Abstract
Given the growing number of older adults with multimorbidity who are prescribed multiple medications, clinicians need to prioritize which medications are most likely to benefit and least likely to harm an individual patient. The concept of time to benefit (TTB) is increasingly discussed in addition to other measures of drug effectiveness in order to understand and contextualize the benefits and harms of a therapy to an individual patient. However, how to glean this information from available evidence is not well established. The lack of such information for clinicians highlights a critical need in the design and reporting of clinical trials to provide information most relevant to decision making for older adults with multimorbidity. We define TTB as the time until a statistically significant benefit is observed in trials of people taking a therapy compared to a control group not taking the therapy. Similarly, time to harm (TTH) is the time until a significantly significant adverse effect is seen in a trial for the treatment group compared to the control group. To determine both TTB and TTH, it is critical that we also clearly define the benefit or harm under consideration. Well-defined benefits or harms are clinically meaningful, measurable outcomes that are desired (or shunned) by patients. In this conceptual review, we illustrate concepts of TTB in randomized controlled trials (RCTs) of statins for the primary prevention of cardiovascular disease. Using published results, we estimate probable TTB for statins with the future goal of using such information to improve prescribing decisions for individual patients. Knowing the relative TTBs and TTHs associated with a patient’s medications could be immensely useful to a clinician in decision-making for their older patients with multimorbidity. We describe the challenges in defining and determining TTB and TTH, and discuss possible ways for analyzing and reporting trial results which would add more information about this aspect of drug effectiveness to the clinician’s evidence base.
Keywords: drug therapy, aged, time to benefit, polypharmacy, multiple chronic conditions, multimorbidity
1.0 Introduction
Our healthcare system now faces the growing challenge of managing large numbers of older people with common problems of aging and multimorbidity. Older individuals are more likely than younger individuals to have multiple chronic diseases. Seventy-five million people have 2 or more chronic conditions, and this number will continue to rise, as 1 in 5 Americans will be 65 or older by 2030.[1]
Because the prevention and treatment of chronic conditions relies heavily on pharmacologic therapy, having multiple chronic conditions, or multimorbidity, results in recommendation of multiple medications.[2, 3] Almost one-fifth of older adults take 10 or more medications in a given week.[4] While medications can be essential in treating chronic disease, prescribing additional medications increases the risk of adverse drug reactions.[5] In observational studies, polypharmacy is associated with functional decline and may negatively impact other chronic conditions.[6]
Most clinical practice guidelines for chronic conditions still focus on one disease.[2, 7–10] While guidelines help to guide the care of patients with that condition, patients with multimorbidity must consider multiple risks and benefits associated with the management of all their conditions.[2, 11] The treatments beneficial for one disease may be harmful for another (e.g., diuretics for heart failure exacerbating urinary incontinence). The benefit of a medication could be more difficult to achieve in the presence of one or more comorbidities due to changes in pharmacokinetics (e.g., decreased clearance due to chronic kidney disease), drug interactions (e.g., warfarin for atrial fibrillation), the patients’ function (e.g., difficulty with adherence due to dementia), or life expectancy (e.g., end-stage heart disease or malignancy).[12]
In patients with multimorbidity taking multiple medications, there are less certain benefits and greater susceptibility to harms. One must also consider whether prescribing all the medications suggested by applicable guidelines is in the best interest of a particular patient, making it the primary care clinician’s role to prioritize the recommended pharmacologic treatments for an older patient with multimorbidity.[3] In such situations, information on a drug’s time to benefit (TTB), which we define as the time until a medication’s effect is evident in a population, could be helpful to increase a medication’s priority. Indeed, as a patient ages and accumulates new conditions, and life expectancy becomes increasingly limited, it may be necessary to re-evaluate current medications and consider de-escalation of treatments that no longer offer net benefits.[13] The lack of information on TTB highlights a critical need in clinical trial design in order to provide information for clinicians that could help this prioritization process.
A number of strategies have been proposed for safely prescribing therapies for older adults with multimorbidity. These include the reduction of high risk or inappropriate medications,[14–16] the careful use of medications most likely to cause adverse drug reactions,[17] the promotion of safer prescribing practices,[18] and the use of algorithms to provide more individualized therapy to high-risk individuals.[19] However, even treatments considered safe and effective for older people need to be prioritized and streamlined, to reduce the burden of medication use, reduce cost, and create a rational treatment strategy based on a patient’s entire profile,[20] rather than recommending all medications from all applicable individual disease guidelines.
Among commonly prescribed drugs, prioritization is most important when considering long-term preventive therapies (which typically have the longest time to benefit) such as hypertension control for prevention of stroke, cholesterol management for prevention of coronary artery disease, and glucose control for management of microvascular complications.[13, 21] Risk-benefit tradeoffs have to be considered, especially if the patient is advanced in age or has diminished life expectancy due to their health status. An early guideline to recommend consideration of life expectancy when prescribing a long-term preventive therapy was California Healthcare Foundation/American Geriatrics Society guidelines for the care of older patients with diabetes.[22]
TTB has previously been proposed as part of a strategy to improve prioritization of medications for individuals.[20, 23–25] The development of statistical computing methods in the 1960s made it possible to detect preventive benefits of cardiovascular medications for large numbers of patients studied in randomized controlled trials (RCTs). The standard for demonstrating clinical benefit became relative risk reduction (RRR) and statistical significance.[26] Later, standards for controlled drug trials proposed routine reporting of an effect size measure related to absolute risk reduction (ARR) rather than relative risk reduction, with suggestions to state the number needed to treat (NNT), which is the reciprocal of ARR.[27] NNT should be reported over a specific amount of time, but the emphasis has been on the effect size, not the time. TTB is a related concept to NNT, focused on the likelihood of realizing an identified, statistically significant, and clinically-relevant benefit (or harm) in a time frame that is likely to impact the patient in their remaining life expectancy (RLE).
In this review, we discuss the concept of TTB as a potentially valuable piece of information that would allow clinicians to better judge a patient’s potential for achieving a benefit associated with a medication under consideration - particularly when the individuals concerned are older, have limited RLE or have multimorbidity. The need for greater individualization in older patients arises because the likelihood of realizing a drug’s benefit is likely to be influenced by whether an individual’s estimated RLE exceeds the time needed to realize a drug’s known benefit. Similarly, for patients with multimorbidity, life expectancy may be decreased due to multiple chronic conditions, and the benefit of treating any specific condition may be uncertain. TTB and time to harm (TTH) have not been rigorously defined and incorporated into the design of RCTs. Much work is needed to develop statistical procedures for estimating TTB and TTH, in order that this information can be routinely reported in clinical trials.
We propose the evaluation of a preventive drug’s TTB using information in a RCT as a function of: (1) a measure of the time required to observe a drug’s clinical effectiveness, and (2) the effect size of the medication in terms of relative risk reduction or NNT. We illustrate how information in existing drug trials can currently be used to estimate TTB information for older persons. In this article we extract elements of TTB using trials of statins, a well-studied preventive medication that is commonly prescribed to both older patients and patients with multimorbidity. We discuss the potential possibilities and limitations of extrapolating this TTB information to clinical care of individuals.
2.0 Search Strategy and Data Evaluation
For this narrative review, we incorporated results from trials of statin medications as specific case examples of TTB data. We focused primarily on randomized controlled trials of statins for primary (rather than secondary) prevention: i.e., people with a low to moderate risk of 10-year cardiovascular mortality, corresponding to a calculated Framingham risk score of <10% or 10–20%, respectively. We identified trials for review if they were included in a 2011 Cochrane Database systematic review and meta-analysis of statin trials for the primary prevention of cardiovascular disease.[28] We then added studies from a second meta-analysis for review based on Cochrane criteria and if the statin trial was conducted on populations with low to moderate cardiovascular risk.[29] Wherever possible, we extracted data from sub-samples of older patients within the trials, if reported. Finally, we conducted general searches with “HMG Co-A reductase inhibitors” as a Medical Subject Heading term, “statin” and “time to benefit” text terms, and combinations of the above terms. This set of searches yielded additional background information on statins and time to benefit.
In total, 7 RCTs from the Cochrane Database systematic review[30–36] and 2 RCTs from the second meta-analysis[37, 38] matched our inclusion criteria. We used a more recent publication of long-term follow-up data for one of the trials originally identified in the Cochrane review.[39] All studies were evaluated for TTB data. We developed a structured data extraction tool (available upon request) for elements of TTB described below (trial length, population of focus, cardiovascular events, relative risk, number needed to treat, visually-representative data of TTB, and effect on older subsamples, if any). Each article was abstracted and compared by at least two authors (HH, LM, MY, and CB). We also identified whether trials made any specific mention of multimorbidity. Finally, we looked for any information relevant to timing of adverse events, or TTH.
3.0 Time to benefit (TTB): definitions in populations versus individuals
Although our aim is to discuss the epidemiologic aspects of TTB, we first acknowledge that the ideal TTB for a medication would be the time necessary for an individual patient to gain various benefits of all kinds that exceed all the potential harms - rather than the average TTB for the population from which the patient belongs.
The patient-specific TTB depends on an individual’s social and biological context. The social environment, such as factors affecting access and adherence to medications has been well documented in prior literature.[40] TTB can be conceived in a biological context as a potentially continuous and cumulative benefit after a drug reaches its intended target and causes enough change to reach a measurable threshold that results in an observed outcome. A maximum amount of benefit or a plateau may exist as the drug may saturate its effect at a receptor or in an organ system. The biological concept of TTB is related to surrogate endpoints that are measured to detect the consequences of drug action at its target. However, the time to effect for surrogate endpoints may not be the same as time to benefit for patient-important outcomes.
Time to benefit is not a new concept in statins.[41] Statins reduce the synthesis of cholesterol by blocking HMG Co-A reductase, which ultimately leads to a measurable reduction in LDL cholesterol. Additional pleiotropic and anti-inflammatory effects of statins also lead to the reduction in arterial intima media thickness as well as stabilization of atherosclerotic plaques.[42, 43]
Because individual TTB depends on multiple social and biologic factors, we believe that true individual TTB cannot be measured. Thus, we must turn to RCTs, scientific studies of populations of patients, to evaluate TTB – defined as the time for a population to realize the intended benefit of the medication. To make results of RCTs relevant to clinical care, we focus on outcomes that are clinically substantial, measurable, and important to patients. In contrast to the ideal concept of individual TTB, the TTB that we can extract from RCTs is tied specifically to each reported outcome. In the case of statins, important outcomes include all-cause mortality, stroke or cardiovascular events. We postulate that population-based TTB can be gleaned from the results of clinical trials. For patients with multimorbidity, the challenge is gleaning results that we can use to estimate their individual TTB, accounting for competing risks posed by their other conditions and treatments for the other conditions.
3.1 Time to harm
Similar conceptually to TTB, time to harm (TTH) is the amount of time required for a therapy to cause harm. A drug’s action at one receptor may cause both benefit and harm, or a drug could cause harm via a separate biologic mechanism. Hypothetically, the TTB and TTH could be similar, but this is not necessarily the case. One example is amiodarone, which slows heart rate and reduces risk for mortality due to sudden cardiac death over relatively short periods of time, but over longer time can result in pulmonary fibrosis.[44] Alternatively, TTH can be unpredictable, and a drug’s harms could derive from an action unique or unrelated to its beneficial actions. An example of this is extrapyramidal side effects of antipsychotic medications, which can occur early or late in the course of long-term treatment.[45]
For some therapies, mild harms are seen early and benefits are seen much later. In such cases, short-term side effects are minor and are worth the long-term gain from benefits. One example is antidepressant medications, where patients endure the immediate gastrointestinal or neuropsychiatric side effects to achieve the more important potential remission from their depression.[46]
If one is trying to balance a harm versus a benefit, and if they are similarly valued, then the next most useful information is to understand whether TTH is shorter or longer than TTB. When harms are seen much later than benefits, knowing the optimum duration of therapy to achieve the maximum benefit with the least harm could help in decision making about whether to continue or stop the therapy. An example of this is seen in bisphosphonate therapy, where benefit in terms of hip fracture prevention occurs in approximately 1.5 years – and thus, not instantaneously – yet atypical fracture risk increases over more than 2, or even 5 years of therapy.[47]
3.2 TTB for the individual patient in a clinical setting
An individual patient can only serve as his or her own control by undertaking a short-term trial, for example, taking doses of medications to test for symptom relief. However, for preventive medications, the potential benefit is the reduction of a rare, undesirable outcome. Benefits of preventive medicine can only occur over the long-term, and we can only observe preventive benefits by comparing treatment and control groups. In the case of prevention, when a medication works, a clinical event of interest either does not occur or its occurrence is delayed. Thus, the time element involved to reach the benefit is critical for a patient to understand the balance between the benefits and harms of a treatment.[48, 49] An important caveat when applying TTB information estimated from a trial is the possibility that TTB could vary greatly among individual trial participants. We may make the assumption that the information obtained at the group level is applicable to all individuals in a trial, but even within the RCT there may be meaningful heterogeneity of treatment effect.[50–53] Thus, applying the results of a trial to an individual patient with multimorbidity could substantially over- or underestimate TTB for the individual.
Understanding the magnitude of benefit is equally important to TTB for clinical decision-making. Measures such as relative risk reduction (RRR), absolute risk reduction (ARR), and number need to treat (NNT) must be extrapolated from clinical trials and applied to the individual patient’s situation. However, participants in trials may have meaningful differences in baseline risk for an outcome, and furthermore, may differ substantially from patients in clinical practice.[50] Because RCTs typically exclude patients with conditions that pose additional risk, patients in RCTs are less likely to have multimorbidity.[54] This means that the baseline risk for any clinically important outcome may be different in trial participants. This raises doubts about the applicability of trial evidence to the individual patient seen in clinical practice. Thus, we must also interpret the results of RCTs in light of the studied population’s baseline risk and estimate what the magnitude of benefit would be for our multimorbid patients. Finally, evaluating measures of effect such as RRR and ARR may also assume that the reduction in risk is constant over time. However, risk reduction (and conversely, the likelihood of adverse effects) could be substantially different at different time points after the initiation of a preventive therapy.
3.3 TTB in clinical trials of statins
The TTB in an RCT is the amount of time required to observe a significant, measurable effect in a group of patients treated with a therapy compared to a control group. We found that few statin trials reported the exact number of years or months to observe statistical benefit. Rather, the only information we had was that the benefit was statistically significant by the end of the trial. For statins, the TTB for reduction in risk of fatal and non-fatal myocardial infarction (MI) in primary prevention ranged between 1.9 and 5.3 years, based on the trial duration of the several trials that showed a significant benefit of statin therapy.[31, 32, 34, 38] We had to assume that if the RCT found benefit, then the TTB was no larger than the trial duration. The trial duration differs between patients: those recruited at the end are sometimes only enrolled for a few months, while those enrolled in the beginning could be followed for 7–8 years. Therefore, we used median or mean follow-up time (whichever was reported) as the TTB for most trials. Thus, our estimate of the range of TTB for statin trials for MI prevention (2 to 5 years) using median trial time as the TTB metric must also be viewed in light of patients’ participation over a varying amount of time. The other limitation of using median or mean trial time is that we only used the trials with positive benefit. It is not clear how to estimate TTB in trials that did not show a benefit. For example, in the ASPEN trial, statin therapy had a relative risk of 0.81 for MI but the results were not significant;[33] thus the TTB is greater than the median or mean trial follow up time. The information about TTB that is taken from a single study or even a body of literature could inaccurately estimate the true TTB unless a meta-analysis is conducted that includes data from positive and negative trials. Pooling data from multiple trials of varying duration may uncover benefits that occur earlier or later than those seen in an individual trial.
The TTB information obtained from statin trials for all-cause mortality is shown in the Table. The information on benefit includes hazard ratio or relative risk, NNT, and TTB, for statin trials that used all-cause mortality as one of their endpoints. Most trials did not show a reduction in all-cause mortality with statin treatment. Two trials showed a benefit: ACAPS showed a mortality benefit in 3 years with RR=0.12 (95% CI 0.02–0.99) and NNT of 65,[30] and JUPITER showed a benefit in 1.9 years with HR=0.80 (95% CI 0.67–0.97) and NNT 182.[38] Neither study reported information on whether a benefit was achieved earlier than the trial duration, thus it cannot be determined whether TTB was shorter than the trial duration. The PROSPER trial included adults age 70–82 with a high prevalence of comorbid conditions, and all-cause mortality was not significantly different for treatment compared to placebo groups.[35] These results were not included in the table because the event rates were not reported separately for the high-risk secondary prevention group and the moderate-risk primary prevention group.
Table.
Results from Statin RCTs for All-Cause Mortality
| Trial Name |
Baseline Risk |
Target Population |
Number of Subjects |
Hazard/ Relative Risk (CI) |
Subgroup Analysis |
NNT | NNT/ yeara |
TTBb | Adverse Events/TTH |
|---|---|---|---|---|---|---|---|---|---|
| ACAPS 1994[30] |
Low to Mod |
Men and women ages 40–79 with early carotid atherosclerosis and moderately elevated LDL (130–159 mg/dL with any number of risk factors or 160–189 with no or one CHD risk factor). |
Treatment group = 460 |
RR = 0.12 (0.02 – 0.99) |
No subgroup analysis performed. |
65 over 3 years |
195 | Curve separation at 1.5 years (VO) |
Early D/C in 3 of treated group, 2 of placebo group; skin problems more common in placebo group. |
| Placebo group = 459 |
No info on TTH. | ||||||||
| 29% had hypertension; 2.3% had diabetes mellitus No mention of multimorbidity. |
|||||||||
| MEGA 2006[31] |
Low | Japanese adults (men and Postmenopausal women) with mild-to-moderate hypertension with mildly elevated TC levels (220– 270 mg/dL) and without coronary heart disease. |
Treatment group = 3866 |
HR = 0.72 (0.51 – 1.01 |
Subgroup analysis for coronary heart disease events but not all-cause mortality. Significant benefit for age ≥ 60, male, high LDL, Nonsmokers and those without hypertension (physician report). No benefit for those with diabetes mellitus. |
NS | NS | N/A |
Elevated AST/ALT in 1.3%/2.8% of treated group, 1.4%/2.8% of placebo group; elevated CK in 3.1% of treated group, 2.6% of placebo group. |
| Placebo group = 3966 |
|||||||||
| 21% had diabetes mellitus. No mention of multimorbidity. |
No info on TTH. | ||||||||
| AFCAPS/TexCAPS 1998[32] |
Low | Men and post-menopausal women with average TC (221 mg/dL), LDL-C (150 mg/dL) and below average HDL-C (36 and 40 mg/dL for men and women, respectively). |
Treatment group = 3304 |
RR = 1.04 (0.76 – 1.41) |
No significant difference for first acute major coronary event (not all-cause mortality) by subgroup analysis that included age, hypertension, and diabetes mellitus. |
NS | NS | N/A |
Adverse events leading to D/C in 13.6% of treated group and 13.8% of placebo group; AST elevation similar; ALT elevation higher in treated group (3.3%) than placebo group (2.1%). |
| Placebo group = 3301 |
|||||||||
| 22% with hypertention, 2.5% with diabetes mellitus, and 3.6% on thyroid replacement. No mention of multimorbidity. |
No info on TTH. | ||||||||
| ASPEN 2006[33] |
Low | Men and women with type II diabetes with LDL cholesterol <140 mg/dL if they previously had an MI or 160 mg/dL if they had not. |
Treatment group = 959 |
RR = 1.09 (0.71 – 1.65) |
No subgroup analysis. |
NS | NS | N/A |
Abnormal liver function tests in 1.4% of treated group and 1.2% of placebo group; myalgia in 3.0% of treated group and 1.6% of placebo group. |
| Placebo group = 946 |
|||||||||
| 55% with hypertension, and many had multiple concomitant medication classes. No mention of multimorbidity. |
No info on TTH | ||||||||
| CARDS 2004[34] |
Low | Men and women from 40–75 years old with a history of type II diabetes mellitus |
Treatment group = 1428 |
HR = 0.73 (0.52–1.01) |
No significant difference in treatment effect by age, sex, lipids, blood pressure, retinopathy, albuminuria, smoking, or HbA1c. |
NS | NS | N/A |
Similar D/C rates, in 9% of treated group, 10% of placebo group; 1.1% in each group had adverse events. |
| Placebo group = 1410 |
|||||||||
| Multimorbidity prevalence unknown. |
No info on TTH. | ||||||||
| WOSCO PS 1995[36] |
Low | Scottish men 45 – 64 years with elevated total plasma and LDL cholesterol levels |
Treatment Group = 3302 |
RR = 0.78 (0.60 – 1.00) |
Subgroup analysis for primary endpoint of MI or coronary heart disease death benefit for younger and older age groups (<55 and ≥ 55), absence of multiple risk factors (which included hypertension or diabetes), and absence of prior vascular disease. |
NS | NS | N/A |
No difference in D/C rates; no difference in myalgia; 4 in treated group. had high CK compared to 1 in placebo group elevated AST and ALT in 26 and 16 of treated group compared to 20 and 12 of placebo. |
| Placebo Group = 3293 |
|||||||||
| 1% had diabetes, and 15.5% had hypertension; 1.4% died of cancer. No mention of multimorbidity. |
No info on TTH. | ||||||||
| JUPITER 2008[38] | Low | Men and women with LDL levels less than 130 mg/dL and elevated high-sensitivity C-reactive protein. |
Treatment Group = 8901 |
HR = 0.80 (0.67 – 0.97) | Subgroup analysis for composite primary endpoint (not all-cause mortality) showed significant benefit in all subgroups, including age ≤65 vs. >65, hypertension, and metabolic syndrome. |
182 over 1.9 years |
346 | Curve separation at between 2.5 – 3 Years (VO) |
No difference in adverse events; myopathy in 19 of treated group, 9 of placebo group; 1 rhabdomyolysis case in treated group; 270 reports of diabetes in treated group compared to 216 in placebo (p=0.01). |
| Placebo group = 8901 |
|||||||||
| 41% with metabolic syndrome. | |||||||||
| No mention of multimorbidity. | No info on TTH | ||||||||
| PREVEN D IT 2011[39] |
Low | Men and women aged 28 to 75 years of age with persistent microalbuminuria and without high cholesterol. |
Treatment group = 433 |
HR = 1.32 (0.82 – 2.14) |
Analysis by urinary albumin excretion; no other subgroup analysis was performed. |
NS | NS | N/A | No report of adverse events. |
| Placebo group = 431 |
No info on TTH. | ||||||||
| 2.6% with DM, and none with prior heart failure. No mention of multimorbidity. |
|||||||||
Abbreviations: NS = not significant; VO = visual only; HR = hazard ratio; RR = relative risk; CI = 95% confidence interval; TTB = time to benefit; TTH = time to harm; MI = myocardial infarction; DM = diabetes mellitus; D/C = discontinuation
NNT / year = the number needed to treat in one year, assuming constant benefit over the years of the trial.
TTB is not reported for trials with non-significant results, and is listed as N/A for trials in which TTB could not be visually inspected from a survival curve or cumulative incidence function.
Unlike TTB, it is more difficult to assess TTH. TTB can be estimated from studies that test hypothesized benefits of a medication based on pre-set beneficial outcomes studied in a trial. Harms could be expected side effects of therapy, or unexpected adverse events that are not attributed to therapy. If adverse events are reported as a total number of events during a trial’s duration, then like TTB, TTH would be considered to be no larger than the study median or mean follow up time. When evaluating TTH, it is possible that the effect of a medication is in the direction of harm, yet not statistically significant. In this case, there is potential TTH for that medication, and if the trial went on for long enough, there would be a statistically significant harm. However, because trials end at their pre-specified time or earlier due to a benefit seen in the primary outcome, the actual TTH is unknowable, and thus the results also cannot be easily compared with TTH information of other trials. Even more importantly is the concern that the applicability of TTH information to patients with multimorbidity is much more problematic than TTB, as patients with multimorbidity may have multiple additional risk factors for adverse effects while on multiple treatments.
In the case of statins, common harms reported in RCTs include myopathy, myalgias, rhabdomyolysis, and transaminase elevations.[30–39] For many studies, there were no significant differences in adverse events in treated and placebo groups. No trials reported any information on the rate at which such events occurred in either group, and it is thus not possible to glean TTH from any of the RCTs. Not surprisingly, no trials reported adverse event rates among subgroups of older adults or those with multimorbidity.
If a clinical trial explores more than one outcome, such as when the primary endpoint is actually a composite of different endpoints (e.g., MI or stroke or revascularization or cardiovascular death), then there is a different TTB for each outcome. If a statistically significant benefit of a therapy is not seen for a particular outcome, then there are two possibilities: (1) there is no true effect on that outcome (TTB = infinity), or (2) the TTB is longer than the length of the trial. The PROSPER study showed a significant reduction in risk of cardiovascular events in a TTB of 3 years, but no significant reduction in risk of stroke. The authors concluded that if PROSPER had continued for a longer duration a significant reduction might have been achieved, similar to other studies that demonstrated a TTB for stroke of more than 3 years.[37] Another possibility is that had PROSPER trial enrolled a larger number of people, they might have seen a TTB for stroke in less than 3 years. In other words, we cannot separate power to detect benefit and TTB. In the case of trials that are stopped early due to achieving a primary outcome, there can be an argument made that pre-specific subgroups that did not meet criteria for stopping should be allowed to continue, because they are still in a state of clinical equipoise, and therefore, allowing them to reach TTB for the main and/or secondary outcomes would benefit future older and multimorbid patients. In the PROSPER trial, for example, women did not have a significant benefit for any cardiovascular outcome for the trial’s duration.[37] If the women had been allowed to continue then we may have observed a reduction in disease, requiring a longer (but still reasonable) TTB if benefit was achieved within 1–2 years of the early stop date. As shown in the table, some trials reported the results of subgroup analyses for specific outcomes (but not all outcomes), among older vs. younger groups, risk categories, and single comorbid conditions, for example. No trials reported the prevalence of multimorbidity, and no trials included a subgroup analysis for those with multimorbidity.
Some trials in which the actual TTB is shorter than the mean follow up time may report the earlier time at which a significant difference in outcome is first seen, in which case this is the TTB for that ARR. Knowing a more precise TTB is more clinically useful than using mean trial time. TTB could be achieved in a shorter time than the end of the trial if monitoring occurred frequently enough to detect the statistically significant benefit earlier. In such a case, a trial might be stopped earlier if the benefit is seen for the primary outcome, and the median or mean follow-up time would be the appropriate TTB for that outcome. When regular data safety monitoring analyses occur frequently, results of TTB could be reported for all outcomes at earlier time points – even if the benefit does not result in stopping the trial. In the Treating to New Targets trial, people with a first cardiovascular event were randomized to high versus low dose atorvastatin and followed for a median time of 4.9 years. The HR for subsequent cardiovascular events (a composite outcome of the occurrence of any of several cardiac events) was significantly lower in the high-dose group by month 16 with an ARR of 2.2%, and the difference remained significant for the duration of the trial.[55] Thus, we consider the TTB for this trial as 16 months, and this is a more clinically useful metric for TTB than mean trial time for the Treating to New Targets trial, which was 4.9 years.
4.0 Recognizing TTB in a RCT from survival and cumulative incidence curves
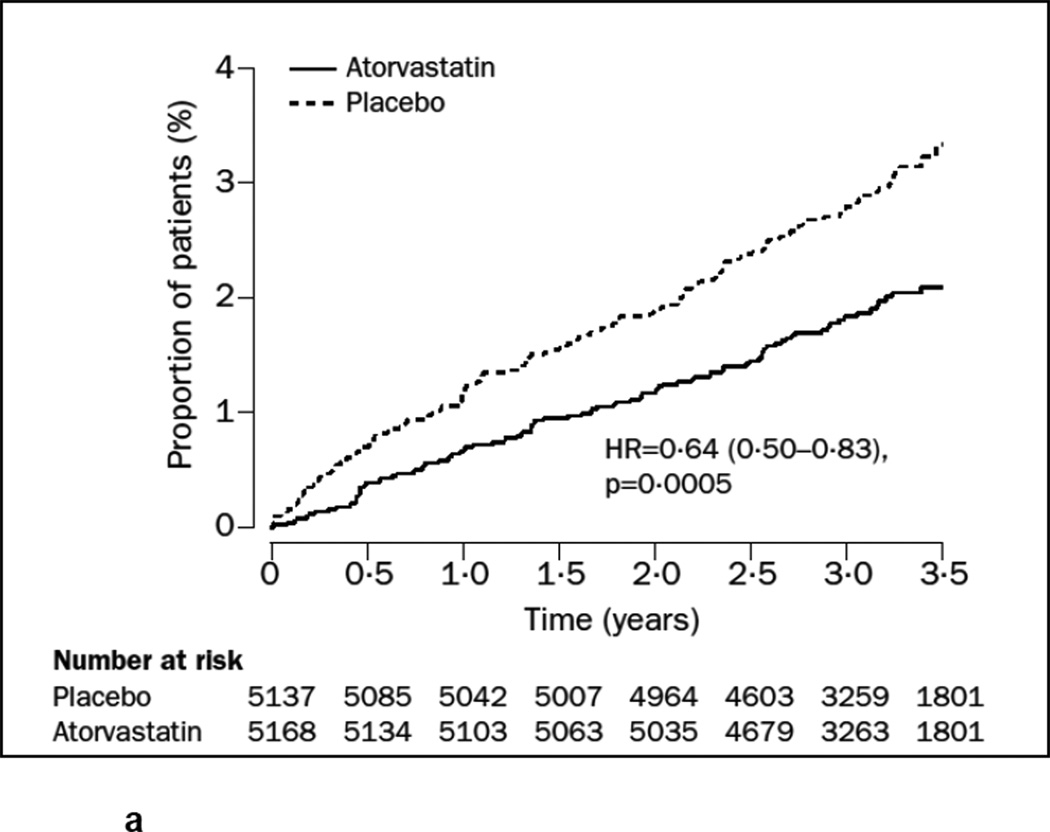
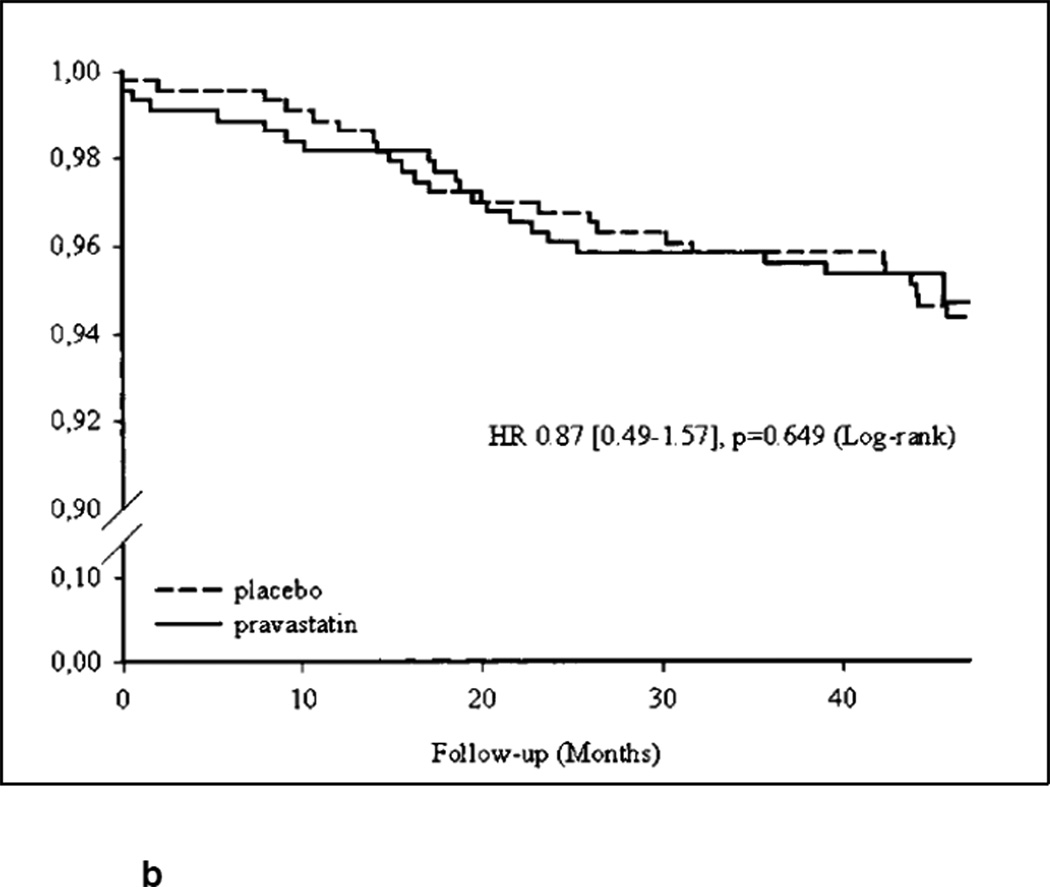
For this review, we also examined graphical representations of survival (usually labeled as Kaplan-Meier survival function) or accumulation of events over time (usually labeled as the cumulative incidence function) in treatment versus control groups. This method of visual observation of the separation of either the survival or incidence curves has been previously described for cancer screening and for statin benefits.[41, 49] We are concerned, however, that trials may not be designed to assess participants for an outcome in a time interval small enough to determine a precise TTB. Visually inspecting a cumulative incidence function or survival curve can suggest TTB but cannot determine whether this TTB was significantly different in the treatment versus the control group at that time point. We found that many trials of statins had statistically insignificant results but visually the survival curves or cumulative incidence functions appeared to separate before the trial was complete. In these cases, a clinician may wonder whether benefit may have been achieved for that particular outcome if the trial had continued for a longer time. Figures 1a and 1b show the survival and cumulative incidence curves for two statin trials, demonstrating the typical visual representation of TTB information when it is available.
Figure 1.
The cumulative incidence function from the ASCOT-LLA trial for the primary endpoint of non-fatal MI and fatal coronary heart disease.[62] Treatment with atorvastatin was associated with a 36% reduction in the risk of the primary endpoint with a median follow-up of 3.3 years. One could speculate, but could not be certain, whether TTB was earlier than 3.3 years, unless it was reported in the trial. One also cannot quantify the ARR at an earlier time point with certainty. Reprinted from The Lancet, Vol. 361 number 9364, Sever PS, Dahlof B, Poulter NR, Wedel H, Beevers G, Caulfield M, et al., Prevention of coronary and stroke events with atorvastatin in hypertensive patients who have average or lower-than-average cholesterol concentrations, in the Anglo-Scandinavian Cardiac Outcomes Trial—Lipid Lowering Arm (ASCOT-LLA): a multicenter randomized controlled trial, Pages 1149–1158, Copyright (2003), with permission from Elsevier.
The survival curve from the PREVEND-IT trial for cardiovascular events in subjects with microalbuminuria.[35] In this case, treatment with pravastatin did not prevent cardiovascular events, and this can be clearly seen on the survival curve, showing no sustained separation between treatment and placebo. Reprinted with permission from Wolters Kluwer Health from Asselbergs FW, Diercks GF, Hillege HL, van Boven AJ, Janssen WM, Voors AA, et al. Effects of fosinopril and pravastatin on cardiovascular events in subjects with microalbuminuria. Circulation 2004; 110 (18): 2809-16.
4.1 TTB complements (and does not replace) other measures of drug effectiveness
It is important to note that existing measures of drug effectiveness are still critical to understanding TTB, and therefore, TTB adds to and does not replace the existing methods in use. We found in our review that we were able to evaluate whether statins were beneficial using relative risk (RR) or odds ratio (OR) for having an outcome for the treatment group compared to the control group, or using hazard ratio (HR) for event per unit of time in the treatment group compared to the control group in the case of time-to-event data. Building on the RR, OR, or HR, the absolute risk reduction (ARR) should also ideally be reported to understand the true magnitude of a benefit. Finally, the NNT is sometimes reported and can be calculated from most reported trial data when it is not readily available, as 1/ARR. One example of how to estimate NNT would be to use the reported RR, and presume that all patients were followed for the mean or median follow-up time for the entire study. In the JUPITER trial, which reported a 20% reduction in risk of mortality and an absolute risk reduction of 0.55%, the NNT is calculated as 1/ARR, or 182. Since the median follow-up time was 1.9 years, we would interpret that 182 patients need to be treated over 2 years to prevent one death.[38] The TTB for avoiding the one death is thus likely within 2 years.
Next, the TTB complements the effect size (NNT) because both can be interpreted in light of clinical meaningfulness. A clinically meaningful effect size is conventionally determined, e.g., by care providers and by society, and is affected by economic trends such as cost of treatment or cost of side effects. For example, a NNT of 30 (or absolute risk reduction of 1/30) might be less acceptable for an expensive or burdensome treatment, but an NNT of 30 might be acceptable for a treatment that prevents a particularly worrisome outcome like stroke or death. However, a NNT of 1000 (or absolute risk reduction of 1/1000) might be hard to justify for a treatment that carries a small benefit but a high amount of risk, but could be suitable for a very inexpensive, low-risk treatment or for one that prevents a common infection like influenza. We propose that with better reporting of TTB, we can begin to weight a clinically acceptable TTB as a complement to NNT.
Last, NNT, ARR, and the TTB provide an absolute sense of the likelihood of benefit. Similarly, TTH and number needed to harm (NNH) are analogous concepts that can help to provide an absolute sense of the likelihood of adverse effects. Because the units of comparison are absolute, in contrast to RRR, having all four measures (NNH, TTH, NNT, and TTB) allows for better overall estimate of net benefit (or net harm).
4.2 The intimate relationship between TTB and trial design
Nearly all aspects of trial design can affect the TTB: sample size, primary endpoint, target effect size, and interim monitoring and stopping rules. A shorter TTB means that a significant beneficial effect is seen sooner.
A trial with a larger sample size would likely result in a shorter TTB, if all other parameters are held constant. Conversely, a smaller target effect size or a stringent stopping rule for the primary endpoint will yield a shorter TTB.
If a study’s duration is shorter than the actual TTB, a significant benefit will not be seen.
If a trial aims to detect a greater magnitude of effect, keeping other parameters of the study design constant, then a longer TTB is required to achieve statistical significance.
Use of an individual endpoint such as stroke, as opposed to a composite endpoint that includes stroke, will result in a longer TTB.
It is important to understand that the TTB estimated from an RCT should not be conflated with the biological TTB. The TTB is set a priori for the RCT based on available information or a plausible estimation of the minimum TTB – which may be based in some part on the biological TTB – needed for the primary outcome.
5.0 Individualizing therapy using TTB and TTH to make clinical decisions about preventive therapies
In preventive medicine, there are many choices for therapies to treat multiple chronic conditions. We posit that if we could measure TTB or TTH accurately, TTB and TTH could be used to prioritize the treatments that are most likely to help and least likely to harm a patient in a shorter time. For patients with limited RLE, we could use the information to preferentially use medications with short TTB in favor of those requiring a longer TTB.[13] Intuitively, individual patients with a short estimated RLE are not likely to achieve benefit from therapies with a long TTB. Similarly if TTH is relatively shorter than RLE, but TTB is possibly longer, than a therapy might be less likely to be used due to concerns that a patient will only see harms but not benefits from a therapy. The lower prioritization of statin therapy has been proposed for patients with a limited RLE, with the recommendation that many patients could discontinue statins on the basis of TTB greatly exceeding RLE.[56] Information on TTB and TTH needs to be more accessible to clinicians to be useful for decision making in the individual patient encounter where multiple therapies are being prioritized in the context of short RLE.
With the increasing recognition that older persons and particularly patients with multimorbidity face a growing complexity of polypharmacy, there is a great need and an interest in finding strategies to optimally manage a patient’s entire profile of medications and conditions. To minimize inappropriate medications, reduce unnecessary therapies, and practice more conservative prescribing, a consistent theme that has emerged is that estimating the absolute benefits and harms of treatments also requires some attempt at understanding the likelihood of achieving these effects in the individual patient.[23, 57] Likelihood of benefit necessitates a comparison of a patient’s estimated RLE with the TTB and TTH. Thus, TTB and TTH along with NNT could aid the complex process of trying to simultaneously consider all of a patient’s competing conditions and treatments.
5.1 Using TTB to aid in treatment decisions in the context of multimorbidity
We speculate that in a patient with multimorbidity, no single analytic model or nomogram can give us a reliable answer regarding how to prioritize all of a patient’s medications. Life expectancy can be estimated using various risk tools available,[58] or global measures of reserve such as functional status or frailty.[59, 60] We propose using remaining life expectancy to frame the information on TTB as the patient’s likelihood of seeing a benefit by using a certain medication in their remaining lifetime. Comparing prognostic information to the information on TTB for multiple therapies for multiple conditions could help a clinician prioritize the therapies most likely to be effective for the individual patient. Using TTB information also necessitates an assumption that benefit accrues linearly over time, and this may not be true.
Knowing what the actual benefit is that corresponds to TTB is critical to interpreting whether the benefit is relevant to a patient with multimorbidity. TTB will be less useful when the benefit relates to surrogate endpoints of disease control and these surrogate end points do not correspond with a patient’s stated preferences. TTB might be estimable for outcomes such as mortality or composite primary endpoints (such as all cardiac and cerebrovascular events), but might not be able to be extrapolated for outcomes like loss of functional status and independence.
TTB information is most needed when a patient has a diminishing RLE (on the order of less than 5 years) and preventive therapies are being considered, for which TTB may exceed RLE. Using the TTB or TTH or a medication that is associated with a benefit that is highly preferred by a patient will help to prioritize multiple medications in people with multimorbidity.
6.0 Conclusions and recommendations
Prescribing for the growing number of older persons with multimorbidity will require careful prioritization among multiple therapies effective for chronic conditions. The use of the concept of TTB in clinical decision-making, and the important methodological issues that underlie it, are in infancy compared to many of the current standards of reporting of randomized controlled trials.[27] TTB can be estimated from RCTs, although more methodological work is needed to understand how the estimation of TTB depends on study design characteristics. TTB can be incorporated into a prescribing decision when considering the multiple therapies a person is eligible to receive. For such information to be available, trials would need to specify a priori earlier time points at which a significant and meaningful benefit might be achieved and would need to demonstrate the TTB as the earliest time measurable when time to event curves diverge for treatment vs. control groups. In addition, populations older and/or multimorbid adults would need to be specified in whom subgroup analysis for the primary and composite outcomes would be conducted, with the consideration of TTB in these groups as well. Ultimately, having more precise information on TTB from trials on more common medications commonly prescribed to patients with multimorbidity, such as statins, will improve clinical decision-making. Last, because our focus is on older patients with multimorbidity, we argue that TTB should be reported in all RCTs of preventive medications for all outcomes, and for subgroups of older (e.g., age 65 and older) and multimorbid adults (e.g., those with the index condition plus at least one other moderate to severe co-morbidity) in the trial. Armed with appropriately conducted subgroup results on TTB, clinicians will be better prepared to estimate what a multimorbid patient’s personal TTB might be.[61]
Acknowledgements
Holly M. Holmes is funded by a K23 from the National Institute on Aging, K23AG038476. Lillian Min is funded by the Agency for Healthcare Research and Quality (R21 HS017621) and career development awards from University of Michigan Older Americans Independence Center (NIA) and the Hartford Foundation. Michael Yee is funded by the Medical Student Training in Aging Research (MSTAR), which is funded through the American Federation for Aging Research (AFAR). Ravi Varadhan is a Brookdale Leadership in Aging Fellow at the Johns Hopkins University School of Medicine. Dr. Boyd is funded by an R21 from the Agency for Healthcare Related Quality, “Improving Clinical Practice Guidelines for Complex Patients” HS018597-01, a Paul Beeson Career Development Award Program from the National Institute on Aging, 1K23AG032910, the American Federation for Aging Research, The John A. Hartford Foundation, The Atlantic Philanthropies, The Starr Foundation, and an anonymous donor. The authors’ work was independent of the funders.
Footnotes
Conflict of Interest
Dr. Holmes, Dr. Min, Mr. Yee, Dr. Varadhan, Dr. Basran, and Dr. Dale have no conflicts of interest to disclose. Dr. Boyd has served as an author for UptoDate on the topic of multimorbidity and has given a talk on multimorbidity to United Health Care’s Medicare Advisory Board.
References
- 1.Parekh AK, Barton MB. The challenge of multiple comorbidity for the US health care system. JAMA : the journal of the American Medical Association. 2010;303(13):1303–1304. doi: 10.1001/jama.2010.381. [DOI] [PubMed] [Google Scholar]
- 2.Boyd CM, Darer J, Boult C, Fried LP, Boult L, Wu AW. Clinical practice guidelines and quality of care for older patients with multiple comorbid diseases: implications for pay for performance. JAMA : the journal of the American Medical Association. 2005;294(6):716–724. doi: 10.1001/jama.294.6.716. [DOI] [PubMed] [Google Scholar]
- 3.Gurwitz JH. The physics of geriatric pharmacotherapy: overcoming therapeutic inertia and momentum. The American journal of medicine. 2012;125(6):523–524. doi: 10.1016/j.amjmed.2012.02.007. [DOI] [PubMed] [Google Scholar]
- 4.A Report from the Slone Survey. Slone Epidemiology Center at the Boston University; 2006. Patterns of medication use in the United States 2006. [Google Scholar]
- 5.Tangiisuran B, Davies JG, Wright JE, Rajkumar C. Adverse drug reactions in a population of hospitalized very elderly patients. Drugs & aging. 2012;29(8):669–679. doi: 10.1007/BF03262282. [DOI] [PubMed] [Google Scholar]
- 6.Shah BM, Hajjar ER. Polypharmacy, adverse drug reactions, and geriatric syndromes. Clinics in geriatric medicine. 2012;28(2):173–186. doi: 10.1016/j.cger.2012.01.002. [DOI] [PubMed] [Google Scholar]
- 7.Fortin M, Contant E, Savard C, Hudon C, Poitras ME, Almirall J. Canadian guidelines for clinical practice: an analysis of their quality and relevance to the care of adults with comorbidity. BMC family practice. 2011;12:74. doi: 10.1186/1471-2296-12-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lugtenberg M, Burgers JS, Clancy C, Westert GP, Schneider EC. Current guidelines have limited applicability to patients with comorbid conditions: a systematic analysis of evidence-based guidelines. PloS one. 2011;6(10):e25987. doi: 10.1371/journal.pone.0025987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mutasingwa DR, Ge H, Upshur RE. How applicable are clinical practice guidelines to elderly patients with comorbidities? Canadian family physician Medecin de famille canadien. 2011;57(7):e253–e262. [PMC free article] [PubMed] [Google Scholar]
- 10.Tatoulis J, Huang NP, Boyden AN. Quality of Australian clinical guidelines and relevance to the care of older people with multiple comorbid conditions. Comment. The Medical journal of Australia. 2009;190(8):459. doi: 10.5694/j.1326-5377.2008.tb02079.x. [DOI] [PubMed] [Google Scholar]
- 11.Guiding principles for the care of older adults with multimorbidity: an approach for clinicians: american geriatrics society expert panel on the care of older adults with multimorbidity. Journal of the American Geriatrics Society. 2012;60(10):E1–E25. doi: 10.1111/j.1532-5415.2012.04188.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fitzgerald SP, Bean NG. An analysis of the interactions between individual comorbidities and their treatments--implications for guidelines and polypharmacy. Journal of the American Medical Directors Association. 2010;11(7):475–484. doi: 10.1016/j.jamda.2010.05.008. [DOI] [PubMed] [Google Scholar]
- 13.Reuben DB. Medical care for the final years of life: "When you're 83, it's not going to be 20 years". JAMA : the journal of the American Medical Association. 2009;302(24):2686–2694. doi: 10.1001/jama.2009.1871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Samsa GP, Hanlon JT, Schmader KE, Weinberger M, Clipp EC, Uttech KM, et al. A summated score for the medication appropriateness index: development and assessment of clinimetric properties including content validity. J Clin Epidemiol. 1994;47(8):891–896. doi: 10.1016/0895-4356(94)90192-9. [DOI] [PubMed] [Google Scholar]
- 15.Gallagher P, Ryan C, Byrne S, Kennedy J, O'Mahony D. STOPP (Screening Tool of Older Person's Prescriptions) and START (Screening Tool to Alert doctors to Right Treatment). Consensus validation. Int J Clin Pharmacol Ther. 2008;46(2):72–83. doi: 10.5414/cpp46072. [DOI] [PubMed] [Google Scholar]
- 16.American Geriatrics Society updated Beers Criteria for potentially inappropriate medication use in older adults. Journal of the American Geriatrics Society. 2012;60(4):616–631. doi: 10.1111/j.1532-5415.2012.03923.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Steinman MA, Hanlon JT. Managing medications in clinically complex elders: "There's got to be a happy medium". JAMA : the journal of the American Medical Association. 2010;304(14):1592–1601. doi: 10.1001/jama.2010.1482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shrank WH, Polinski JM, Avorn J. Quality indicators for medication use in vulnerable elders. Journal of the American Geriatrics Society. 2007;55(Suppl 2):S373–S382. doi: 10.1111/j.1532-5415.2007.01345.x. [DOI] [PubMed] [Google Scholar]
- 19.Garfinkel D, Mangin D. Feasibility study of a systematic approach for discontinuation of multiple medications in older adults: addressing polypharmacy. Archives of internal medicine. 2010;170(18):1648–1654. doi: 10.1001/archinternmed.2010.355. [DOI] [PubMed] [Google Scholar]
- 20.Holmes HM, Hayley DC, Alexander GC, Sachs GA. Reconsidering medication appropriateness for patients late in life. Archives of internal medicine. 2006;166(6):605–609. doi: 10.1001/archinte.166.6.605. [DOI] [PubMed] [Google Scholar]
- 21.Durso S. The next frontier: quantifying risks for interventions with no end in sight. Archives of internal medicine. 2008;168(11):1230–1231. doi: 10.1001/archinte.168.11.1230-b. author reply 1. [DOI] [PubMed] [Google Scholar]
- 22.Brown AF, Mangione CM, Saliba D, Sarkisian CA. Guidelines for improving the care of the older person with diabetes mellitus. Journal of the American Geriatrics Society. 2003;51(5 Suppl Guidelines):S265–S280. doi: 10.1046/j.1532-5415.51.5s.1.x. [DOI] [PubMed] [Google Scholar]
- 23.Scott IA, Gray LC, Martin JH, Mitchell CA. Minimizing inappropriate medications in older populations: a 10-step conceptual framework. The American journal of medicine. 2012;125(6):529–537. doi: 10.1016/j.amjmed.2011.09.021. e4. [DOI] [PubMed] [Google Scholar]
- 24.Stevenson J, Abernethy AP, Miller C, Currow DC. Managing comorbidities in patients at the end of life. BMJ (Clinical research ed) 2004;329(7471):909–912. doi: 10.1136/bmj.329.7471.909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stevenson JP, Currow DC, Abernethy AP. Frameworks for prescribing in comorbid illness. Journal of pain and symptom management. 2007;34(2):117–118. doi: 10.1016/j.jpainsymman.2007.04.013. [DOI] [PubMed] [Google Scholar]
- 26.A proposal for structured reporting of randomized controlled trials. The Standards of Reporting Trials Group. JAMA : the journal of the American Medical Association. 1994;272(24):1926–1931. [PubMed] [Google Scholar]
- 27.Schulz KF, Altman DG, Moher D. CONSORT 2010 statement: updated guidelines for reporting parallel group randomized trials. Annals of internal medicine. 2010;152(11):726–732. doi: 10.7326/0003-4819-152-11-201006010-00232. [DOI] [PubMed] [Google Scholar]
- 28.Taylor F, Ward K, Moore T, Burke M, Davey SG, Casas J, et al. Statins for the primary prevention of cardiovascular disease (Review) The Cochrane Collaboration. 2012 doi: 10.1002/14651858.CD004816.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Brugts JJ, Yetgin T, Hoeks SE, Gotto AM, Shepherd J, Westendorp RG, et al. The benefits of statins in people without established cardiovascular disease but with cardiovascular risk factors: meta-analysis of randomised controlled trials. BMJ (Clinical research ed) 2009;338:b2376. doi: 10.1136/bmj.b2376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Furberg CD, Adams HP, Jr, Applegate WB, Byington RP, Espeland MA, Hartwell T, et al. Effect of lovastatin on early carotid atherosclerosis and cardiovascular events. Asymptomatic Carotid Artery Progression Study (ACAPS) Research Group. Circulation. 1994;90(4):1679–1687. doi: 10.1161/01.cir.90.4.1679. [DOI] [PubMed] [Google Scholar]
- 31.Nakamura H, Arakawa K, Itakura H, Kitabatake A, Goto Y, Toyota T, et al. Primary prevention of cardiovascular disease with pravastatin in Japan (MEGA Study): a prospective randomised controlled trial. Lancet. 2006;368(9542):1155–1163. doi: 10.1016/S0140-6736(06)69472-5. [DOI] [PubMed] [Google Scholar]
- 32.Downs JR, Clearfield M, Weis S, Whitney E, Shapiro DR, Beere PA, et al. Primary prevention of acute coronary events with lovastatin in men and women with average cholesterol levels: results of AFCAPS/TexCAPS. Air Force/Texas Coronary Atherosclerosis Prevention Study. JAMA : the journal of the American Medical Association. 1998;279(20):1615–1622. doi: 10.1001/jama.279.20.1615. [DOI] [PubMed] [Google Scholar]
- 33.Knopp RH, d'Emden M, Smilde JG, Pocock SJ. Efficacy and safety of atorvastatin in the prevention of cardiovascular end points in subjects with type 2 diabetes: the Atorvastatin Study for Prevention of Coronary Heart Disease Endpoints in non-insulin-dependent diabetes mellitus (ASPEN) Diabetes care. 2006;29(7):1478–1485. doi: 10.2337/dc05-2415. [DOI] [PubMed] [Google Scholar]
- 34.Colhoun HM, Betteridge DJ, Durrington PN, Hitman GA, Neil HA, Livingstone SJ, et al. Primary prevention of cardiovascular disease with atorvastatin in type 2 diabetes in the Collaborative Atorvastatin Diabetes Study (CARDS): multicentre randomised placebo-controlled trial. Lancet. 2004;364(9435):685–696. doi: 10.1016/S0140-6736(04)16895-5. [DOI] [PubMed] [Google Scholar]
- 35.Asselbergs FW, Diercks GF, Hillege HL, van Boven AJ, Janssen WM, Voors AA, et al. Effects of fosinopril and pravastatin on cardiovascular events in subjects with microalbuminuria. Circulation. 2004;110(18):2809–2816. doi: 10.1161/01.CIR.0000146378.65439.7A. [DOI] [PubMed] [Google Scholar]
- 36.Shepherd J, Cobbe SM, Ford I, Isles CG, Lorimer AR, MacFarlane PW, et al. Prevention of coronary heart disease with pravastatin in men with hypercholesterolemia. West of Scotland Coronary Prevention Study Group. The New England journal of medicine. 1995;333(20):1301–1307. doi: 10.1056/NEJM199511163332001. [DOI] [PubMed] [Google Scholar]
- 37.Shepherd J, Blauw GJ, Murphy MB, Bollen EL, Buckley BM, Cobbe SM, et al. Pravastatin in elderly individuals at risk of vascular disease (PROSPER): a randomised controlled trial. Lancet. 2002;360(9346):1623–1630. doi: 10.1016/s0140-6736(02)11600-x. [DOI] [PubMed] [Google Scholar]
- 38.Ridker PM, Danielson E, Fonseca FA, Genest J, Gotto AM, Jr, Kastelein JJ, et al. Rosuvastatin to prevent vascular events in men and women with elevated C-reactive protein. The New England journal of medicine. 2008;359(21):2195–2207. doi: 10.1056/NEJMoa0807646. [DOI] [PubMed] [Google Scholar]
- 39.Brouwers FP, Asselbergs FW, Hillege HL, de Boer RA, Gansevoort RT, van Veldhuisen DJ, et al. Long-term effects of fosinopril and pravastatin on cardiovascular events in subjects with microalbuminuria:Ten years of follow-up of Prevention of Renal and Vascular End-stage Disease Intervention Trial (PREVEND IT) American heart journal. 2011;161(6):1171–1178. doi: 10.1016/j.ahj.2011.03.028. [DOI] [PubMed] [Google Scholar]
- 40.Parris ES, Lawrence DB, Mohn LA, Long LB. Adherence to statin therapy and LDL cholesterol goal attainment by patients with diabetes and dyslipidemia. Diabetes care. 2005;28(3):595–599. doi: 10.2337/diacare.28.3.595. [DOI] [PubMed] [Google Scholar]
- 41.Ray KK, Cannon CP. Time to benefit: an emerging concept for assessing the efficacy of statin therapy in cardiovascular disease. Critical pathways in cardiology. 2005;4(1):43–45. doi: 10.1097/01.hpc.0000154979.98731.5d. [DOI] [PubMed] [Google Scholar]
- 42.Mercuri M, Bond MG, Sirtori CR, Veglia F, Crepaldi G, Feruglio FS, et al. Pravastatin reduces carotid intima-media thickness progression in an asymptomatic hypercholesterolemic mediterranean population: the Carotid Atherosclerosis Italian Ultrasound Study. The American journal of medicine. 1996;101(6):627–634. doi: 10.1016/s0002-9343(96)00333-6. [DOI] [PubMed] [Google Scholar]
- 43.Ray KK, Cannon CP. Early time to benefit with intensive statin treatment: could it be the pleiotropic effects? The American journal of cardiology. 2005;96(5A):54F–60F. doi: 10.1016/j.amjcard.2005.06.027. [DOI] [PubMed] [Google Scholar]
- 44.Effect of prophylactic amiodarone on mortality after acute myocardial infarction and in congestive heart failure: meta-analysis of individual data from 6500 patients in randomised trials. Amiodarone Trials Meta-Analysis Investigators. Lancet. 1997;350(9089):1417–1424. [PubMed] [Google Scholar]
- 45.Dayalu P, Chou KL. Antipsychotic-induced extrapyramidal symptoms and their management. Expert opinion on pharmacotherapy. 2008;9(9):1451–1462. doi: 10.1517/14656566.9.9.1451. [DOI] [PubMed] [Google Scholar]
- 46.Wilson KC, Mottram PG, Vassilas CA. Psychotherapeutic treatments for older depressed people. Cochrane Database Syst Rev. 2008;(1):CD004853. doi: 10.1002/14651858.CD004853.pub2. [DOI] [PubMed] [Google Scholar]
- 47.Schilcher J, Michaelsson K, Aspenberg P. Bisphosphonate use and atypical fractures of the femoral shaft. The New England journal of medicine. 2011;364(18):1728–1737. doi: 10.1056/NEJMoa1010650. [DOI] [PubMed] [Google Scholar]
- 48.Braithwaite RS, Concato J, Chang CC, Roberts MS, Justice AC. A framework for tailoring clinical guidelines to comorbidity at the point of care. Archives of internal medicine. 2007;167(21):2361–2365. doi: 10.1001/archinte.167.21.2361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Walter LC, Covinsky KE. Cancer screening in elderly patients: a framework for individualized decision making. JAMA : the journal of the American Medical Association. 2001;285(21):2750–2756. doi: 10.1001/jama.285.21.2750. [DOI] [PubMed] [Google Scholar]
- 50.Kent DM, Hayward RA. Limitations of applying summary results of clinical trials to individual patients: the need for risk stratification. JAMA : the journal of the American Medical Association. 2007;298(10):1209–1212. doi: 10.1001/jama.298.10.1209. [DOI] [PubMed] [Google Scholar]
- 51.Rothwell PM. Treating individuals 2. Subgroup analysis in randomised controlled trials: importance, indications, and interpretation. Lancet. 2005;365(9454):176–186. doi: 10.1016/S0140-6736(05)17709-5. [DOI] [PubMed] [Google Scholar]
- 52.Rothwell PM. Can overall results of clinical trials be applied to all patients? Lancet. 1995;345(8965):1616–1619. doi: 10.1016/s0140-6736(95)90120-5. [DOI] [PubMed] [Google Scholar]
- 53.Weiss CO, Segal JB, Varadhan R. Assessing the applicability of trial evidence to a target sample in the presence of heterogeneity of treatment effect. Pharmacoepidemiology and drug safety. 2012;21(Suppl 2):121–129. doi: 10.1002/pds.3242. [DOI] [PubMed] [Google Scholar]
- 54.Boyd CM, Vollenweider D, Puhan MA. Informing evidence-based decision-making for patients with comorbidity: availability of necessary information in clinical trials for chronic diseases. PloS one. 2012;7(8):e41601. doi: 10.1371/journal.pone.0041601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.LaRosa JC, Deedwania PC, Shepherd J, Wenger NK, Greten H, DeMicco DA, et al. Comparison of 80 versus 10 mg of atorvastatin on occurrence of cardiovascular events after the first event (from the Treating to New Targets [TNT] trial) The American journal of cardiology. 2010;105(3):283–287. doi: 10.1016/j.amjcard.2009.09.025. [DOI] [PubMed] [Google Scholar]
- 56.Vollrath AM, Sinclair C, Hallenbeck J. Discontinuing cardiovascular medications at the end of life: lipid-lowering agents. Journal of palliative medicine. 2005;8(4):876–881. doi: 10.1089/jpm.2005.8.876. [DOI] [PubMed] [Google Scholar]
- 57.Schiff GD, Galanter WL, Duhig J, Lodolce AE, Koronkowski MJ, Lambert BL. Principles of conservative prescribing. Archives of internal medicine. 2011;171(16):1433–1440. doi: 10.1001/archinternmed.2011.256. [DOI] [PubMed] [Google Scholar]
- 58.Yourman LC, Lee SJ, Schonberg MA, Widera EW, Smith AK. Prognostic indices for older adults: a systematic review. JAMA : the journal of the American Medical Association. 2012;307(2):182–192. doi: 10.1001/jama.2011.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Min L, Yoon W, Mariano J, Wenger NS, Elliott MN, Kamberg C, et al. The vulnerable elders-13 survey predicts 5-year functional decline and mortality outcomes in older ambulatory care patients. Journal of the American Geriatrics Society. 2009;57(11):2070–2076. doi: 10.1111/j.1532-5415.2009.02497.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Fried LP, Ferrucci L, Darer J, Williamson JD, Anderson G. Untangling the concepts of disability, frailty, and comorbidity: implications for improved targeting and care. The journals of gerontology Series A, Biological sciences and medical sciences. 2004;59(3):255–263. doi: 10.1093/gerona/59.3.m255. [DOI] [PubMed] [Google Scholar]
- 61.Sun X, Briel M, Busse JW, You JJ, Akl EA, Mejza F, et al. The influence of study characteristics on reporting of subgroup analyses in randomised controlled trials: systematic review. BMJ (Clinical research ed) 2011;342:d1569. doi: 10.1136/bmj.d1569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sever PS, Dahlof B, Poulter NR, Wedel H, Beevers G, Caulfield M, et al. Prevention of coronary and stroke events with atorvastatin in hypertensive patients who have average or lower-than-average cholesterol concentrations, in the Anglo-Scandinavian Cardiac Outcomes Trial--Lipid Lowering Arm (ASCOT-LLA): a multicentre randomised controlled trial. Lancet. 2003;361(9364):1149–1158. doi: 10.1016/S0140-6736(03)12948-0. [DOI] [PubMed] [Google Scholar]