also plays a crucial role in transforming resident stem cells into tumor cells. Dysregulation of several pathways has also been identified in gastric tumor cells. An in-depth understanding of the interaction between GCSCs and their niches, as well as targeting stem cell pathways and identifying new candidate therapies that target the CSCs, may lead to the development of novel therapeutic strategies that eradicate GCSC populations, thereby curing GC.
Acknowledgments
This work was supported by the Intramural Research Program of the National Cancer Institute, National Institutes of Health. I would like to thank Dr. Chhavi Chauhan for critical reading and Ashley DeVine for editing the manuscript. I apologize to all colleagues whose relevant contributions were not cited due to space limitations.
Footnotes
Conflict of Interest
The author has no conflicts of interest to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin. 2005;55:74–108. doi: 10.3322/canjclin.55.2.74. [DOI] [PubMed] [Google Scholar]
- 2.Jemal A, Bray F, Center MM, Ferlay J, et al. Global caner statistics. CA Cancer J Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 3.American Cancer Society. Cancer Facts and Figures 2012. Atlanta, GA: American Cancer Society; 2012. [Google Scholar]
- 4.Peek RM, Jr, Blaser MJ. Helicobacter pylori and gastrointestinal tract adenocarcinomas. Nat Rev Cancer. 2002;2:28–37. doi: 10.1038/nrc703. [DOI] [PubMed] [Google Scholar]
- 5.Suzuki H, Iwasaki E, Hibi T. Helicobacter pylori and gastric cancer. Gastric Cancer. 2009;12:79–87. doi: 10.1007/s10120-009-0507-x. [DOI] [PubMed] [Google Scholar]
- 6.Milne AN, Carneiro F, O’Morain C, Offerhaus GJ. Nature meets nurture: molecular genetics of gastric cancer. Hum Genet. 2009;126:615–28. doi: 10.1007/s00439-009-0722-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tsugawa H, Suzuki H, Saya H, Hatakeyama M, et al. Reactive oxygen species-induced autophagic degradation of Helicobacter pylori CagA is specifically suppressed in cancer stem-like cells. Cell Host Microbe. 2012;12:764–77. doi: 10.1016/j.chom.2012.10.014. [DOI] [PubMed] [Google Scholar]
- 8.Wroblewski LE, Peek RM, Jr, Wilson KT. Helicobacter pylori and gastric cancer: factors that modulate disease risk. Clin Microbiol Rev. 2010;23:713–39. doi: 10.1128/CMR.00011-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gomceli I, Demiriz B, Tez M. Gastric carcinogenesis. World J Gastroenterol. 2012;18:5164–70. doi: 10.3748/wjg.v18.i37.5164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ooi CH, Ivanova T, Wu J, Lee M, Tan IB, et al. Oncogenic pathway combinations predict clinical prognosis in gastric cancer. PLoS Genet. 2009;5:e1000676. doi: 10.1371/journal.pgen.1000676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Resende C, Thiel A, Machado JC, Ristimäki A. Gastric cancer: basic aspects. Helicobacter. 2011;16:38–44. doi: 10.1111/j.1523-5378.2011.00879.x. [DOI] [PubMed] [Google Scholar]
- 12.Marshall BJ, Warren JR. Unidentified curved bacilli in the stomach of patients with gastritis and peptic ulceration. Lancet. 1984;1:1311–5. doi: 10.1016/s0140-6736(84)91816-6. [DOI] [PubMed] [Google Scholar]
- 13.Tomita H, Yamada Y, Oyama T, Hata K, Hirose Y, et al. Development of gastric tumors in Apc(Min/+) mice by the activation of the beta-catenin/Tcf signaling pathway. Cancer Res. 2007;67:4079–87. doi: 10.1158/0008-5472.CAN-06-4025. [DOI] [PubMed] [Google Scholar]
- 14.Ohnishi N, Yuasa H, Tanaka S, Sawa H, et al. Transgenic expression of Helicobacter pylori CagA induces gastrointestinal and hematopoieticneoplasms in mouse. Proc Natl Acad Sci USA. 2008;105:1003–8. doi: 10.1073/pnas.0711183105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kushima R, Vieth M, Borchard F, Stolte M, Mukaisho K, Hattori T. Gastric-type well-differentiated adenocarcinoma and pyloric gland adenoma of the stomach. Gastric Cancer. 2006;9:177–84. doi: 10.1007/s10120-006-0381-8. [DOI] [PubMed] [Google Scholar]
- 16.Yasui W, Sentani K, Sakamoto N, Anami K, Naito Y, Oue N. Molecular pathology of gastric cancer: research and practice. Pathol Res Pract. 2011;207:608–12. doi: 10.1016/j.prp.2011.09.006. [DOI] [PubMed] [Google Scholar]
- 17.Zouridis H, Deng N, Ivanova T, Zhu Y, Wong B, et al. Methylation subtypes and large-scale epigenetic alterations in gastric cancer. Sci Transl Med. 2012;4:156ra140. doi: 10.1126/scitranslmed.3004504. [DOI] [PubMed] [Google Scholar]
- 18.Lauren P. The two histological main types of gastric carcinoma: diffuse and so-called intestinal-type carcinoma. An attempt at a histopclinical classification. Acta Pathol Microbiol Scand. 1965;64:31–49. doi: 10.1111/apm.1965.64.1.31. [DOI] [PubMed] [Google Scholar]
- 19.Nakamura K, Sugano H, Takagi K. Carcinoma of the stomach in incipient phase: its histogenesis and histological appearances. Gann. 1968;59:251–8. [PubMed] [Google Scholar]
- 20.Mariotto AB, Yabroff KR, Shao Y, Feuer EJ, Brown ML. Projections of the cost of cancer care in the United States: 2010–2020. J Natl Cancer Inst. 2011;103:117–28. doi: 10.1093/jnci/djq495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hartgrink HH, Jansen EP, van Grieken NC, van de Velde CJ. Gastric cancer. Lancet. 2009;374:477–90. doi: 10.1016/S0140-6736(09)60617-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rocco A, Compare D, Nardone G. Cancer stem cell hypothesis and gastric carcinogenesis: Experimental evidence and unsolved questions. World J Gastrointest Oncol. 2012;4:54–9. doi: 10.4251/wjgo.v4.i3.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Visvader JE, Lindeman GJ. Cancer stem cells: current status and evolving complexities. Cell Stem Cell. 2012;10:717–28. doi: 10.1016/j.stem.2012.05.007. [DOI] [PubMed] [Google Scholar]
- 24.Hoffmann W. Regeneration of the gastric mucosa and its glands from stem cells. Curr Med Chem. 2008;15:3133–44. doi: 10.2174/092986708786848587. [DOI] [PubMed] [Google Scholar]
- 25.Hoffmann W. Stem cells, self-renewal and cancer of the gastric epithelium. Curr Med Chem. 2012;19:5975–83. [PubMed] [Google Scholar]
- 26.Barker N, Huch M, Kujala P, van de Wetering M, Snippert HJ, van Es JH, et al. Lgr5+ve stem cells drive self-renewal in the stomach and build long-lived gastric units in vitro. Cell Stem Cell. 2010;6:25–36. doi: 10.1016/j.stem.2009.11.013. [DOI] [PubMed] [Google Scholar]
- 27.Leedham SJ, Brittan M, Preston SL, McDonald SA, Wright NA. The stomach periglandular fibroblast sheath: all present and correct. Gut. 2006;55:295–6. [PMC free article] [PubMed] [Google Scholar]
- 28.Karam SM. Cellular origin of gastric cancer. Ann N Y Acad Sci. 2008;1138:162–8. doi: 10.1196/annals.1414.023. [DOI] [PubMed] [Google Scholar]
- 29.Lee ER, Leblond CP. Dynamic histology of the antral epithelium in the mouse stomach: II. Ultrastructure and renewal of isthmal cells. Am J Anat. 1985;172:205–24. doi: 10.1002/aja.1001720304. [DOI] [PubMed] [Google Scholar]
- 30.Lee ER, Leblond CP. Dynamic histology of the antral epithelium in the mouse stomach: IV. Ultrastructure and renewal of gland cells. Am J Anat. 1985;172:241–59. doi: 10.1002/aja.1001720306. [DOI] [PubMed] [Google Scholar]
- 31.Qiao XT, Gumucio DL. Current molecular markers for gastric progenitor cells and gastric cancer stem cells. J Gastroenterol. 2011;46:855–65. doi: 10.1007/s00535-011-0413-y. [DOI] [PubMed] [Google Scholar]
- 32.Mills JC, Shivdasani RA. Gastric epithelial stem cells. Gastroenterology. 2011;140:412–24. doi: 10.1053/j.gastro.2010.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bjerknes M, Cheng H. Multipotential stem cells in adult mouse gastric epithelium. Am J Physiol: Gastrointest Liver Physiol. 2002;283:G767–G777. doi: 10.1152/ajpgi.00415.2001. [DOI] [PubMed] [Google Scholar]
- 34.Karam SM, Straiton T, Hassan WM, Leblond CP. Defining epithelial cell progenitors in the human oxyntic mucosa. Stem Cells. 2003;21:322–336. doi: 10.1634/stemcells.21-3-322. [DOI] [PubMed] [Google Scholar]
- 35.van den Brink GR, Hardwick JC, Tytgat GN, Brink MA, et al. Sonic hedgehog regulates gastric gland morphogenesis in man and mouse. Gastroenterology. 2001;121:317–28. doi: 10.1053/gast.2001.26261. [DOI] [PubMed] [Google Scholar]
- 36.Karam SM, Leblond CP. Dynamics of epithelial cells in the corpus of the mouse stomach. I. Identification of proliferative cell types and pinpointing of the stem cell. Anat Rec. 1993;236:259–79. doi: 10.1002/ar.1092360202. [DOI] [PubMed] [Google Scholar]
- 37.Karam SM, Leblond CP. Dynamics of epithelial cells in the corpus of the mouse stomach. II. Outward migration of pit cells. Anat Rec. 1993;236:280–96. doi: 10.1002/ar.1092360203. [DOI] [PubMed] [Google Scholar]
- 38.Karam SM, Leblond CP. Dynamics of epithelial cells in the corpus of the mouse stomach. III. Inward migration of neck cells followed by progressive transformation into zymogenic cells. Anat Rec. 1993;236:297–313. doi: 10.1002/ar.1092360204. [DOI] [PubMed] [Google Scholar]
- 39.Karam SM, Leblond CP. Dynamics of epithelial cells in the corpus of the mouse stomach. V. Behavior of entero-endocrine and caveolated cells: general conclusions on cell kinetics in the oxyntic epithelium. Anat Rec. 1993;236:333–40. doi: 10.1002/ar.1092360206. [DOI] [PubMed] [Google Scholar]
- 40.McDonald SA, Greaves LC, Gutierrez-Gonzalez L, Rodriguez-Justo M, et al. Mechanisms of field cancerization in the human stomach: the expansion and spread of mutatedgastric stem cells. Gastroenterology. 2008;134:500–10. doi: 10.1053/j.gastro.2007.11.035. [DOI] [PubMed] [Google Scholar]
- 41.Li Q, Karam SM, Gordon JI. Diphtheria toxin-mediated ablation of parietal cells in the stomach of transgenic mice. J Biol Chem. 1996;271:3671–6. [PubMed] [Google Scholar]
- 42.Mills JC, Andersson N, Hong CV, Stappenbeck TS, Gordon JI. Molecular characterization of mouse gastric epithelial progenitor cells. Proc Natl Acad Sci USA. 2002;99:14819–24. doi: 10.1073/pnas.192574799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Akasaka Y, Saikawa Y, Fujita K, Kubota T, et al. Expression of a candidate marker for progenitor cells, Musashi-1, in the proliferative regions of human antrum and its decreased expression in intestinal metaplasia. Histopathology. 2005;47:348–56. doi: 10.1111/j.1365-2559.2005.02223.x. [DOI] [PubMed] [Google Scholar]
- 44.Giannakis M, Stappenbeck TS, Mills JC, Leip DG, et al. Molecular properties of adult mouse gastric and intestinal epithelial progenitors in their niches. J Biol Chem. 2006;281:11292–300. doi: 10.1074/jbc.M512118200. [DOI] [PubMed] [Google Scholar]
- 45.Qiao XT, Ziel JW, McKimpson W, Madison BB, Todisco A, et al. Prospective identification of a multilineage progenitor in murine stomach epithelium. Gastroenterology. 2007;133:1989–98. doi: 10.1053/j.gastro.2007.09.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Barker N, Bartfeld S, Clevers H. Tissue-resident adult stem cell populations of rapidly self-renewing organs. Cell Stem Cell. 2010;7:656–70. doi: 10.1016/j.stem.2010.11.016. [DOI] [PubMed] [Google Scholar]
- 47.Quante M, Marrache F, Goldenring JR, Wang TC. TFF2 mRNA transcript expression marks a gland progenitor cell of the gastric oxyntic mucosa. Gastroenterology. 2010;139:2018–2027. doi: 10.1053/j.gastro.2010.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Means AL, Xu Y, Zhao A, Ray KC, Gu G. A CK19(CreERT) knockin mouse line allows for conditional DNA recombination in epithelial cells in multiple endodermal organs. Genesis. 2008;46:318–23. doi: 10.1002/dvg.20397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nam KT, Lee HJ, Sousa JF, Weis VG, O’Neal RL, et al. Mature chief cells are cryptic progenitors for metaplasia in the stomach. Gastroenterology. 2010;139:2028–2037. doi: 10.1053/j.gastro.2010.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ramsey VG, Doherty JM, Chen CC, Stappenbeck TS, Konieczny SF, Mills JC. The maturation of mucus-secreting gastric epithelial progenitors into digestive-enzyme secreting zymogenic cells requires Mist1. Development. 2007;134:211–22. doi: 10.1242/dev.02700. [DOI] [PubMed] [Google Scholar]
- 51.Arnold K, Sarkar A, Yram MA, Polo JM, et al. Sox2(+) adult stem and progenitor cells are important for tissue regeneration and survival of mice. Cell Stem Cell. 2011;9:317–29. doi: 10.1016/j.stem.2011.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kouznetsova I, Kalinski T, Meyer F, Hoffmann W. Self-renewal of the human gastric epithelium: new insights from expression profiling using laser microdissection. Mol BioSyst. 2011;7:1105–1112. doi: 10.1039/c0mb00233j. [DOI] [PubMed] [Google Scholar]
- 53.van den Brink GR. Hedgehog signaling in development and homeostasis of the gastrointestinal tract. Physiol Rev. 2007;87:1343–75. doi: 10.1152/physrev.00054.2006. [DOI] [PubMed] [Google Scholar]
- 54.Shinohara M, Mao M, Keeley TM, El-Zaatari M, Lee HJ, et al. Bone morphogenetic protein signaling regulates gastric epithelial cell development and proliferation in mice. Gastroenterology. 2010;139:2050–2060. doi: 10.1053/j.gastro.2010.08.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kim TH, Shivdasani RA. Notch signaling in stomach epithelial stem cell homeostasis. J Exp Med. 2011;208:677–88. doi: 10.1084/jem.20101737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Voon DC, Wang H, Koo JK, Nguyen TA, Hor YT, et al. Runx3 protects gastric epithelial cells against epithelial-mesenchymal transition-induced cellular plasticity and tumorigenicity. Stem Cells. 2012;30:2088–99. doi: 10.1002/stem.1183. [DOI] [PubMed] [Google Scholar]
- 57.Daley GQ. The promise and perils of stem cell therapeutics. Cell Stem Cell. 2012;10:740–9. doi: 10.1016/j.stem.2012.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Singh SR. Stem cell niche in tissue homeostasis, aging and cancer. Curr Med Chem. 2012;19:5965–74. doi: 10.2174/092986712804485917. [DOI] [PubMed] [Google Scholar]
- 59.Bomken S, Fiser K, Heidenreich O, Vormoor J. Understanding the cancer stem cell. Br J Cancer. 2010;103:439–45. doi: 10.1038/sj.bjc.6605821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Nguyen LV, Vanner R, Dirks P, Eaves CJ. Cancer stem cells: an evolving concept. Nat Rev Cancer. 2012;12(2):133–43. doi: 10.1038/nrc3184. [DOI] [PubMed] [Google Scholar]
- 61.Brunner TB, Kunz-Schughart LA, Grosse-Gehling P, Baumann M. Cancer stem cells as a predictive factor in radiotherapy. Semin Radiat Oncol. 2012;22:151–74. doi: 10.1016/j.semradonc.2011.12.003. [DOI] [PubMed] [Google Scholar]
- 62.Magee JA, Piskounova E, Morrison SJ. Cancer stem cells: impact, heterogeneity, and uncertainty. Cancer Cell. 2012;21:283–96. doi: 10.1016/j.ccr.2012.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Borovski T, De Sousa E, Melo F, Vermeulen L, Medema JP. Cancer stem cell niche: the place to be. Cancer Res. 2011;71:634–9. doi: 10.1158/0008-5472.CAN-10-3220. [DOI] [PubMed] [Google Scholar]
- 64.Bonnet D, Dick JE. Human acute myeloid leukemia is organized as a hierarchy that originates from a primitive hematopoietic cell. Nat Med. 1997;3:730–7. doi: 10.1038/nm0797-730. [DOI] [PubMed] [Google Scholar]
- 65.Singh SK, Hawkins C, Clarke ID, Squire JA, et al. Identification of human brain tumour initiating cells. Nature. 2004;432:396–401. doi: 10.1038/nature03128. [DOI] [PubMed] [Google Scholar]
- 66.Al-Hajj M, Wicha MS, Benito-Hernandez A, Morrison SJ, Clarke MF. Prospective identification of tumorigenic breast cancer cells. Proc Natl Acad Sci USA. 2003;100:3983–8. doi: 10.1073/pnas.0530291100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ritchie KE, Nör JE. Perivascular stem cell niche in head and neck cancer. Cancer Lett. 2013 doi: 10.1016/j.canlet.2012.07.025. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Bussolati B, Dekel B, Azzarone B, Camussi G. Human renal cancer stem cells. Cancer Lett. 2013 doi: 10.1016/j.canlet.2012.05.007. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 69.Lin SP, Lee YT, Yang SH, Miller SA, Chiou SH, Hung MC, Hung SC. Colon cancer stem cells resist antiangiogenesis therapy-induced apoptosis. Cancer Lett. 2013;328:226–34. doi: 10.1016/j.canlet.2012.08.036. [DOI] [PubMed] [Google Scholar]
- 70.Li Y, Kong D, Ahmad A, Bao B, Sarkar FH. Pancreatic cancer stem cells: Emerging target for designing novel therapy. Cancer Lett. 2013 doi: 10.1016/j.canlet.2012.03.018. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lee TK, Castilho A, Cheung VC, Tang KH, Ma S, Ng IO. CD24(+) liver tumor-initiating cells drive self-renewal and tumor initiation through STAT3-mediated NANOG regulation. Cell Stem Cell. 2011;9:50–63. doi: 10.1016/j.stem.2011.06.005. [DOI] [PubMed] [Google Scholar]
- 72.Civenni G, Walter A, Kobert N, Mihic-Probst D, Zipser M, et al. Human CD271-positive melanoma stem cells associated with metastasis establish tumor heterogeneity and long-term growth. Cancer Res. 2011;71:3098–109. doi: 10.1158/0008-5472.CAN-10-3997. [DOI] [PubMed] [Google Scholar]
- 73.Lundin A, Driscoll B. Lung cancer stem cells: Progress and prospects. Cancer Lett. 2013 doi: 10.1016/j.canlet.2012.08.014. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Driessens G, Beck B, Caauwe A, Simons BD, Blanpain C. Defining the mode of tumour growth by clonal analysis. Nature. 2012;488:527–30. doi: 10.1038/nature11344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Chen J, Li Y, Yu TS, McKay RM, Burns DK, Kernie SG, Parada LF. A restricted cell population propagates glioblastoma growth after chemotherapy. Nature. 2012;488:522–6. doi: 10.1038/nature11287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Schepers AG, Snippert HJ, Stange DE, van den Born M, van Es JH, van de Wetering M, Clevers H. Lineage tracing reveals Lgr5+ stem cell activity in mouse intestinal adenomas. Science. 2012;337:730–5. doi: 10.1126/science.1224676. [DOI] [PubMed] [Google Scholar]
- 77.Xu G, Shen J, Ou Yang X, Sasahara M, Su X. Cancer stem cells: the ‘heartbeat’ of gastric cancer. J Gastroenterol. 2012 doi: 10.1007/s00535-012-0712-y. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 78.Houghton J, Stoicov C, Nomura S, Rogers AB, Carlson J, et al. Gastric cancer originating from bone marrow-derived cells. Science. 2004;306:1568–71. doi: 10.1126/science.1099513. [DOI] [PubMed] [Google Scholar]
- 79.Takaishi S, Okumura T, Wang TC. Gastric cancer stem cells. J Clin Oncol. 2008;26:2876–82. doi: 10.1200/JCO.2007.15.2603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Quante M, Tu SP, Tomita H, Gonda T, Wang SS, Takashi S, et al. Bone marrow-derived myofibroblasts contribute to the mesenchymal stem cell niche and promote tumor growth. Cancer Cell. 2011;19:257–72. doi: 10.1016/j.ccr.2011.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Shibata W, Ariyama H, Westphalen CB, Worthley DL, et al. Stromal cell-derived factor-1 overexpression induces gastric dysplasia through expansion of stromal myofibroblasts and epithelial progenitors. Gut. 2013;62:192–200. doi: 10.1136/gutjnl-2011-301824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Varon C, Dubus P, Mazurier F, Asencio C, Chambonnier L, et al. Helicobacter pylori infection recruits bone marrow-derived cells that participate in gastric preneoplasia in mice. Gastroenterology. 2012;142:281–91. doi: 10.1053/j.gastro.2011.10.036. [DOI] [PubMed] [Google Scholar]
- 83.Simon E, Petke D, Böger C, Behrens HM, Warneke V, Ebert M, Röcken C. The spatial distribution of LGR5+ cells correlates with gastric cancer progression. PLoS One. 2012;7:e35486. doi: 10.1371/journal.pone.0035486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Li Q, Jia Z, Wang L, Kong X, Li Q, et al. Disruption of Klf4 in villin-positive gastric progenitor cells promotes formation and progression of tumors of the antrum in mice. Gastroenterology. 2012;142:531–42. doi: 10.1053/j.gastro.2011.11.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Quante M, Bhagat G, Abrams JA, Marache F, Good P, et al. Bile acid and inflammation activate gastric cardia stem cells in a mouse model of Barrett-like metaplasia. Cancer Cell. 2012;21:36–51. doi: 10.1016/j.ccr.2011.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Takaishi S, Okumura T, Tu S, Wang SS, Shibata W, et al. Identification of gastric cancer stem cells using the cell surface marker CD44. Stem Cells. 2009;27:1006–20. doi: 10.1002/stem.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Zhang C, Li C, He F, Cai Y, Yang H. Identification of CD44+CD24+ gastric cancer stem cells. J Cancer Res Clin Oncol. 2011;137:1679–86. doi: 10.1007/s00432-011-1038-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Yang L, Ping YF, Yu X, Qian F, Guo ZJ, Qian C, Cui YH, Bian XW. Gastric cancer stem-like cells possess higher capability of invasion and metastasis in association with a mesenchymal transition phenotype. Cancer Lett. 2011;310:46–52. doi: 10.1016/j.canlet.2011.06.003. [DOI] [PubMed] [Google Scholar]
- 89.Chen T, Yang K, Yu J, Meng W, Yuan D, et al. Identification and expansion of cancer stem cells in tumor tissues and peripheral blood derived fromgastric adenocarcinoma patients. Cell Res. 2012;22:248–58. doi: 10.1038/cr.2011.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Han ME, Jeon TY, Hwang SH, Lee YS, Kim HJ, et al. Cancer spheres from gastric cancer patients provide an ideal model system for cancer stem cell research. Cell Mol Life Sci. 2011;68:3589–605. doi: 10.1007/s00018-011-0672-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Jiang J, Zhang Y, Chuai S, Wang Z, Zheng D, Xu F, Zhang Y, Li C, Liang Y, Chen Z. Trastuzumab (herceptin) targets gastric cancer stem cells characterized by CD90 phenotype. Oncogene. 2012;31:671–82. doi: 10.1038/onc.2011.282. [DOI] [PubMed] [Google Scholar]
- 92.Jiang Y, He Y, Li H, Li HN, Zhang L, Hu W, Sun YM, Chen FL, Jin XM. Expressions of putative cancer stem cell markers ABCB1, ABCG2, and CD133 are correlated with the degree of differentiation of gastric cancer. Gastric Cancer. 2012;15:440–50. doi: 10.1007/s10120-012-0140-y. [DOI] [PubMed] [Google Scholar]
- 93.Katsuno Y, Ehata S, Yashiro M, Yanagihara K, Hirakawa K, Miyazono K. Coordinated expression of REG4 and aldehyde dehydrogenase 1 regulating tumourigenic capacity of diffuse-type gastric carcinoma-initiating cells is inhibited by TGF-β. J Pathol. 2012;228:391–404. doi: 10.1002/path.4020. [DOI] [PubMed] [Google Scholar]
- 94.Ohkuma M, Haraguchi N, Ishii H, Mimori K, Tanaka F, et al. Absence of CD71 transferrin receptor characterizes human gastric adenosquamous carcinoma stem cells. Ann Surg Oncol. 2012;19:1357–64. doi: 10.1245/s10434-011-1739-7. [DOI] [PubMed] [Google Scholar]
- 95.Liu J, Ma L, Xu J, Liu C, Zhang J, Liu J, Chen R, Zhou Y. Spheroid body-forming cells in the human gastric cancer cell line MKN-45 possess cancer stem cell properties. Int J Oncol. 2013;42:453–9. doi: 10.3892/ijo.2012.1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Fujii Y, Yoshihashi K, Suzuki H, Tsutsumi S, Mutoh H, Maeda S, et al. CDX1 confers intestinal phenotype on gastric epithelial cells via induction of stemness-associated reprogramming factors SALL4 and KLF5. Proc Natl Acad Sci USA. 2012;109:20584–9. doi: 10.1073/pnas.1208651109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Zhang Y, Huang X. Investigation of doublecortin and calcium/calmodulin-dependent protein kinase-like-1-expressing cells in the mouse stomach. J Gastroenterol Hepatol. 2010;25:576–82. doi: 10.1111/j.1440-1746.2009.06114.x. [DOI] [PubMed] [Google Scholar]
- 98.Kikuchi M, Nagata H, Watanabe N, Watanabe H, Tatemichi M, Hibi T. Altered expression of a putative progenitor cell marker DCAMKL1 in the rat gastric mucosa in regeneration, metaplasia and dysplasia. BMC Gastroenterol. 2010;10:65. doi: 10.1186/1471-230X-10-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Okumura T, Ericksen RE, Takaishi S, Wang SS, Dubeykovskiy Z, Shibata W, Betz KS, Muthupalani S, Rogers AB, Fox JG, Rustgi AK, Wang TC. K-ras mutation targeted to gastric tissue progenitor cells results in chronic inflammation, an altered microenvironment, and progression to intraepithelial neoplasia. Cancer Res. 2010;70:8435–45. doi: 10.1158/0008-5472.CAN-10-1506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Matsuoka J, Yashiro M, Sakurai K, Kubo N, Tanaka H, Muguruma K, Sawada T, Ohira M, Hirakawa K. Role of the stemness factors sox2, oct3/4, and nanog in gastric carcinoma. J Surg Res. 2012;174:130–5. doi: 10.1016/j.jss.2010.11.903. [DOI] [PubMed] [Google Scholar]
- 101.Sashikawa Kimura M, Mutoh H, Sugano K. SOX9 is expressed in normal stomach, intestinal metaplasia, and gastric carcinoma in humans. J Gastroenterol. 2011;46:1292–9. doi: 10.1007/s00535-011-0443-5. [DOI] [PubMed] [Google Scholar]
- 102.Fukui T, Kishimoto M, Nakajima A, Yamashina M, Nakayama S, Kusuda T, Sakaguchi Y, Yoshida K, Uchida K, Nishio A, Matsuzaki K, Okazaki K. The specific linker phosphorylation of Smad2/3 indicates epithelial stem cells in stomach; particularly increasing in mucosae of Helicobacter-associated gastritis. J Gastroenterol. 201;46:456–68. doi: 10.1007/s00535-010-0364-8. [DOI] [PubMed] [Google Scholar]
- 103.Gupta A, Wodziak D, Tun M, Bouley DM, Lowe AW. Loss of anterior gradient 2 (Agr2) expression results in hyperplasia and defective lineage maturation in the murine stomach. J Biol Chem. 2013;288:4321–33. doi: 10.1074/jbc.M112.433086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Reinisch C, Kandutsch S, Uthman A, Pammer J. BMI-1: a protein expressed in stem cells, specialized cells and tumors of the gastrointestinal tract. Histol Histopathol. 2006;21:1143–9. doi: 10.14670/HH-21.1143. [DOI] [PubMed] [Google Scholar]
- 105.Al-Marzoqee FY, Khoder G, Al-Awadhi H, John R, Beg A, Vincze A, Branicki F, Karam SM. Upregulation and inhibition of the nuclear translocation of Oct4 during multistep gastric carcinogenesis. Int J Oncol. 2012;41:1733–43. doi: 10.3892/ijo.2012.1608. [DOI] [PubMed] [Google Scholar]
- 106.Zhao P, Li Y, Lu Y. Aberrant expression of CD133 protein correlates with Ki-67 expression and is a prognostic marker in gastric adenocarcinoma. BMC Cancer. 2010;10:218. doi: 10.1186/1471-2407-10-218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Fukamachi H, Shimada S, Ito K, Ito Y, Yuasa Y. CD133 is a marker of gland-forming cells in gastric tumors and Sox17 is involved in its regulation. Cancer Sci. 2011;102:1313–21. doi: 10.1111/j.1349-7006.2011.01947.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Wakamatsu Y, Sakamoto N, Oo HZ, Naito Y, Uraoka N, Anami K, Sentani K, Oue N, Yasui W. Expression of cancer stem cell markers ALDH1, CD44 and CD133 in primary tumor and lymph node metastasis of gastric cancer. Pathol Int. 2012;62:112–9. doi: 10.1111/j.1440-1827.2011.02760.x. [DOI] [PubMed] [Google Scholar]
- 109.Nishikawa S, Konno M, Hamabe A, Hasegawa S, Kano Y, Ohta K, Fukusumi T, Sakai D, Kudo T, Haraguchi N, Satoh T, Takiguchi S, Mori M, Doki Y, Ishii H. Aldehyde dehydrogenasehigh gastric cancer stem cells are resistant to chemotherapy. Int J Oncol. 2013;42:1437–42. doi: 10.3892/ijo.2013.1837. [DOI] [PubMed] [Google Scholar]
- 110.Wang T, Ong CW, Shi J, Srivastava S, Yan B, Cheng CL, Yong WP, Chan SL, Yeoh KG, Iacopetta B, Salto-Tellez M. Sequential expression of putative stem cell markers in gastric carcinogenesis. Br J Cancer. 2011;105:658–65. doi: 10.1038/bjc.2011.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Lennerz JK, Kim SH, Oates EL, Huh WJ, Doherty JM, Tian X, Bredemeyer AJ, Goldenring JR, Lauwers GY, Shin YK, Mills JC. The transcription factor MIST1 is a novel human gastric chief cell marker whose expression is lost in metaplasia, dysplasia, and carcinoma. Am J Pathol. 2010 Sep;177(3):1514–33. doi: 10.2353/ajpath.2010.100328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Peterson AJ, Menheniott TR, O’Connor L, Walduck AK, Fox JG, Kawakami K, Minamoto T, Ong EK, Wang TC, Judd LM, Giraud AS. Helicobacter pylori infection promotes methylation and silencing of trefoil factor 2, leading to gastric tumor development in mice and humans. Gastroenterology. 2010;139:2005–17. doi: 10.1053/j.gastro.2010.08.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Haraguchi N, Utsunomiya T, Inoue H, Tanaka F, Mimori K, Barnard GF, Mori M. Characterization of a side population of cancer cells from human gastrointestinal system. Stem Cells. 2006;24:506–13. doi: 10.1634/stemcells.2005-0282. [DOI] [PubMed] [Google Scholar]
- 114.Fukuda K, Saikawa Y, Ohashi M, Kumagai K, Kitajima M, Okano H, Matsuzaki Y, Kitagawa Y. Tumor initiating potential of side population cells in human gastric cancer. Int J Oncol. 2009;34:1201–7. [PubMed] [Google Scholar]
- 115.Nishii T, Yashiro M, Shinto O, Sawada T, Ohira M, Hirakawa K. Cancer stem cell-like SP cells have a high adhesion ability to the peritoneum in gastric carcinoma. Cancer Sci. 2009;100:1397–402. doi: 10.1111/j.1349-7006.2009.01211.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Schmuck R, Warneke V, Behrens HM, Simon E, Weichert W, Röcken C. Genotypic and phenotypic characterization of side population of gastric cancer cell lines. Am J Pathol. 2011;178:1792–804. doi: 10.1016/j.ajpath.2010.12.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Ehata S, Johansson E, Katayama R, Koike S, Watanabe A, et al. Transforming growth factor-β decreases the cancer-initiating cell population within diffuse-type gastric carcinoma cells. Oncogene. 2011;30:1693–705. doi: 10.1038/onc.2010.546. [DOI] [PubMed] [Google Scholar]
- 118.She JJ, Zhang PG, Wang X, Che XM, Wang ZM. Side population cells isolated from KATO III human gastric cancer cell line have cancer stem cell-like characteristics. World J Gastroenterol. 2012;18:4610–7. doi: 10.3748/wjg.v18.i33.4610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Zhang H, Xi H, Cai A, Xia Q, Wang XX, Lu C, et al. Not all side population cells contain cancer stem-like cells in human gastric cancer cell lines. Dig Dis Sci. 2013;58(1):132–9. doi: 10.1007/s10620-012-2330-1. [DOI] [PubMed] [Google Scholar]
- 120.Cai C, Zhu X. The Wnt/β-catenin pathway regulates self-renewal of cancer stem-like cells in human gastric cancer. Mol Med Report. 2012;5:1191–6. doi: 10.3892/mmr.2012.802. [DOI] [PubMed] [Google Scholar]
- 121.Ishimoto T, Oshima H, Oshima M, Kai K, Torii R, Masuko T, Baba H, Saya H, Nagano O. CD44+ slow-cycling tumor cell expansion is triggered by cooperative actions of Wnt and prostaglandin E2 in gastric tumorigenesis. Cancer Sci. 2010;101:673–8. doi: 10.1111/j.1349-7006.2009.01430.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Song Z, Yue W, Wei B, Wang N, Li T, Guan L, Shi S, Zeng Q, Pei X, Chen L. Sonic hedgehog pathway is essential for maintenance of cancer stem-like cells in human gastric cancer. PLoS One. 2011;6:e17687. doi: 10.1371/journal.pone.0017687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Ding SZ, Zheng PY. Helicobacter pylori infection induced gastric cancer; advance in gastric stem cell research and the remaining challenges. Gut Pathog. 2012;4:18. doi: 10.1186/1757-4749-4-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Correa P, Houghton J. Carcinogenesis of Helicobacter pylori. Gastroenterology. 2007;133:659–72. doi: 10.1053/j.gastro.2007.06.026. [DOI] [PubMed] [Google Scholar]
- 125.Uehara T, Ma D, Yao Y, Lynch JP, Morales K, Ziober A, Feldman M, Ota H, Sepulveda AR. H. pylori Infection Is Associated with DNA Damage of Lgr5-Positive Epithelial Stem Cells in the Stomach of Patients with Gastric Cancer. Dig Dis Sci. 2013;58:140–9. doi: 10.1007/s10620-012-2360-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Kuipers EJ, Perez-Perez GI, Meuwissen SG, Blaser MJ. Helicobacter pylori and atrophic gastritis: importance of the cagA status. J Natl Cancer Inst. 1995;87:1777–1780. doi: 10.1093/jnci/87.23.1777. [DOI] [PubMed] [Google Scholar]
- 127.Oh JD, Karam SM, Gordon JI. Intracellular Helicobacter pylori in gastric epithelial progenitors. Proc Natl Acad Sci USA. 2005;102:5186–91. doi: 10.1073/pnas.0407657102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Giannakis M, Chen SL, Karam SM, Engstrand L, Gordon JI. Helicobacter pylori evolution during progression from chronic atrophic gastritis to gastric cancer and its impact on gastric stem cells. Proc Natl Acad Sci USA. 2008;105:4358–63. doi: 10.1073/pnas.0800668105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Ferrand J, Lehours P, Schmid-Alliana A, Mégraud F, Varon C. Helicobacter pylori infection of gastrointestinal epithelial cells in vitro induces mesenchymal stem cell migration through an NF-κB-dependent pathway. PLoS One. 2011;6:e29007. doi: 10.1371/journal.pone.0029007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Pilpilidis I, Kountouras J, Zavos C, Katsinelos P. Upper gastrointestinal carcinogenesis: H. pylori and stem cell cross-talk. J Surg Res. 2011;166:255–64. doi: 10.1016/j.jss.2010.02.012. [DOI] [PubMed] [Google Scholar]
- 131.Noto JM, Khizanishvili T, Chaturvedi R, Piazuelo MB, Romero-Gallo J, et al. Helicobacter pylori promotes the expression of krüppel-like factor 5, a mediator of carcinogenesis, in vitro and in vivo. PLoS One. 2013;8:e54344. doi: 10.1371/journal.pone.0054344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Reya T, Morrison SJ, Clarke MF, Weissman IL. Stem cells, cancer, and cancer stem cells. Nature. 2001;414:105–11. doi: 10.1038/35102167. [DOI] [PubMed] [Google Scholar]
- 133.Li L, Neaves WB. Normal stem cells and cancer stem cells: the niche matters. Cancer Res. 2006;66:4553–7. doi: 10.1158/0008-5472.CAN-05-3986. [DOI] [PubMed] [Google Scholar]
- 134.Cabarcas SM, Mathews LA, Farrar WL. The cancer stem cell niche--there goes the neighborhood? Int J Cancer. 2011;129:2315–27. doi: 10.1002/ijc.26312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Voog J, Jones DL. Stem cells and the niche: a dynamic duo. Cell Stem Cell. 2010;6:103–15. doi: 10.1016/j.stem.2010.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Zeng W, Wan R, Zheng Y, Singh SR, Wei Y. Hypoxia, stem cells and bone tumor. Cancer Lett. 2011;313:129–36. doi: 10.1016/j.canlet.2011.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Sampieri K, Fodde R. Cancer stem cells and metastasis. Semin Cancer Biol. 2012;22:187–93. doi: 10.1016/j.semcancer.2012.03.002. [DOI] [PubMed] [Google Scholar]
- 138.Guo X, Oshima H, Kitmura T, Taketo MM, Oshima M. Stromal fibroblasts activated by tumor cells promote angiogenesis in mouse gastric cancer. J Biol Chem. 2008;283:19864–71. doi: 10.1074/jbc.M800798200. [DOI] [PubMed] [Google Scholar]
- 139.Kolev Y, Uetake H, Iida S, Ishikawa T, Kawano T, Sugihara K. Prognostic significance of VEGF expression in correlation with COX-2, microvessel density, and clinicopathological characteristics in human gastric carcinoma. Ann Surg Oncol. 2007;14:2738–47. doi: 10.1245/s10434-007-9484-7. [DOI] [PubMed] [Google Scholar]
- 140.Suzuki S, Dobashi Y, Hatakeyama Y, Tajiri R, Fujimura T, Heldin CH, Ooi A. Clinicopathological significance of platelet-derived growth factor (PDGF)-B and vascular endothelial growth factor-A expression, PDGF receptor-β phosphorylation, and microvessel density in gastriccancer. BMC Cancer. 2010;10:659. doi: 10.1186/1471-2407-10-659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Li M, Gu Y, Zhang Z, Zhang S, Zhang D, Saleem AF, Zhao X, Sun B. Vasculogenic mimicry: a new prognostic sign of gastric adenocarcinoma. Pathol Oncol Res. 2010;16:259–66. doi: 10.1007/s12253-009-9220-7. [DOI] [PubMed] [Google Scholar]
- 142.Jiang J, Liu W, Guo X, Zhang R, Zhi Q, Ji J, et al. IRX1 influences peritoneal spreading and metastasis via inhibiting BDKRB2-dependent neovascularization on gastric cancer. Oncogene. 2011;30:4498–508. doi: 10.1038/onc.2011.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Liu L, Ning X, Sun L, Zhang H, Shi Y, Guo C, Han S, Liu J, Sun S, Han Z, Wu K, Fan D. Hypoxia-inducible factor-1 alpha contributes to hypoxia-induced chemoresistance in gastriccancer. Cancer Sci. 2008;99:121–8. doi: 10.1111/j.1349-7006.2007.00643.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Komuro A, Yashiro M, Iwata C, Morishita Y, Johansson E, et al. Diffuse-type gastric carcinoma: progression, angiogenesis, and transforming growth factor beta signaling. J Natl Cancer Inst. 2009;101:592–604. doi: 10.1093/jnci/djp058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Johansson E, Komuro A, Iwata C, Hagiwara A, Fuse Y, et al. Exogenous introduction of tissue inhibitor of metalloproteinase 2 reduces accelerated growth of TGF-β-disrupted diffuse-type gastric carcinoma. Cancer Sci. 2010;101:2398–403. doi: 10.1111/j.1349-7006.2010.01688.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Van Cutsem E, de Haas S, Kang YK, Ohtsu A, et al. Bevacizumab in combination with chemotherapy as first-line therapy in advanced gastric cancer: a biomarker evaluation from the AVAGAST randomized phase III trial. J Clin Oncol. 2012;30:2119–27. doi: 10.1200/JCO.2011.39.9824. [DOI] [PubMed] [Google Scholar]
- 147.Yakisich JS. Challenges and limitations of targeting cancer stem cells and/or the tumour microenvironment. Drugs Ther Stud. 2012;2:e10. [Google Scholar]
- 148.Liu X, Sun Y, Guo J, Ma H, Li J, Dong B, Jin G, Zhang J, Wu J, Meng L, Shou C. Expression of hiwi gene in human gastric cancer was associated with proliferation of cancer cells. Int J Cancer. 2006;118(8):1922–9. doi: 10.1002/ijc.21575. [DOI] [PubMed] [Google Scholar]
- 149.Zhi QM, Chen XH, Ji J, Zhang JN, Li JF, Cai Q, Liu BY, Gu QL, Zhu ZG, Yu YY. Salinomycin can effectively kill ALDH(high) stem-like cells on gastric cancer. Biomed Pharmacother. 2011;65:509–15. doi: 10.1016/j.biopha.2011.06.006. [DOI] [PubMed] [Google Scholar]
- 150.Akagi H, Higuchi H, Sumimoto H, Igarashi T, Kabashima A, Mizuguchi H, et al. Suppression of myeloid cell leukemia-1 (Mcl-1) enhances chemotherapy-associated apoptosis ingastric cancer cells. Gastric Cancer. 2013;16:100–10. doi: 10.1007/s10120-012-0153-6. [DOI] [PubMed] [Google Scholar]
- 151.Ruan J, Ji J, Song H, Qian Q, Wang K, Wang C, Cui D. Fluorescent magnetic nanoparticle-labeled mesenchymal stem cells for targeted imaging and hyperthermia therapy of in vivo gastric cancer. Nanoscale Res Lett. 2012;7:309. doi: 10.1186/1556-276X-7-309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Ruan J, Song H, Li C, Bao C, Fu H, Wang K, Ni J, Cui D. DiR-labeled Embryonic Stem Cells for Targeted Imaging of in vivo Gastric Cancer Cells. Theranostics. 2012;2:618–28. doi: 10.7150/thno.4561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Ruan J, Song H, Qian Q, Li C, Wang K, Bao C, Cui D. HER2 monoclonal antibody conjugated RNase-A-associated CdTe quantum dots for targeted imaging and therapy of gastric cancer. Biomaterials. 2012;33:7093–102. doi: 10.1016/j.biomaterials.2012.06.053. [DOI] [PubMed] [Google Scholar]
- 154.Park J, Ku M, Kim E, Park Y, Hong Y, Haam S, Cheong JH, et al. CD44-specific supramolecular hydrogels for fluorescence molecular imaging of stem-like gastric cancer cells. Integr Biol. 2013;5:669–672. doi: 10.1039/c3ib20203h. [DOI] [PubMed] [Google Scholar]
- 155.Zieker D, Bühler S, Ustündag Z, Königsrainer I, et al. Induction of tumor stem cell differentiation-novel strategy to overcome therapy resistance in gastric cancer. Langenbecks Arch Surg. 2013 doi: 10.1007/s00423-013-1058-5. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 156.Wang B, Liu J, Ma LN, Xiao HL, Wang YZ, et al. Chimeric 5/35 adenovirus-mediated Dickkopf-1 overexpression suppressed tumorigenicity of CD44(+) gastric cancer cells via attenuating Wnt signaling. J Gastroenterol. 2012 doi: 10.1007/s00535-012-0711-z. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 157.Zhan YY, He JP, Chen HZ, Wang WJ, Cai JC. Orphan receptor TR3 is essential for the maintenance of stem-like properties in gastric cancer cells. Cancer Lett. 2013;329:37–44. doi: 10.1016/j.canlet.2012.09.022. [DOI] [PubMed] [Google Scholar]
- 158.Tian T, Zhang Y, Wang S, Zhou J, Xu S. Sox2 enhances the tumorigenicity and chemoresistance of cancer stem-like cells derived from gastric cancer. J Biomed Res. 2012;26:336–34. doi: 10.7555/JBR.26.20120045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Kim KY, Yi BR, Lee HR, Kang NH, Jeung EB, Kim SU, Choi KC. Stem cells with fused gene expression of cytosine deaminase and interferon-β migrate to human gastric cancer cells and result in synergistic growth inhibition for potential therapeutic use. Int J Oncol. 2012;40:1097–104. doi: 10.3892/ijo.2011.1288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Zhu X, Su D, Xuan S, Ma G, Dai Z, Liu T, Tang D, Mao W, Dong C. Gene therapy of gastric cancer using LIGHT-secreting human umbilical cord blood- derived mesenchymal stem cells. Gastric Cancer. 2012 doi: 10.1007/s10120-012-0166-1. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]