Abstract
The risk/benefit of adding fludarabine to a 2 Gy total body irradiation nonmyeloablative regimen is unknown. For this reason we conducted a prospective randomized trial comparing 2 Gy TBI alone or in combination with 90mg/m2 fludarabine (FLU/TBI) before transplantation of peripheral blood stem cells from HLA-matched related donors. Eighty-five patients with hematological malignancies were randomized to be conditioned with TBI alone (n=44) or FLU/TBI (n=41). All patients had initial engraftment. Two graft rejections were observed, both in the TBI group. Infection rates, nonrelapse mortality, and GVHD were similar between groups. Three-year overall survival was lower in the TBI group (54% vs. 65%; hazard ratio (HR) 0.57; p=0.09), with higher incidences of relapse/progression (55% vs. 40%; HR 0.55; p=0.06) and relapse-related mortality (37% vs. 28%; HR 0.53; p=0.09), and a lower progression-free survival (36% vs. 53%; HR 0.56; p=0.05). Median donor T-cell chimerism levels were significantly lower in the TBI group at days 28 (61% vs. 90%; p<0.0001) and 84 (68% vs. 92%; p<0.0001), as was NK-cell chimerism on day 28 (75% vs. 96%, p=0.0005). In conclusion, this randomized trial demonstrates the importance of fludarabine in augmenting the graft-versus-tumor effect by ensuring prompt and durable high level donor engraftment early post-transplant.
Introduction
During the development of the widely used nonmyeloablative conditioning regimen based on 2 Gy low-dose total body irradiation (TBI) and 90 mg/m2 fludarabine (FLU), the first 44 patients in the initial clinical trial were conditioned with a regimen directly translated from our canine studies [1-3]. The regimen consisted of 2 Gy TBI alone, and although results were encouraging a non-fatal graft rejection rate of 20% was observed [4]. In order to reduce the high rejection rate FLU (30 mg/m2/day for 3 days) was added to the 2 Gy TBI, which resulted in a decrease in rejections to 3% [5]. However, in a retrospective analysis of the first 176 patients with hematologic malignancies treated with nonmyeloablative HCT from HLA-identical related donors, higher nonrelapse mortality (NRM) was observed among patients conditioned with 2 Gy TBI and FLU (FLU/TBI) (FLU/TBI 31% vs. TBI 14% at 2 years; p=0.02). The increased NRM was due to increased infectious events with or without graft-versus-host disease (GVHD) [5].
As rejections were mainly observed in patients who had not been treated with significant myelosuppressive chemotherapy [5] (myeloid malignancies or multiple myeloma) prior to allogeneic HCT, we hypothesized that in patients at a low risk of rejection, conditioning with 2 Gy TBI alone could be sufficient to allow stable long term engraftment. However, these results needed to be taken with caution as they were retrospective and not from concurrently transplanted cohorts. To investigate the question more definitively, we initiated this phase III trial, where patients at low/moderate risk of rejection were randomized between conditioning with 2 Gy TBI alone or in combination with FLU (30 mg/m2/day for 3 days) /FLU/TBI) prior to transplantation with peripheral blood stem cells (PBSC) from human leukocyte antigen (HLA) matched related donors.
Patients and Methods
The study was a randomized phase III trial including 9 transplant centers: Fred Hutchinson Cancer Research Center (FHCRC), Medical College of Wisconsin, University of Leipzig, Oregon Health and Science University, VA Puget Sound Health Care System Seattle, Huntsman Cancer Institute/University of Utah, University of Torino School of Medicine, LDS Hospital, University of Tuebingen, and University of Cologne. The FHCRC acted as the coordinating center. The study was approved by the institutional review board at each center and written informed consent was obtained from all patients prior to start of treatment.
Randomization, study endpoints, and accrual
Patients were randomized equally between conditioning with 2 Gy TBI alone or in combination with FLU 30mg/m2/day for 3 days. The randomization was performed at the FHCRC and was stratified balanced over time for institution, disease risk (indolent vs. aggressive) [6], and a history of prior high-dose HCT.
The initial primary endpoint was to compare NRM at one year between arms. However, as accrual was slow, and it was unlikely that the enrollment goal of 200 patients would be met within a reasonable timeframe, the data safety monitoring board (DSMB) recommended a change of the primary objective to overall survival at 3 years to accommodate a lower accrual goal of 110 patients. Apart from including NRM at 1 year in the secondary objectives, the rest of the secondary objectives remained the same (disease progression; relapse-related mortality; graft rejection, grades II-IV acute and chronic GVHD; infections, and immune reconstitution). The protocol was opened for accrual in December 2003 and closed by the PI (with DSMB approval) in May 2011, after accruing 85 patients, due to a difference in relapse and progression rates between the two arms.
The database was analyzed as of December 12, 2012.
Eligibility criteria
Included in this study were patients with hematological malignancies treatable by allogeneic HCT who were not curable by high-dose conditioning with autologous stem cell support and ineligible for high-dose allogeneic HCT due to age or comorbidities. Donors were related and at least genotypically HLA-identical at one haplotype and phenotypically or genotypically identical at the allele level at HLA-A, -B, -C, -DRB1 and -DQB1 for the second haplotype [7]. The hematological malignancies allowed were aggressive non-Hodgkin lymphomas (NHL); low grade NHL with <6 months duration of complete remission (CR) between courses of therapy; mantle cell lymphoma; chronic lymphocytic leukemia (CLL) that did not meet the National Cancer Institute's Working Group criteria for CR or PR or relapse within 12 months after FLU or other nucleoside analogue-containing therapy; failed FLU-cyclophosphamide-rituximab therapy, had 17p deletion; progressed to prolymphocytic leukemia (PLL) or T-cell CLL or PLL; Hodgkin lymphoma (HL) that had at least failed frontline therapy and were ineligible for or had failed high-dose conditioning with autologous stem cell support; multiple myeloma (MM) that was chemotherapy-sensitive after failed high-dose conditioning with autologous stem cell support; acute myeloid leukemia (AML) or acute lymphoblastic leukemia (ALL) beyond first CR; chronic myeloid leukemia (CML) beyond first chronic phase after myelosuppressive therapy; myelodysplastic syndrome (MDS) or myeloproliferative disease (MPD) after myelosuppressive therapy; or Waldenstrom's macroglobulinemia after failing two courses of therapy. Patients with CML, AML, ALL, MDS or MPD had to have <5% marrow blasts at time of transplant. Myelosuppressive therapy less than 3 weeks prior to conditioning or high-dose conditioning with stem cell support less than 6 months prior to allogeneic HCT was not allowed.
Patients were excluded from the trial if they were pregnant or breast-feeding; had rapidly progressing intermediate- or high-grade NHL unless in minimal disease state; chronic myelomonocytic leukemia, leukemic blasts in the peripheral blood detected by standard pathology; central nervous system involvement refractory to intrathecal chemotherapy; infection with human immunodeficiency virus, bacterial, viral or fungal infections unresponsive to therapy; decompensated liver disease; lung carbon monoxide diffusion capacity (DLCO) < 30%, total lung capacity <30%, forced expiratory volume (FEV1) <30, or dependency on supplementary oxygen; symptomatic coronary artery disease or cardiac ejection fraction < 35%; poorly controlled hypertension; or a Karnofsky performance score < 50%.
Treatment and evaluations
Patients allocated to the TBI-only arm received 2 Gy at a rate of 0.07-0.10 Gy/minute from a linear accelerator on the day of HCT (day 0) with PBSC, while patients in the FLU/TBI arm in addition received FLU (30 mg/m2/day) on days −4, −3, and −2 before 2 Gy TBI and HCT. PBSC were collected from related donors on days −1 and 0 (CD34+ target cell dose was 5 × 106 cells/kg of recipient weight) following administration of granulocyte colony stimulating factor (G-CSF) (16 μg/kg) on days −4 to 0. Post-grafting immunosuppression consisted of oral cyclosporine (CSP; 5 mg/kg twice daily from days −3 to +56, and in the absence of GVHD tapered by 6% weekly until day +180) and mycophenolate mofetil (MMF; 15 mg/kg twice daily from day 0 to +27). CSP levels were monitored by immunoassay, and whole blood trough levels were targeted at 500 ng/ml for the first 28 days post-transplant and at 150-450 ng/ml until start of taper (Abbott, TDX, Abbott Park, IL). If there was evidence of persistent/progressive disease or relapse in the absence of GVHD on day 56 post-transplant, all immunosuppressive agents were rapidly tapered to allow graft-versus-tumor effects to occur. If relapse was observed, patients were considered treatment failures and taken off protocol. Donor lymphocyte infusion (DLI) was not offered on this protocol, and patients with low chimerism or disease progression were eligible for ongoing DLI protocols or treatment plans. For the purpose of survival analysis, patients were followed past the time point of relapse or DLI. Chimerism analysis was performed as previously described [8]. Peripheral blood CD3+ T-cell chimerism studies were performed on days +28, +84 and +365, and if the patient had <50% donor chimerism on day 28, additional analyses were performed on days +56 and +180. If the patient was not >95% CD3+ T-cell donor chimerism at 1 year, analyses were repeated annually. Natural killer (NK) cell (CD56) and granulocyte (CD33) chimerisms were obtained on days +28 and +84, respectively. Full donor chimerism was defined as > 95% donor CD3+ T cells, and graft rejection was defined as the inability to detect at least 5% donor CD3+ T cells in peripheral blood. Toxicities were determined using the Common Toxicity Criteria, Version 2.0 [9].
All patients received standard prophylaxis against infections as previously published [10]. Diagnosis, clinical grading, and treatment of acute and chronic GVHD were performed by local investigators according to established criteria [11,12]. Tumor responses were assessed using standard criteria and PCR, cytogenetics, FISH and flow cytometric based methods as appropriate.
Analysis of peripheral blood lymphocytes
Immunnophenotyping of peripheral blood lymphocytes was only performed in a subset of patient/donor pairs transplanted at the FHCRC. Peripheral blood was obtained from donors pre-G-CSF and from patients before transplant and at days +28, +84, +180 and +365. Enumeration of mononuclear cell (MNC) subsets [13] and immunophenotyping by flow cytometry for naïve and memory B cells; naïve and memory/effector CD4+ and CD8+ T cells; monocytes, NK cells; and myeloid and plasmacytoid dendritic cells, were performed as previously described [13,14].
Statistical analysis
Survival was estimated by the Kaplan-Meier method. Cumulative incidence was estimated by standard methods in the competing risk setting. Non-relapse death was a competing risk for the analysis of relapse/progression and relapse-related mortality, and, conversely, relapse/progression was a competing risk for the analysis of non-relapse death. Death was a competing risk for the analysis of acute and chronic GVHD and infection. All statistical comparisons of time-to-event endpoints are based on hazard ratio (HR) analysis using Cox regression. Comparisons of chimerism are based on two-sample Wilcoxon test. Comparisons of immune reconstitution are based on two-sample t-test. All p-values are two-sided.
Results
Patients
Forty-four patients were accrued into the TBI-only arm, and 41 into the FLU/TBI arm. Patient demographics are summarized in Table 1. Overall median patient age was 55 (range 17-73) years with a predominance of male gender (68%). Patients received G-CSF-mobilized PBSC containing a median of 7.9 (range, 1.9-22.7) ×106 CD34+ cells/kg and 3.6 (range, 1.0–40.9) ×108 CD3+ cells/kg. Underlying diseases were AML (n=15); MDS (n=4) NHL (n=32), CLL (n=9); MM (n=9) and HL (n=16) with slightly more patients with NHL compared to AML and lower relapse risk score [15] in the FLU/TBI arm, but this was not statistically different and within the limits expected by chance. More than half of the patients (55%) had failed at least one high-dose HCT. As randomization was stratified upon failed prior high-dose HCT, the number of patients was evenly distributed between arms (TBI only, n=26 (59%); FLU/TBI, n=21 (51%)). In the TBI arm, 3 of 26 patients had failed an allogeneic HCT (all from different HLA-matched siblings) compared to none in the FLU/TBI arm. There was no difference in median HCT comorbidity index between arms [16]. Four patients in the TBI arm and 3 in the FLU/TBI arm, all with refractory or relapsed CD20+ B-cell lymphomas, were concurrently enrolled on a protocol (ClinicalTrials.gov identifier: NCT00867529) studying the effects of peri-transplant rituximab (days −3, 10, 24 and 38).
Table 1.
Pre-transplant demographics
| Characteristic | TBI only (n= 44) | Flu/TBI (n= 41) |
|---|---|---|
| Patient age, median (range), years | 54 (17-73) | 56 (18-72) |
| Male patient gender, no. (%) | 32 (73) | 26 (63) |
| Donor age, median (range), years | 53 (17-73) | 54 (15-71) |
| Sex of patient / donor | ||
| Male / Female, no. (%) | 15 (34) | 15 (37) |
| Other combinations, no. (%) | 29 (66) | 26 (63) |
| CMV serostatus of patient/donor | ||
| Negative/negative, no. (%) | 14 (32) | 12 (29) |
| Other combinations, no. (%) | 30 (68) | 29 (71) |
| Prior failed high dose HCT | ||
| Autologous | 23 (52) | 21 (51) |
| Allogeneic | 3 (7) | 0 |
| Number of previous regimens, median (range) | 5 (1-17) | 5 (1-19) |
| Diagnoses, no. (%) | ||
| Non-Hodgkin lymphoma, no. (%) | 14 (32) | 18 (44) |
| Acute myeloid leukemia, no. (%) | 10 (23) | 5 (12) |
| Multiple myeloma, no. (%) | 5 (11) | 4 (10) |
| Chronic lymphocytic leukemia, no. (%) | 5 (11) | 4 (10) |
| Myelodysplastic syndrome, no. (%) | 2 (5) | 2 (5) |
| Hodgkin lymphoma, no. (%) | 8 (18) | 8 (20) |
| Relapse risk [15] | ||
| Low, no. (%) | 9 (20) | 14 (34) |
| Standard, no. (%) | 18 (41) | 12 (29) |
| High, no. (%) | 17 (39) | 15 (37) |
| HCT comorbidity index, number (%) | ||
| 0 | 13 (30) | 6 (15) |
| 1,2 | 6 (14) | 13 (33) |
| 3+ | 24 (56) | 20 (51) |
| CD34+ cells × 106 / kg, median (range) | 7.9 (2.6-19.8) | 7.7 (1.9-22.7) |
| CD3+ cells × 108 / kg, median (range) | 3.7 (1.4-40.9) | 3.5 (1.0-9.7) |
Peripheral blood cell changes, rejections, and chimerism
All patients had initial engraftment. While no patients in the FLU/TBI arm rejected their grafts, two patients in the TBI arm, one with AML in second CR and one with MDS (refractory anemia with excess of blasts-2), experienced graft rejection 31 and 262 days after transplant, respectively. The patient with AML had failed a prior allogeneic high-dose HCT from a different HLA-matched sibling, and after rejecting the graft from the current 2 Gy TBI conditioned HCT, went on to get a third allogeneic HCT from the same sibling and subsequently died of disease progression. The patient with MDS was in complete remission prior to transplant having received only one cycle of cytarabine and mitoxantrone. After rejecting the graft on this trial, the patient received a second myeloablative transplant from a syngeneic donor. The patient was still alive and in remission at last follow-up.
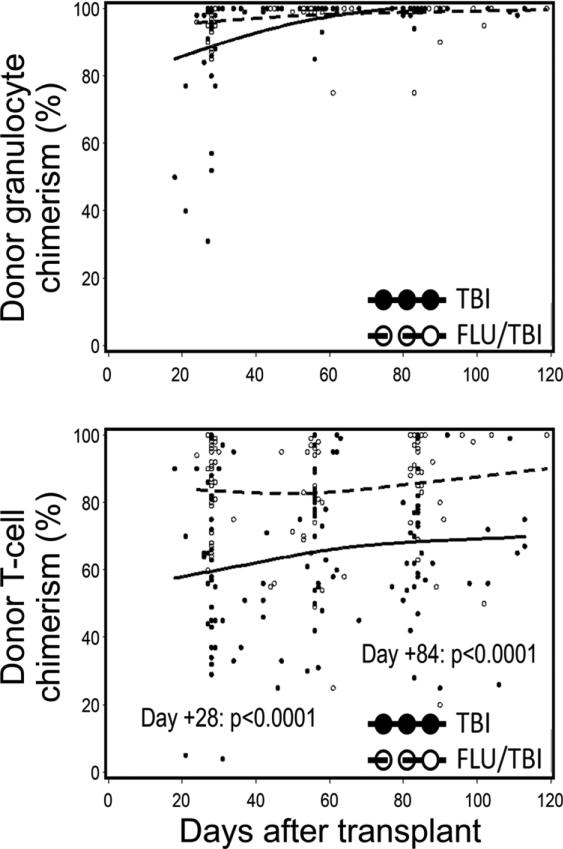
Near-complete donor granulocyte chimerism was achieved promptly, with no significant differences between arms (Figure 1A). Median donor T-cell chimerism levels were significantly higher in the FLU/TBI arm compared to the TBI arm at days +28 (90% vs. 61%, p<0.0001) and +84 (92% vs. 68%, p<0.0001) (Figure 1B). Median day +28 NK cell donor chimerism was also significantly higher in FLU/TBI conditioned patients (FLU/TBI (n=14), 96%; TBI (n=15), 75%; p=0.0005) and correlated with day +28 donor T-cell chimerism (Pearsons correlation coefficient = 0.82; p<0.0001).
Figure 1. Donor granulocyte and T-cell chimerism.
Percent donor granulocyte (A) and T-cell chimerism (B) in patients conditioned with 2 Gy total body irradiation only (TBI, n=44) or in combination with 90 mg/m2 fludarabine (FLU/TBI, n=41). Horizontal lines represent medians and dots, the individual data points. P-values are two-tailed.
Median absolute granulocyte count nadirs were similar in the two groups (TBI: 396 (range, 0-2340) cells/μl; FLU/TBI: 270 (0-1090) cells/μl; p=0.32). However, the median number of days with absolute granulocyte counts below 500 cells/μl was significantly higher in the FLU/TBI group (TBI: 0 (range 0-40) days; FLU/TBI: 4 (range 0-17) days; p=0.05), but the number of patients who required G-CSF treatment for prolonged neutropenia (persistence or development of granulocyte counts below 500 cell/μl past day +21 post-transplant) was similar (TBI: 12%; FLU/TBI: 18%; p=0.45). Platelet nadirs (TBI: 59,000 [range 6,000-251,000] platelets/μl; FLU/TBI: 59,000 [range 6,000-209,000] platelets/μl; p=0.62) and days below platelet counts of 20,000 platelets/μl (TBI: 0 [range 0-24] days; FLU/TBI: 0 [range 0-3] days; p=0.63) were similar in both arms. The percentages of patients who needed red blood cell transfusions trended to be higher in the FLU/TBI arm (TBI 43%; FLU/TBI 63%; p=0.06), while the percentages of patients who needed platelet transfusions were similar in both arms (TBI 23%; FLU/TBI 27%; p=0.66).
DLI
None of the patients in the FLU/TBI arm received DLI. A total of 8 patients in the TBI arm received DLI: two patients due to low chimerism (both died of relapsed AML) and six patients due to relapse or progression (four died of relapse, while one with HL and one with small lymphocytic lymphoma were alive at last follow-up).
Immune reconstitution
In the TBI arm, samples for immunophenotyping were available from 16 patients at day 28 and from 8 patients at day 90, while in the FLU/TBI arm samples were available from 7 and 13 patients at days 28 and 90, respectively.
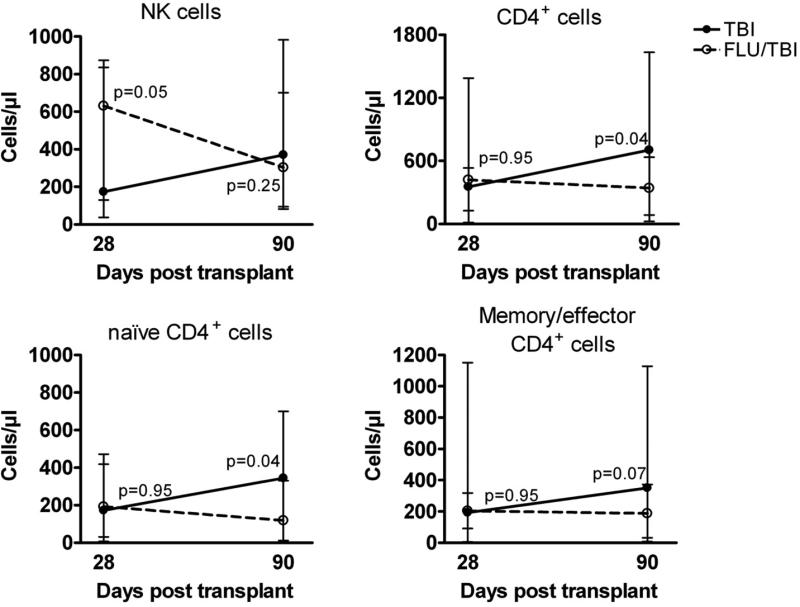
Immunophenotyping showed that the median absolute number of NK cells was significantly higher at day +28 after transplantation in the FLU/TBI arm compared to the TBI arm (Figure 2). The difference disappeared by day +90. No differences between arms were observed for the median absolute number of CD4+ cells at day +28, but at day +90 lower levels were observed in patients in the FLU/TBI arm compared to the TBI arm, [significant for naïve CD4+ cells and a trend for the CD4 memory/effector population (Figure 2)]. There were no significant differences between arms in absolute counts of naïve and memory/effector CD8+ T cells; naïve and memory B cells; monocytes; and myeloid and plasmacytoid dendritic cells at any post-transplant time point, and in the counts of NK cells and naïve and memory/effector CD4 T cells on day 180 and 365 (data not shown).
Figure 2. Immune reconstitution.
Mean absolute numbers of natural killer (NK) cells, CD4+, naïve CD4+, and memory/effector CD4+ cells at days 28 (TBI n=16, FLU/TBI n=7) and 90 (TBI n=8, FLU/TBI n=13) post-transplant in patients conditioned with 2 Gy total body irradiation only (TBI) or in combination with 90 mg/m2 fludarabine (FLU/TBI). Bars represent standard error of the mean. P-values are two-tailed.
GVHD
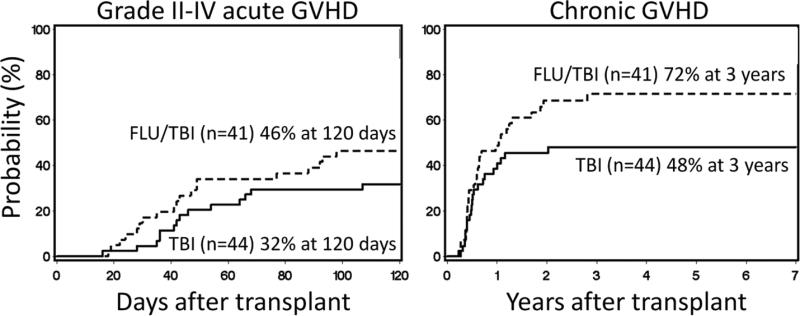
The cumulative incidences of grade II-IV and III-IV acute GVHD at 120 days in the TBI arm were 32% and 11%, respectively, and in the FLU/TBI arm 46% (grade II-IV acute GVHD HR 1.60 [95% confidence interval (CI) 0.8-3.1]; p=0.16) and 7% (grade III-IV acute GVHD HR 0.66 [95% CI 0.2-2.7]; p=0.56), respectively (Figure 3A). Although the cumulative incidence of chronic GVHD was higher in the FLU/TBI arm at 3 years (72% vs. 48%), the difference did not reach statistical significance, (HR=1.52 [0.9-2.7]; p=0.14) (Figure 3B).
Figure 3. Graft-versus-host disease.
Cumulative incidences of grade II-IV acute (A) and chronic (B) GVHD among patients conditioned with 2 Gy total body irradiation only (TBI) or in combination with 90 mg/m2 fludarabine (FLU/TBI).
Regimen related toxicities, infections, and NRM
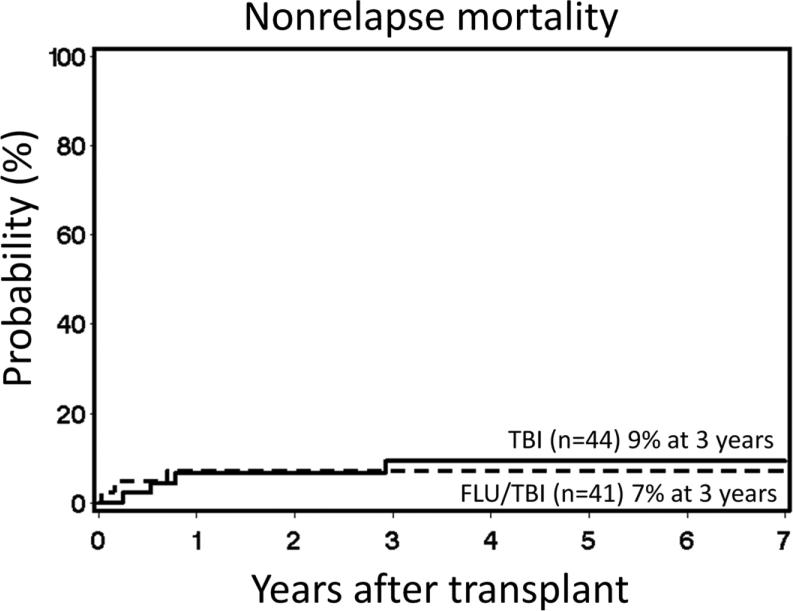
The most common toxicities were, as expected, reversible neutropenia and thrombocytopenia. In general, toxicities unrelated to the blood and bone marrow were mild with 14 patients in both arms (TBI arm 32%; FLU/TBI arm 34%) experiencing one or more grade III to IV toxicities. No differences in distribution of toxicities were observed between arms (supplemental data table 1). One patient in the FLU/TBI arm developed a squamous cell carcinoma 3.5 years after transplantation. The cumulative 3-year incidences of bacterial (TBI 64%, FLU/TBI 68%, p=0.52), viral (TBI 60%, FLU/TBI 68%, p=0.27) and fungal (TBI 21%, FLU/TBI 22%, p=0.87) infections were similar in the two arms. NRM at 3 years was 9% in the TBI arm and 7% in the FLU/TBI arm (HR 0.67 [95% CI 0.1-3.0]; p=0.59) (Figure 4). Of the 4 NRM deaths in the TBI arm, 1 was due to chronic GVHD and 1 was due to multiple pulmonary emboli and hemolytic uremic syndrome, while 2 were due to GVHD complicated with severe sepsis on days 194 and 287 post-transplant. In the FLU/TBI arm, 3 NRM deaths were observed on days 9, 59 and 254, all associated with sepsis.
Figure 4. Non-relapse mortality.
Cumulative incidence of non-relapse mortality among patients conditioned with 2 Gy total body irradiation only (TBI) or in combination with 90 mg/m2 fludarabine (FLU/TBI).
Relapse and survival
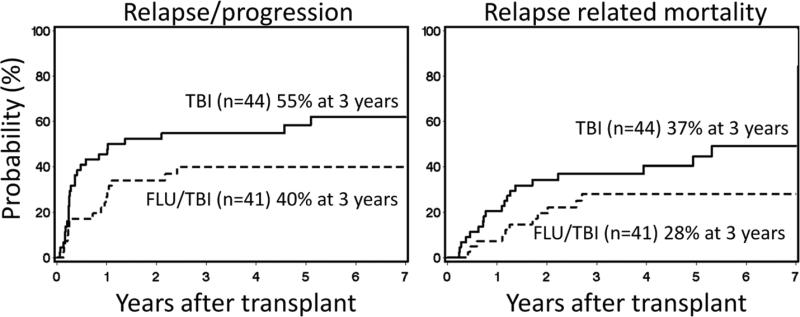
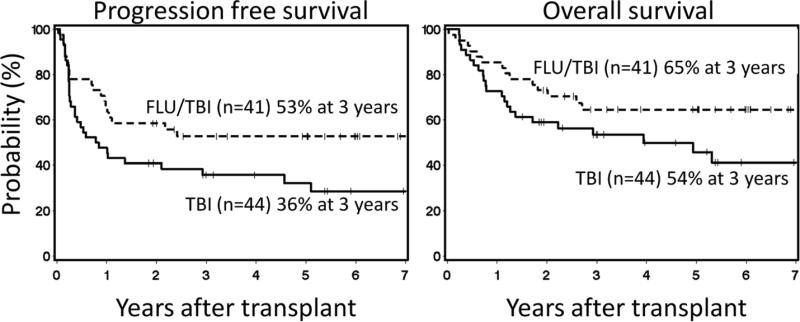
At the time of analysis, median follow-up of 48 surviving patients was 5 (range, 1.5-8) years. There was a trend for a higher progression/relapse rate in the TBI arm than in the FLU/TBI arm (55% vs. 40% at 3 years; HR 0.55 [95% CI 0.3-1.0], p=0.06) (Figure 5A), which translated into a trend towards a higher relapse-related mortality (37% vs. 28% at 3 years; HR 0.53 [95% CI 0.3-1.1], p=0.09) (Figure 5B) and worse progression-free survival (36% vs. 53% at 3 years; HR 0.56 [95% CI 0.3-1.0], p=0.05) (Figure 6A). Due to limited sample size, evaluation of whether the effects of FLU vary according to disease was not possible; however, similar trends of increased relapse in the TBI arm were observed when patients with lymphoid and myeloid malignancies were analyzed separately (data not shown).
Figure 5. Relapse or progression incidence and relapse related mortality.
Cumulative incidences of relapse or progression (A) and relapse related mortality among patients conditioned with 2 Gy total body irradiation only (TBI) or in combination with 90 mg/m2 fludarabine (FLU/TBI).
Figure 6. Progression free survival and overall survival.
Cumulative incidences of progression free survival (A) and overall survival (B) among patients conditioned with 2 Gy total body irradiation only (TBI) or in combination with 90 mg/m2 fludarabine (FLU/TBI).
Twenty-six patients relapsed in the TBI arm. At the end of follow-up, 3 were alive with relapsed disease (HL, n=2; NHL, n=1), while 4 (HL, n=1; NHL, n=2; MM, n=1) were brought back into remission. In the FLU/TBI arm, 16 patients relapsed of whom 5 were alive at end of follow-up. One of the 5 who had HL was brought back into remission, 3 had progressive disease and one patient with CLL had stable disease.
Compared to the TBI arm, there was a trend towards higher overall survival in the FLU/TBI arm (54% vs. 65% at 3 years; HR 0.57 [95% CI 0.3-1.1], p=0.09) (Figure 6B).
Discussion
Historically, the addition of FLU to 2 Gy TBI to the conditioning regimen successfully reduced the rejection rate from 20 to 3%, by augmenting the pretransplant immunosuppression [4,5,17]. However, in a retrospective analysis of the first 176 patients, the question was raised of whether the addition of FLU to 2 Gy TBI exposed patients at low to moderate risk of rejection to unnecessary toxicity [5]. In the current study, 85 patients at low to moderate risk of rejection were randomized to conditioning with either 2 TBI alone or in combination with FLU. Baseline characteristics were balanced between groups, except for a slight imbalance towards more patients with NHL compared to AML in the FLU/TBI group. Although number of prior treatment regimens and transplantations were similar in both groups, 21 of 41 patients in the FLU/TBI arm had failed autologous transplants. In the TBI group, 23 of 44 patients failed autografts and 3 failed allografts. Only two rejections were observed in the trial, both in the TBI only group. One had a prior allograft from a different donor, possibly indicating that donor cells given in the first transplant were not adequately myelosuppressed with TBI only. For this reason, the current practice is to condition patients who have failed prior allografts with 3 Gy TBI in addition to FLU. The second patient that rejected their graft had only received one cycle of chemotherapy prior to transplant, which possibly was insufficiently myelosuppressive.
All patients had initial engraftment, even the 2 who experienced rejection. Donor granulocyte chimerism was prompt, with no difference between arms. However, significant differences were observed in the rate of achieving donor T- and NK-cell chimerism. Patients conditioned with FLU/TBI had significantly higher levels of donor T-cell chimerism at days 28 and 84 post-transplant. Day 28 NK-cell chimerism was also higher in the FLU/TBI group. Low levels of donor T- and NK-cell chimerism have previously been associated with graft rejection in settings of nonmyeloablative, reduced intensity and in high-dose conditioning. In a cohort of 38 patients transplanted after conditioning with FLU/TBI, Keil and coauthors [18] observed that donor T-cell chimerism <90% at day 28 was associated with a higher rejection rate. Similarly, analyses by Baron et al. have demonstrated that day-14 donor T- and NK-cell chimerism levels <50%, conditioning with 2 Gy TBI with or without FLU (90 mg/m2) were associated with increased graft rejection [19,20]. In a recent study of pediatric patients conditioned with a variety of high-dose and reduced-intensity regimens, early low donor T- and NK-cell chimerism levels were also associated with increased risk of graft rejection, irrespective of conditioning intensity [21].
Although, the addition of FLU was associated with increased number of days with absolute granulocyte counts below 500 cells/μl and a trend towards increased red blood cell transfusion needs, no increases in NRM or bacterial, viral or fungal infection rates were observed. However, it is possible that the two early septic deaths in the FLU/TBI arm could be related to FLU. NRM rates in both arms were lower than in the previous retrospective analysis [5] that prompted the current study, probably reflecting the recent years’ overall improvement in supportive care which have lowered NRM in general and, in particular, also negated the effect of FLU on NRM. Although 55% of the patients in the current study had failed a high dose transplant prior to entering the trial, NRM rates still compared favorably to previously published data on nonmyeloablative regimens, such as fludarabine/busulfan in AML/MDS (NRM 26% at 2 years) [22] and fludarabine/cyclophosphamide/rituximab in follicular lymphoma (NRM 15% at 5 years) [23]. A recent registry study investigating outcome of low-intensity conditioning allogeneic transplant in patients with NHL who had relapsed after autologous transplants, reported a NRM of 44% at 3 years [24].
In this study, the relapse/progression incidence, relapse-related mortality and PFS were superior in the FLU/TBI arm, while only non-significant trends towards higher rates of acute and chronic GVHD were observed. It is possible that some of the effect on relapse may be due to the antineoplastic effects of the FLU, although this is unlikely in this patient population.
The observed differences in outcome between arms in our study were likely due to differences in donor T- and NK-cell chimerism kinetics. Our results are in agreement with previous observations by both Keil et al. [18] and our own group [19], where donor T-cell chimerism <90% (day 28 post-transplant) and <75% (day 84 post-transplant) was associated with a higher risk of relapse and lower progression-free survival. Although it is agreed upon that the graft-versus-tumor effects and GVHD after HLA-identical HCT are mainly a product of T-cell activity [25], the roles of NK cells are far less explored. Killer cell immunoglobulin-like receptor (KIR) genes are inherited independently from HLA, and will be mismatched in 75% of matched related transplants [26]. In two studies by Baron et al. where patients were conditioned with FLU/TBI, and NK-cell chimerism was investigated along with T-cell chimerism, T-cell chimerism was mainly associated with the development GVHD, while rapid development of NK-cell chimerism was associated with lower relapse rates and better progression-free survival [20,27]. Notably, no association between NK-cell chimerism and GVHD was observed [27]. As T- and NK-cell chimerism correlated closely at day +28 in our study, it is an open question whether the superior outcomes in the FLU/TBI arm were due to faster, more complete donor T-cell chimerism, NK-cell chimerism or both.
Reconstitution of immune cells was similar between arms, except for NK and CD4+ T cells. Why only CD4+ T-cell counts were affected in the current study is not clear, but these results are in line with findings by De Bock et al. that showed levels of CD4+ cells, including naïve CD4+, regenerated slower than other cell subsets after nonmyeloablative conditioning with fludarabine and low-dose TBI [28]. Although our findings should be interpreted with caution due to small sample size, it is possible that they represent an effect induced by fludarabine as both CD8+ and CD4+ cells have been shown to be highly sensitive to depletion by fludarabine in CLL [29], whereas NK cells have been shown to be more resistant [30], explaining the higher NK and lower CD4+ cell counts in the FLU/TBI arm. Conversely, with 2 Gy TBI alone conditioning killing less T (including CD4+) cells, the higher levels of CD4+ cells could be a consequence of a longer period with mixed chimerism.
In conclusion, the current study showed that in the setting of a randomized phase III clinical trial, the addition of FLU (30 mg/m2) for 3 days to conditioning with 2 Gy TBI prior to allogeneic HCT from HLA-matched related donors with CSP and MMF as post-grafting GVHD prophylaxis is safe and efficacious. FLU did not increase NRM or incidence of infections and was associated with lower relapse and better PFS, probably due to the induction of higher levels of donor T- and NK-cell chimerism early post-transplant. Furthermore, the study suggests that donor engraftment in heavily pretreated patients is possible with only 2 Gy TBI, but that a lower conditioning intensity correlates to a slower speed of engraftment, which may be associated with a decrease in successful outcome.
Although NRM is low with the current nonmyeloablative regimens, relapse still represents a challenge. In the context of reduced intensity regimens, where conditioning intensity and antineoplastic activity is increased compared to the nonmyeloablative regimens, the possible beneficial effects on relapse rates are counterbalanced by an increase in NRM at 19-25% [31-33].
A possible solution to this problem could be further studies with minimal conditioning intensity, where the issue of slow development of donor chimerism could be approached by augmenting the antitumor effect by adding disease specific agents, such as anti-CD20 antibodies [34] or receptor tyrosine kinase inhibitors [35], in the post-transplant period thereby allowing full donor chimerism ample time to develop. A different approach could also include substituting external beam radiation with targeted α-emitter labeled anti-CD45 based radioimmunotherapy, which reduces off-target radiation toxicity and increases radiation dose selectively in cells responsible for rejection and tumor cells [36].
Supplementary Material
Acknowledgments
Foremost we thank the patients who participated in the clinical trial. We also thank the members of the research staff, clinical staff, and referring physicians at all the participating sites for their dedication to the research and care of the patients after hematopoietic cell transplantation.
Financial Disclosure Statement: Research funding was provided by the National Institutes of Health, Bethesda, MD, grants, CA078902, HL036444, and CA015704. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health nor its subsidiary Institutes and Centers. BK was supported by a fellowship from the Danish Cancer Society (DP08135), Frøken Amalie Jørgensens Mindelegat and Anders Hasselbalchs Fond. BB was supported by Ricerca Sanitaria Finalizzata RF-PIE-2008-1206999 and RF-PIE-2009-1491359.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors have no conflicts of interest to declare.
References
- 1.Storb R, Raff RF, Appelbaum FR, et al. Comparison of fractionated to single-dose total body irradiation in conditioning canine littermates for DLA-identical marrow grafts. Blood. 1989;74:1139–1143. [PubMed] [Google Scholar]
- 2.Storb R, Yu C, Wagner JL, et al. Stable mixed hematopoietic chimerism in DLA-identical littermate dogs given sublethal total body irradiation before and pharmacological immunosuppression after marrow transplantation. Blood. 1997;89:3048–3054. [PubMed] [Google Scholar]
- 3.Storb R, Raff RF, Graham T, et al. Marrow toxicity of fractionated versus single dose total body irradiation is identical in a canine model. International Journal of Radiation Oncology, Biology , Physics. 1993;26:275–283. doi: 10.1016/0360-3016(93)90207-c. [DOI] [PubMed] [Google Scholar]
- 4.McSweeney PA, Niederwieser D, Shizuru JA, et al. Hematopoietic cell transplantation in older patients with hematologic malignancies: replacing high-dose cytotoxic therapy with graft-versus-tumor effects. Blood. 2001;97:3390–3400. doi: 10.1182/blood.v97.11.3390. [DOI] [PubMed] [Google Scholar]
- 5.Sandmaier BM, Maloney DG, Gooley TA, et al. Low dose TBI conditioning for hematopoietic stem cell transplants (HSCT) from HLA-matched related donors for patients with hematologic malignancies: influence of fludarabine or cytoreductive autografts on outcome. Blood. 2002;100(Part 1):145a. #544[abstr.] [Google Scholar]
- 6.Panse JP, Heimfeld S, Guthrie KA, et al. Allogeneic peripheral blood stem cell graft composition affects early T-cell chimaerism and later clinical outcomes after nonmyeloablative conditioning. Br J Haematol. 2005;128:659–667. doi: 10.1111/j.1365-2141.2005.05363.x. [DOI] [PubMed] [Google Scholar]
- 7.Petersdorf EW, Gooley TA, Anasetti C, et al. Optimizing outcome after unrelated marrow transplantation by comprehensive matching of HLA class I and II alleles in the donor and recipient. Blood. 1998;92:3515–3520. [PubMed] [Google Scholar]
- 8.Maris MB, Niederwieser D, Sandmaier BM, et al. HLA-matched unrelated donor hematopoietic cell transplantation after nonmyeloablative conditioning for patients with hematologic malignancies. Blood. 2003;102:2021–2030. doi: 10.1182/blood-2003-02-0482. [DOI] [PubMed] [Google Scholar]
- 9.The Revised Common Toxicity Criteria: Version 2.0. DCTD, NCI, NIH, DHHS; 1999. [Google Scholar]
- 10.Junghanss C, Marr KA, Carter RA, et al. Incidence and outcome of bacterial and fungal infections following nonmyeloablative compared with myeloablative allogeneic hematopoietic stem cell transplantation: a matched control study. Biol Blood Marrow Transplant. 2002;8:512–520. doi: 10.1053/bbmt.2002.v8.pm12374456. [DOI] [PubMed] [Google Scholar]
- 11.Przepiorka D, Weisdorf D, Martin P, et al. 1994 Consensus Conference on Acute GVHD Grading. Bone Marrow Transplant. 1995;15:825–828. [PubMed] [Google Scholar]
- 12.Sullivan KM. Graft-versus-host disease. In: Thomas ED, Blume KG, Forman SJ, editors. Hematopoietic Cell Transplantation. Blackwell Sciences, Inc.; Malden, MA: 1999. pp. 515–536. [Google Scholar]
- 13.Storek J, Dawson MA, Storer B, et al. Immune reconstitution after allogeneic marrow transplantation compared with blood stem cell transplantation. Blood. 2001;97:3380–3389. doi: 10.1182/blood.v97.11.3380. [DOI] [PubMed] [Google Scholar]
- 14.Storek J, Zhao Z, Lin E, et al. Recovery from and consequences of severe iatrogenic lymphopenia (induced to treat autoimmune diseases). Clinical Immunology. 2004;113:285–298. doi: 10.1016/j.clim.2004.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kahl C, Storer BE, Sandmaier BM, et al. Relapse risk among patients with malignant diseases given allogeneic hematopoietic cell transplantation after nonmyeloablative conditioning. Blood. 2007;110:2744–2748. doi: 10.1182/blood-2007-03-078592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sorror ML, Maris MB, Storb R, et al. Hematopoietic cell transplantation (HCT)-specific comorbidity index: a new tool for risk assessment before allogeneic HCT. Blood. 2005;106:2912–2919. doi: 10.1182/blood-2005-05-2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Niederwieser D, Maris M, Shizuru JA, et al. Low-dose total body irradiation (TBI) and fludarabine followed by hematopoietic cell transplantation (HCT) from HLA-matched or mismatched unrelated donors and postgrafting immunosuppression with cyclosporine and mycophenolate mofetil (MMF) can induce durable complete chimerism and sustained remissions in patients with hematological diseases. Blood. 2003;101:1620–1629. doi: 10.1182/blood-2002-05-1340. [DOI] [PubMed] [Google Scholar]
- 18.Keil F, Prinz E, Moser K, et al. Rapid establishment of long-term culture-initiating cells of donor origin after nonmyeloablative allogeneic hematopoietic stem-cell transplantation, and significant prognostic impact of donor T-cell chimerism on stable engraftment and progression-free survival. Transplantation. 2003;76:230–236. doi: 10.1097/01.TP.0000071862.42835.76. [DOI] [PubMed] [Google Scholar]
- 19.Baron F, Maris MB, Sandmaier BM, et al. Graft-versus-tumor effects after allogeneic hematopoietic cell transplantation with nonmyeloablative conditioning. J Clin Oncol. 2005;23:1993–2003. doi: 10.1200/JCO.2005.08.136. [DOI] [PubMed] [Google Scholar]
- 20.Baron F, Baker JE, Storb R, et al. Kinetics of engraftment in patients with hematologic malignancies given allogeneic hematopoietic cell transplantation after nonmyeloablative conditioning. Blood. 2004;104:2254–2262. doi: 10.1182/blood-2004-04-1506. [DOI] [PubMed] [Google Scholar]
- 21.Breuer S, Preuner S, Fritsch G, et al. Early recipient chimerism testing in the T- and NK-cell lineages for risk assessment of graft rejection in pediatric patients undergoing allogeneic stem cell transplantation. Leukemia. 2012;26:509–519. doi: 10.1038/leu.2011.244. [DOI] [PubMed] [Google Scholar]
- 22.Alyea EP, Kim HT, Ho V, et al. Impact of conditioning regimen intensity on outcome of allogeneic hematopoietic cell transplantation for advanced acute myelogenous leukemia and myelodysplastic syndrome. Biol Blood Marrow Transplant. 2006;12:1047–1055. doi: 10.1016/j.bbmt.2006.06.003. [DOI] [PubMed] [Google Scholar]
- 23.Khouri IF, McLaughlin P, Saliba RM, et al. Eight-year experience with allogeneic stem cell transplantation for relapsed follicular lymphoma after nonmyeloablative conditioning with fludarabine, cyclophosphamide, and rituximab. Blood. 2008;111:5530–5536. doi: 10.1182/blood-2008-01-136242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Freytes CO, Zhang MJ, Carreras J, et al. Outcome of lower-intensity allogeneic transplantation in non-Hodgkin lymphoma after autologous transplantation failure. Biol Blood Marrow Transplant. 2012;18:1255–1264. doi: 10.1016/j.bbmt.2011.12.581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kolb HJ, Schmidt C, Barrett AJ, Schendel DJ. Graft-versus-leukemia reactions in allogeneic chimeras. Blood. 2004;103:767–776. doi: 10.1182/blood-2003-02-0342. [DOI] [PubMed] [Google Scholar]
- 26.Parham P, McQueen KL. Alloreactive killer cells: hindrance and help for haematopoietic transplants (Review). Nat Rev Immunol. 2003;3:108–122. doi: 10.1038/nri999. [DOI] [PubMed] [Google Scholar]
- 27.Baron F, Petersdorf EW, Gooley T, et al. What is the role for donor NK cells after nonmyeolablative conditioning? Biol Blood Marrow Transplant. 2009;15:580–588. doi: 10.1016/j.bbmt.2009.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.De BM, Fillet M, Hannon M, et al. Kinetics of IL-7 and IL-15 levels after allogeneic peripheral blood stem cell transplantation following nonmyeloablative conditioning. PLoS ONE [Electronic Resource] 2013;8:e55876. doi: 10.1371/journal.pone.0055876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Keating MJ, O'Brien S, Lerner S, et al. Long-term follow-up of patients with chronic lymphocytic leukemia (CLL) receiving fludarabine regimens as initial therapy. Blood. 1998;92:1165–1171. [PubMed] [Google Scholar]
- 30.Ysebaert L, Gross E, Kuhlein E, et al. Immune recovery after fludarabine-cyclophosphamide-rituximab treatment in B-chronic lymphocytic leukemia: implication for maintenance immunotherapy. Leukemia. 2010;24:1310–1316. doi: 10.1038/leu.2010.89. [DOI] [PubMed] [Google Scholar]
- 31.Hari P, Carreras J, Zhang MJ, et al. Allogeneic transplants in follicular lymphoma: higher risk of disease progression after reduced-intensity compared to myeloablative conditioning. Biol Blood Marrow Transplant. 2008;14:236–245. doi: 10.1016/j.bbmt.2007.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ringdén O, Labopin M, Ehninger G, et al. Reduced intensity conditioning compared with myeloablative conditioning using unrelated donor transplants in patients with acute myeloid leukemia. J Clin Oncol. 2009;27:4570–4577. doi: 10.1200/JCO.2008.20.9692. [DOI] [PubMed] [Google Scholar]
- 33.Dreger P, Brand R, Milligan D, et al. Reduced-intensity conditioning lowers treatment-related mortality of allogeneic stem cell transplantation for chronic lymphocytic leukemia: a population-matched analysis. Leukemia. 2005;19:1029–1033. doi: 10.1038/sj.leu.2403745. [DOI] [PubMed] [Google Scholar]
- 34.Michallet M, Socie G, Mohty M, et al. Rituximab, fludarabine, and total body irradiation as conditioning regimen before allogeneic hematopoietic stem cell transplantation for advanced chronic lymphocytic leukemia: long-term prospective multicenter study. Exp Hematol. 2013;41:127–133. doi: 10.1016/j.exphem.2012.10.008. [DOI] [PubMed] [Google Scholar]
- 35.Takahashi K, Kantarjian H, Pemmaraju N, et al. Salvage therapy using FLT3 inhibitors may improve long-term outcome of relapsed or refractory AML in patients with FLT3-ITD. Br J Haematol. 2013;161:659–666. doi: 10.1111/bjh.12299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chen Y, Kornblit B, Hamlin DK, et al. Durable donor engraftment after radioimmunotherapy using α-emitter astatine-211-labeled anti-CD45 antibody for conditioning in allogeneic hematopoietic cell transplantation. Blood. 2012;119:1130–1138. doi: 10.1182/blood-2011-09-380436. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.