Abstract
Objective
The present study is designed to understand the contribution of peripheral vascular disease and peripheral neuropathy to the wound-healing impairment associated with diabetes. Using a rabbit model of diabetic neuroischemic wound-healing we investigated rate of healing, leukocyte infiltration and expression of cytokines, Interleukin (IL)-8 and IL-6, and, neuropeptides, Substance P (SP) and Neuropeptide Y (NPY).
Design of study
Diabetes was induced in White New Zealand rabbits by administering alloxan while control rabbits received saline. Ten days later animals in both groups underwent surgery. One ear served as a sham and the other was made ischemic (ligation of central+rostral arteries), or neuroischemic (ischemia+ resection of central+rostral nerves). Four, 6mm punch biopsy wounds were created in both ears and wound-healing was followed for ten days using computerized planimetry.
Results
Non-diabetic sham and ischemic wounds healed significantly more rapidly than diabetic sham and ischemic wounds. Healing was slowest in neuroischemic wounds, irrespective of diabetic status. A high M1/M2 macrophage ratio and a high pro-inflammatory cytokine expression, both indicators of chronic-proinflammatory state, and low neuropeptide expression were seen in pre-injury diabetic skin. Post-injury, in diabetic wounds M1/M2 ratio remained high, the reactive increase in cytokine expression was low and neuropeptide expression was further decreased in neuroischemic wounds.
Conclusion
This rabbit model illustrates how a combination of a high M1/M2 ratio, a failure to mount post-injury cytokine response as well as a diminished neuropeptide expression contribute to wound-healing impairment in diabetes. The addition of neuropathy to ischemia leads to equivalently severe impaired wound-healing irrespective of diabetes status, suggesting that in the presence of ischemia, loss of neuropeptide function contributes to the impaired healing associated with diabetes.
INTRODUCTION
One of the most common complications of both, Type I and Type II diabetes is the development of chronic non-healing foot ulcerations leading to severe morbidity and mortality 1. The cause of impaired wound-healing in diabetes is multi-factorial and includes vascular, neurologic and inflammatory alterations 2. Impaired diabetic wound-healing is increased in skin areas that are affected by peripheral neuropathy and there is growing evidence that cutaneous peripheral nerves regulate immune and cytokine response via mediators such as neuropeptides Substance P and Neuropeptide Y 3, 4. Our group has shown that these neuropeptides not only control cytokine release from leukocytes but also affect endothelial cell function 5. A recent clinical study by our group also shows that increased inflammation and aberrant growth factor expression in the skin are associated with failure to heal diabetic foot ulcers 6.
In vivo investigation of diabetes-related impaired wound-healing is most commonly performed in rodent models because of their economic feasibility, shorter reproduction times and accessibility to genetically engineered mice models. However, skin healing in rodents is largely mediated through contraction while in humans it occurs through re-epithelialization. Rabbit ear skin is similar to human skin, having blood vessels that serve as a major heat-exchange surface and are controlled by the autonomic nervous system for thermoregulation 7-9. Both SP- and NPY-releasing nerves innervate the rabbit ear and are involved in controlling the vascular tone, more specifically for thermoregulation 10. A previous study from our lab that employed the rabbit ear wound-healing model showed that diabetes affects the focused inflammatory cytokine response to injury and dysregulates neuropeptide gene expression but this study did not assess the role of vascular ischemia and neuropathy, two of the major contributing factors of diabetes-associated complications 11.
Our hypothesis is that in addition to vascular ischemia, neuropeptide dysregulation resulting in the alteration of inflammatory response will impair wound-healing in diabetic rabbits.
To test our hypothesis, we used a rabbit neuroischemic model of ear wound-healing similar to the model used by Chien et.al 12where, the intact cartilage stents the wound open and hence most of the healing occurs through re-epithelialization 13. Our study is the first to use this model to provide a comprehensive analysis of the combined effects of diabetes, ischemia and denervation on wound-healing. Changes in immune cell infiltration, gene and protein expression of inflammatory cytokines, neuropeptides, their receptors and associated proteins were evaluated. Specifically, the infiltration of HLA-DR+ M1 macrophages, responsible for development of chronic wounds, CD206+ M2 macrophages, involved in tissue repair, CD177+ neutrophils and CD3+ T cells were evaluated. The expression of cytokines, IL-6, IL-8 and, IL-8 receptor, CXCR1, all of which are pivotal for wound-healing were assessed. We also analyzed the expression of SP and its pre-dominant receptor NK1R, NPY and its pro-angiogenic receptors NPY2R and NPY5R, the enzyme Neprilysin (NEP) that metabolizes SP and the enzyme Dipeptidyl Peptidase IV (DPPIV) that cleaves the 36 amino acid NPY to the angiogenic form, NPY3-36 4.
MATERIALS AND METHODS
Rabbits
New Zealand White rabbits (3.0–3.2kg, Millbrook Farms, Amherst, MA) received 75mg/kg, i.v. alloxan monohydrate (diabetic group) or saline (non-diabetic group) on day (D)0. After 48 hours, rabbits that did not become diabetic with the first alloxan injection received another 75mg/kg alloxan monohydrate, i.v. Normal blood glucose levels for rabbits varied between 100mg/dl to 150mg/dl. Rabbits with blood glucose over 250mg/dl were considered diabetic 14. There were no significant changes in the weights of the non-diabetic and diabetic rabbits (Table S1).
Surgical Models
In each surgical group on D10, one ear served as an experimental ear and other as a sham ear (Fig.S1A). In one group, ischemia was created in the experimental ear by ligating the central and the rostral artery leaving the caudal artery and all the veins intact. In the second surgical group, central and rostral arteries were ligated along with, central and rostral nerve resection creating the neuroischemia. In the sham ears, arteries and nerves were dissected out but not ligated or resected. Following surgery, in both ears, four full thickness circular wounds were created using a 6mm punch biopsy. The cartilage was kept intact since it stents the wound open, and minimizes tissue contracture to less than 3%, allowing the wound to heal by re-epthelialization 15. On D20, rabbits were euthanized and wound tissue was harvested for analysis. This time-point was chosen based on the results of our previous study where we observed that at least 50% wounds heal in the non-diabetic sham group 11. The Harvard Medical Area Standing Committee on Animals approved all study procedures.
Wound area measurement
Post-injury, wound-healing was monitored for ten days (from D10 to D20). Wounds were photographed using Medical Hyperspectral Imaging camera (Hypermed Inc. Burlington MA) and wound area was measured as change in pixels.
Tissue Analysis
Punch biopsies and the wound samples (1cm × 1cm, cut into 4 pieces) from each ear were analyzed for gene (qRT-PCR) and protein (Immunohistochemistry-paraffin & -frozen and western blot) expression analysis.
Gene Expression
Standard qRT-PCR was used to quantitate and compare levels of specific RNA transcripts. Primers (Table S7) were obtained from Integrated DNA Technologies (Coralville, IA). For quantitative analysis, target gene levels were normalized to beta-actin levels. Gene expression in the wound samples was measured as fold change over the baseline gene expression.
Histology and Immunohistochemistry (IHC)
Tissue samples were either formalin-fixed paraffin-embedded or were OCT-embedded and frozen. Standard histology and IHC techniques were used. Antibody information is in Table S8. To confirm specific staining for each primary antibody, an isotype negative control and a no-primary antibody control was used. All H&E staining and IHC readings were performed in a blinded fashion and an arbitrary scale of 1-5 was used to grade them. H&E grading was performed on the basis of extent of leukocyte infiltration (distance cells migrated from the wound margin) and intensity of leukocyte infiltration (# of infiltrating cells). For immune cell infiltration, neuropeptide and cytokine IHC, data are presented as fold change over the baseline infiltration. For macrophage studies, M1/M2 ratio is calculated from the arbitrary raw scores. It is important to note that because of very high numbers of positively stained total leukocytes (H&E) and macrophages (both M1 and M2) it was not possible to count the number of cells but to use an arbitrary scale as explained above.
Western blot
Standard western blot techniques were used and specific antibody information is in Supplemental Table S8. Densitometry was performed using Quantity One™ (Bio-Rad, Hercules, CA) software with protein levels corrected to β-Actin levels. Data are presented in the form of densitometric analysis. Protein expression in the wound samples was measured as fold change over the baseline protein expression.
Data Analysis and Statistics
Data were compared between non-diabetic and diabetic rabbits grouped into their specific surgery. Representative images of western blot and immunohistochemistry are presented in the supplemental data (Fig.S3-S6). All data are expressed as the mean±SEM and are analyzed using either One-Way or Two-Way ANOVA test. An unpaired t-test is performed and p<0.05 is considered statistically significant. Statistical analysis was done using Statview (SAS Inc.). In addition to graphical representation of the data, numerical data is presented in Supplementary Tables S1-S6. We have also presented graphical data showing comparisons between sham, ischemic and neuroischemic wounds within non-diabetic or diabetic rabbits in the Supplementary document (Figs. S7-S11).
RESULTS
Compared to Non-Diabetic Sham and Ischemic Wounds, Wound-Healing is Impaired in Diabetic Sham and Ischemic Wounds
Wound-healing was impaired in the diabetic sham and ischemic wounds at D15 and D20 when compared to non-diabetic wounds (Figs. 1, S1B and S7). Interestingly, no differences between non-diabetic and diabetic neuroischemic wounds were noted. However, amongst non-diabetic animals neuroischemic wounds showed the most impaired healing when compared to the other non-diabetic wounds, comparable to that observed in diabetic wounds. Across all diabetic animals groups minimal differences in the rate of wound-healing were noted. These results suggest that neuropathy and ischemia have additive effects and that diabetes does not further impair neuroischemic wound-healing.
Figure 1. Wound-healing: Comparison between non-diabetic and diabetic rabbit sham, ischemic and neuroischemic wounds.
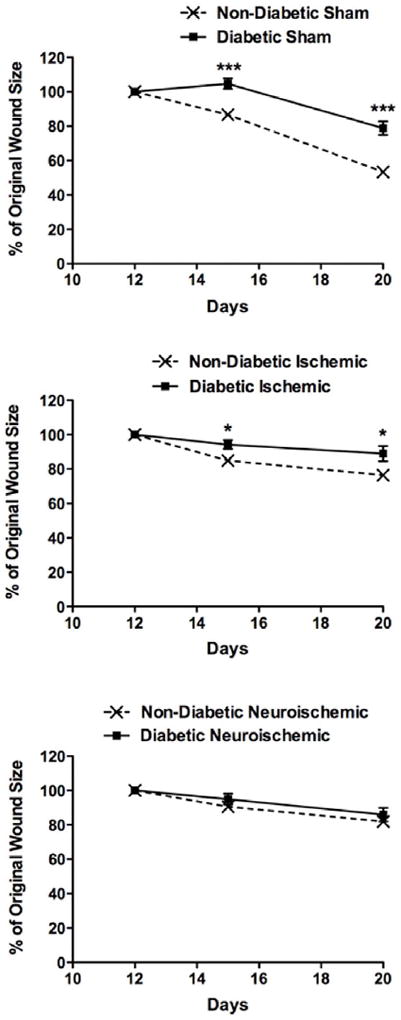
Wound-healing was monitored over 10-day period from D10-D20. Photographs were obtained on D12, D15 and D20 using Medical Hyperspectral Imaging camera. Using Adobe Photoshop®, wound area was measured in pixels. Data are expressed as percent change in wound area from D12 to D20 (N=6-18, mean±SEM, ***P<0.001 & *P<0.05).
Pre-Injury Diabetic Rabbit Skin Shows Signs of Inflammation (Assessed By Leukocyte Infiltration) But Post-Injury Fails to Mount a Satisfactory Inflammatory Response
In the present study, we evaluated the pre-injury (D10) and post-injury (D20 of experiment and ten days after surgery) leukocyte infiltration in the wounds, respectively. In agreement with our previous findings, baseline pre-injury skin leukocyte infiltration (extent and intensity) was higher in diabetic compared to non-diabetic rabbits (Figs. 2A and S2). Post-injury, the fold increase over baseline infiltration (extent and intensity) was higher in non-diabetic sham, ischemic and neuroischemic wounds compared to diabetic wounds, clearly indicating an impaired ability in the diabetic animals to mount an appropriate, focused leukocyte response after injury (Figs. 2B, S2 and S8).
Figure 2. Leukocyte Infiltration: Comparison between non-diabetic and diabetic.
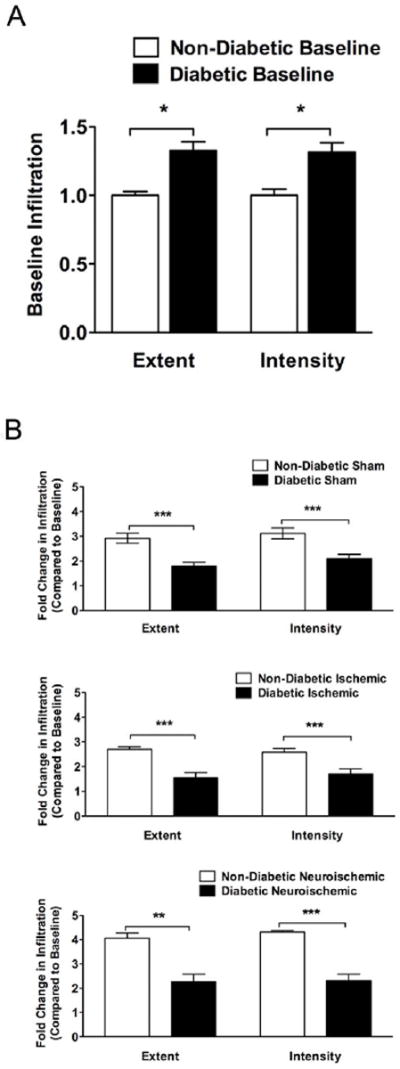
H&E stained cross-sections were analyzed for leukocyte infiltration. An arbitrary scale of 1-5 was used for grading entire cross-sections. Grading was performed on the basis of extent of infiltration (distance the cells migrated from the wound margin) and intensity of infiltration (# of infiltrating cells). Data are presented in the form of arbitrary scores.
A. Baseline comparison: Data is expressed as fold change in infiltration (extent and intensity) of diabetic rabbit skin compared to non-diabetic rabbit skin (N=6-12, mean±SEM, *P<0.05).
B. Post-injury comparison within sham, ischemic and neuroischemic wounds: Data is expressed as post-injury change in infiltration (extent and intensity) over the baseline in non-diabetic and diabetic rabbit wounds (N=5-6, mean±SEM, ***P<0.001 & **P<0.01).
Diabetic Rabbits Have Increased M1/M2 Macrophage Ratio In Their Skin and Wounds
Since macrophages play important role in both, tissue repair (M2 activation pathway) and development of chronic wounds (M1 activation pathway), we calculated M1/M2 ratio in the tissues by analyzing HLA-DR+/CD18+ (M1) and CD206+/CD18+ (M2) macrophages at baseline in the skin at D10 and in the wounds at D20 (Figs. 3A-3C and S3). Compared to non-diabetic, diabetic rabbits had more M1 macrophages at baseline in the skin at D10 and in the sham wounds at D20 (Fig. 3A). M2 macrophages were less in diabetic rabbits both, at D10 and in all wounds at D20 (Fig. 3B). This resulted in a higher M1/M2 ratio in diabetic rabbits in all surgical conditions (Figs. 3C and S9). Moreover, there were higher numbers of M1 macrophages in diabetic rabbit skin at D10 than there were M2 macrophages in non-diabetic rabbit skin at D10 probably resulting in overall higher number of leukocytes observed in diabetic rabbit skin at D10. CD177+ neutrophils or CD3+ T cells were not observed in the skin of both, non-diabetic and diabetic rabbits on D10 and there were no differences in their numbers between non-diabetic and diabetic rabbits in any wounds at D20. However, in both, non-diabetic and diabetic rabbits, the neuroischemic wounds had the highest number of CD177+ neutrophils (Fig. 4A) and CD3+ T cells (Fig. 4B). Higher M1/M2 ratio indicated pro-inflammatory, impaired healing environment in the diabetic rabbits and presence of CD177+ neutrophils suggests that the neuroischemic wounds remained in a prolonged inflammatory phase of wound-healing.
Figure 3. Macrophage Infiltration: Comparison between non-diabetic and diabetic.
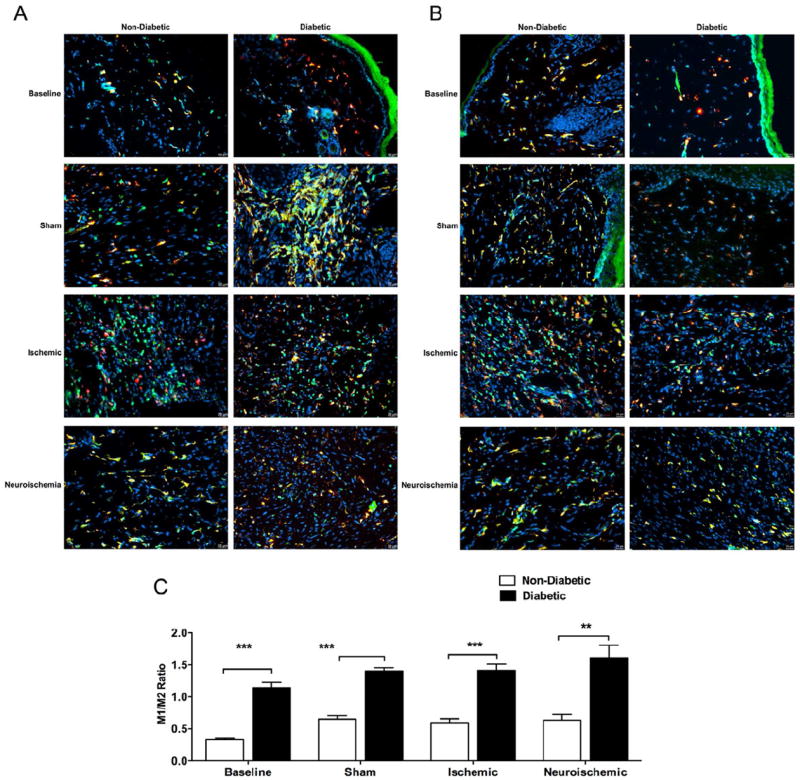
OCT embedded 6μm frozen tissue sections were stained for macrophage marker CD18 and, co-stained for either, M1 macrophage activation marker HLA-DR or M2 macrophage activation marker CD206. Merged images (yellow) of HLA-DR+ (red) and CD18+ (green) or CD206+ (red) and CD18+ (green) macrophages were obtained close to the wound margins using standard fluorescent microscopy.
A. Representative images of M1+ macrophages and, B. Representative images of M2+ macrophages: Representative images are from non-diabetic and diabetic treatment groups at Baseline and, Sham, Ischemic and Neuroischemic wounds (Mag =20X).
C. M1/M2 ratio: An arbitrary scale of 1-5 was used for grading cross-section images. M1/M2 ratio was obtained based on arbitrary scores. (N=3-4, mean±SEM, ***P<0.001, **P<0.01).
Figure 4. Representative images of A) CD177+ (neutrophil) and, B) CD3+ (T cell) infiltration.
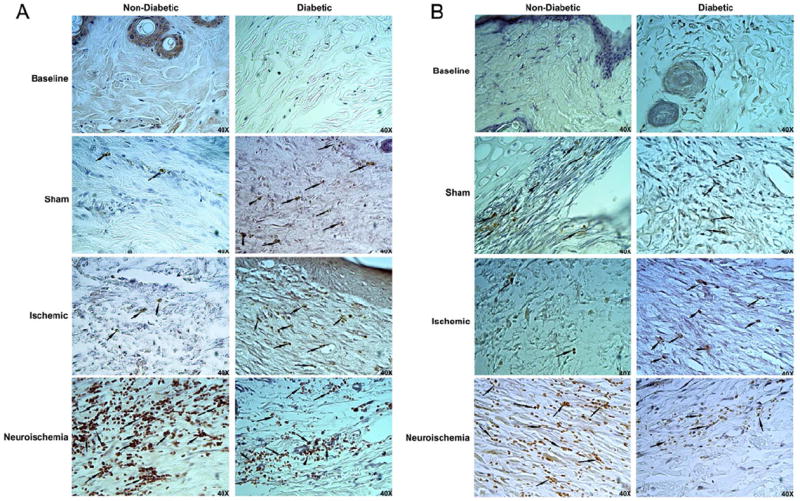
Formalin fixed paraffin embedded 6μm cross-sections were stained with CD177 or CD3 primary antibody and analyzed for neutrophils and T cell infiltration respectively. Representative images close to the wound margin are from non-diabetic and diabetic treatment groups at Baseline and, Sham, Ischemic and Neuroischemic wounds (Mag=40X). Arrows indicate staining for CD177+ or CD3+ cells.
Gene and Protein Expression of Inflammatory Cytokines Involved in Wound-Healing Follow the Same Pattern as Leukocyte Infiltration
We evaluated the gene and protein expression of the main cytokines that are involved in wound-healing. At baseline in the skin at D10, diabetic rabbits showed higher gene expression of IL-8, CXCR1 and IL-6 with no differences in the protein expression (Figs. 5, S4 and S5). After creation of the wound, the reactive fold change in gene expression over baseline of IL-6, IL-8 and CXCR1 was lower, or trended to be lower in all diabetic wounds when compared to non-diabetic wounds (Figs. 6A and S10A). The fold change in protein expression over baseline was lowest for IL-6 followed by IL-8 and CXCR1 in diabetic wounds when compared to non-diabetic wounds (Figs. 6B, S4, S5 and S10B).
Figure 5. Cytokine, Neuropeptide and Their Receptor Expression: Comparison at baseline in the expression of IL-8, CXCR1, IL-6, SP, NK1R, NEP, NPY, NPY2R, NPY5R and DPPIV between non-diabetic and diabetic rabbits.
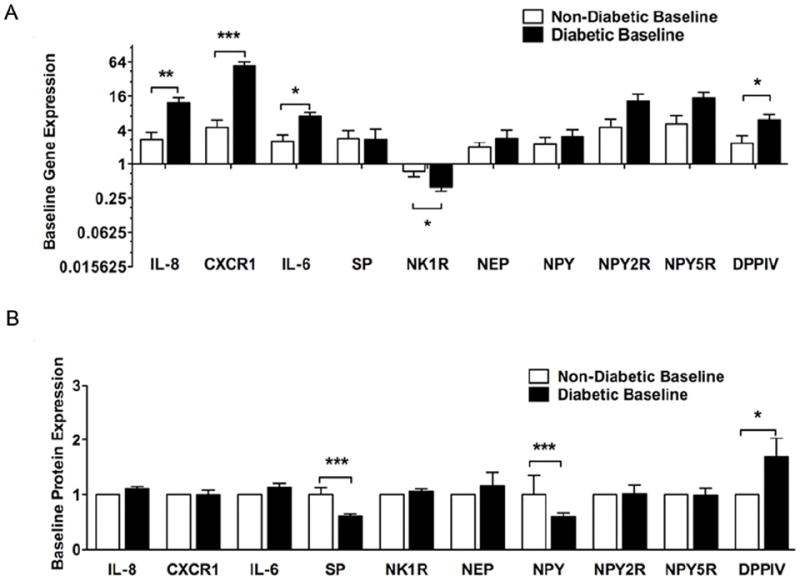
A. Gene expression: Using standard Q-RT-PCR and 2-ΔΔCt analysis method, gene expression was analyzed. Data are expressed as fold change over non-diabetic expression. (N=12-15, mean±SEM, ***P<0.001, **P<0.01, *P<0.05).
B. Protein expression: Using standard western blot and immunohistochemistry methods, protein expression was analyzed. For western blot quantification, densitometry was used. For immunohistochemistry quantification, an arbitrary scale of 1-5 was used for grading entire cross-sections. Data are expressed as fold change over non-diabetic expression (N=6-12, mean±SEM, ***P<0.001, **P<0.01, *P<0.05).
Figure 6. Cytokines And Their Receptors: Post-injury comparison in the expression of IL-8, CXCR1 and IL-6 between non-diabetic and diabetic rabbits within sham, ischemic and neuroischemic wounds.
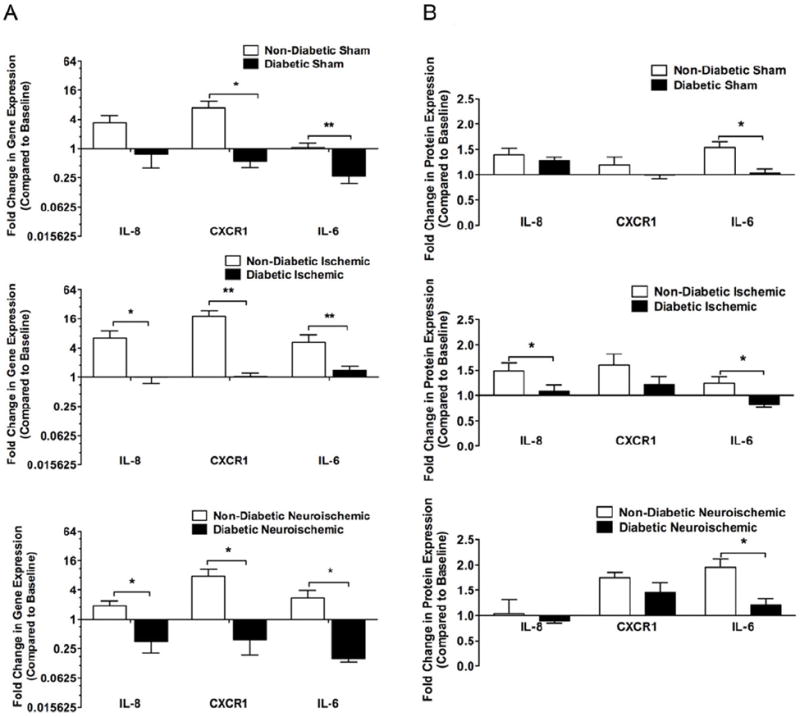
A. Gene Expression: Using standard Q-RT-PCR and 2-ΔΔCt analysis method, gene expression was analyzed. Data are expressed as change of expression over the baseline in non-diabetic and diabetic rabbit wounds. (N=5-6, mean±SEM, **P<0.01, *P<0.05).
B. Protein Expression: Using standard western blot and immunohistochemistry methods, protein expression was analyzed. For western blot quantification, densitometry was used. For immunohistochemistry quantification, an arbitrary scale of 1-5 was used for grading entire cross-sections. Data expressed as change of expression over the baseline in non-diabetic and diabetic rabbit wounds (N=4-6, mean±SEM, **P<0.01, *P<0.05).
Gene and Protein Expression of Neuropeptides, Their Receptors, and Enzymes That Are Involved in Their Turnover Are Altered in Diabetes
At baseline in the skin at D10, gene expression of DPPIV was higher while that of NK1R was lower in diabetic compared to non-diabetic rabbits while no other differences were observed in the other factors. SP and NPY protein expression was lower and DPPIV was higher in diabetic compared to non-diabetic rabbits (Figs. 5A, 5B, S4 and S6).
Compared to non-diabetic wounds, the fold change in gene expression over baseline of SP was lower in the diabetic neuroischemic wounds, while the expression of NPY was lower in the diabetic ischemic and neuroischemic wounds. DPPIV gene expression was lower in all types of diabetic wounds. Interestingly, NK1R, NPY2R, NPY5R gene expression was not changed in any diabetic wounds. NEP gene expression was reduced in the diabetic neuroischemic wounds (Figs. 7A and S11A).
Figure 7. Neuropeptides, Their receptors and Related Enzymes: Post-injury comparison in the expression of SP, NK1R, NEP, NPY, NPY2R, NPY5R and DPPIV between non-diabetic and diabetic rabbits within sham, ischemic and neuroischemic wounds.
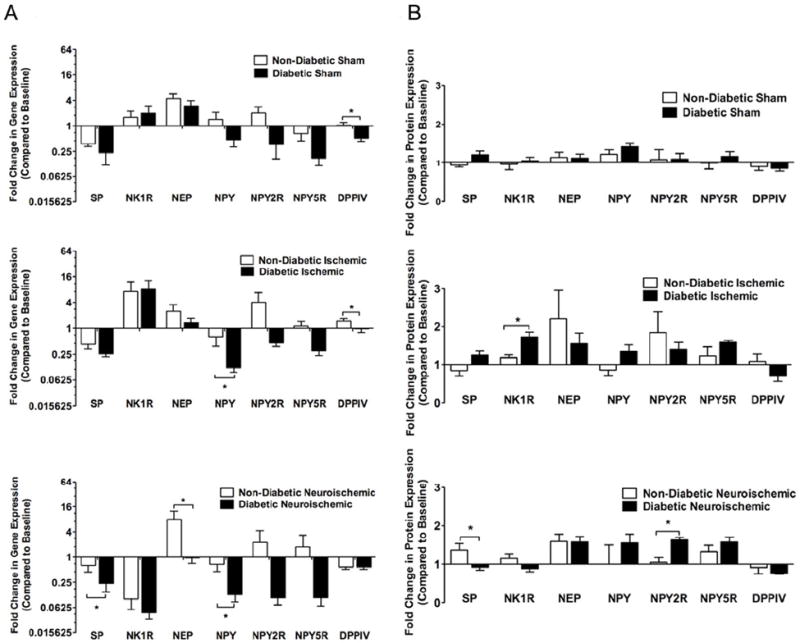
A. Gene Expression: Using standard Q-RT-PCR and 2-ΔΔCt analysis method, gene expression was analyzed. Data expressed as change of expression over the baseline in non-diabetic and diabetic rabbit wounds (N=5-6, mean±SEM, *P<0.05).
B. Protein Expression: Using standard western blot and immunohistochemistry methods, protein expression was analyzed. For western blot quantification, densitometry was used. For immunohistochemistry quantification, an arbitrary scale of 1-5 was used for grading entire cross-sections. Data expressed as change of expression over the baseline in non-diabetic and diabetic rabbit wounds (N=4-6, mean±SEM, *P<0.05).
Overall, fold change over baseline of SP protein expression was not different between non-diabetic and diabetic wounds except in neuroischemic wounds. NK1R protein expression was increased in diabetic ischemic wounds. NPY2R and NPY5R protein expression were increased in diabetic neuroischemic wounds. (Figs. 7B, S4, S6 and S11B).
DISCUSSION
In this model we have shown that the presence of diabetes results in impaired wound-healing in both sham and ischemic wounds. Additionally ischemia alone reduces wound-healing in non-diabetic animals with minimal reduction in diabetic rabbits. The presence of neuroischemia results in the greatest impairment of healing in all animals and is so severe that additional presence of diabetes does not further impair wound-healing. This may be partially explained by the fact, that two of the most relevant long-term diabetes complications are tissue ischemia and neuropathy. Diabetic animals have chronic cutaneous inflammation, which can be detected prior to injury. However, they fail to mount an adequate post-injury focal inflammatory response indicated by failure to switch from M1 to M2 macrophage phenotype and reduced cytokine expression, both of which are required to achieve wound-healing.
In the present study we used a clinically relevant model that can serve to understand the pathophysiology underlying diabetic wound-healing impairment and can be used for testing future therapeutics. Although Chien et. al have used the neuroischemic model to study tissue phosphate contents, our study is the first to compare the differences in wound-healing between the ischemic and neuroischemic models 13. Moreover, our study is the first to analyze and compare in depth the expression of neuroimmune markers in these models.
Diabetes is known to be associated with a systemic pro-inflammatory state. Our recent clinical work shows that diabetic patients have increased skin leukocyte infiltration 6. Similar to the clinical findings, in the present study we observed an increased leukocyte infiltration in the diabetic rabbit skin. Most likely this increase was due to the increased M1 macrophage infiltration that is associated with chronic inflammation. Although pre-injury chronic inflammation is deleterious for wound-healing, post-injury inflammation, mainly achieved by adequate leukocyte infiltration and cytokine release, is needed for complete wound-healing. In almost all wound types, the extent and intensity of acute infiltration was higher in all non-diabetic wounds compared to diabetic. Neuroischemic wounds that heal the slowest had the highest baseline macrophage infiltration compared to other wounds. These results suggest that, to achieve successful wound-healing, there is a critical threshold for an increase in focused acute infiltration in response to injury. Moreover, diabetes is associated with leukocyte dysregulation with irregular and/or insufficient immune cell activation and cytokine and growth factor secretion. Therefore, in diabetic wounds, even though there may be a comparable number of infiltrating leukocytes, they may not reflect an adequate response to injury. Further analysis of types of cells infiltrating the skin and wounds suggests that, pre-injury, diabetic rabbit skin has a higher number of M1 while lower number of M2 macrophages. Although there was no difference in the number of M1 macrophages between non-diabetic and diabetic (ischemic and neuroischemic) wounds, there were lower number of M2 macrophages in all of the diabetic wounds leading to an overall higher M1/M2 ratio in the diabetic wounds suggesting a chronic wound environment. On D20, almost all of the infiltrating cells found in sham and ischemic wounds were macrophages with a few CD177+ neutrophils and CD3+ T cells. Only the neuroischemic wounds had high numbers of CD177+ neutrophils and CD3+ T cells. Presence of neutrophils beyond the initial inflammatory stage is a hallmark of chronic wounds.
Similar to our prior study, we found an up-regulation of IL-8, CXCR1 and IL-6 gene expression in diabetic rabbits at baseline, suggesting a chronic pro-inflammatory effect of diabetes.
Post-injury, the fold change from baseline gene and protein expression of IL-8, CXCR1 and IL-6 was blunted in all diabetic wounds compared to non-diabetic wounds indicating that the diabetes-related chronic up-regulation of cytokine expression at baseline may hamper the much-needed focused up-regulation post-injury.
Neuroischemia may not have had any additional effect on wound-healing in the diabetic wounds due to the already reduced baseline expression of neuropeptides SP and NPY. Similar to other studies 16, 17, the gene expression of the NEP, the enzyme that breaks down SP was higher in diabetic animals although it did not reach statistical significance. This might further reduce the levels of SP and therefore negatively affected wound-healing. Similar to previous studies 18, 19, the present study showed decreased NK1R gene expression, the receptor for SP. Current information regarding the expression of NPY receptors in diabetes is inconsistent 19, 20. In the present study, at baseline, there was no difference in the gene expression of NPY2R and NPY5R between non-diabetic and diabetic rabbits. We did find that pre-injury, diabetes up-regulated both, gene and protein expression of DPPIV.
Post-injury, there was a decrease from baseline in gene expression of SP and NPY in all wounds. These results suggest that injury itself, irrespective of diabetic status can lead to down-regulation of neuropeptides gene expression. The decrease in SP gene expression was significant between non-diabetic and diabetic rabbits only in the neuroischemic wounds whereas the decrease in NPY was significant between non-diabetic and diabetic rabbits in almost all wound types. NK1R, NPY2R and NPY5R gene expression were not different between non-diabetic and diabetic rabbits in any wounds. Gene expression of NEP was increased over baseline in all wound types with no differences between non-diabetic and diabetic wounds except in the neuroischemic wounds, where NEP expression is decreased in diabetic wounds compared to non-diabetic wounds, maybe to counteract loss of SP. The relative gene expression of DPPIV in sham and ischemic wounds was lower in diabetic compared to non-diabetic wounds with no difference in neuroischemic wounds. Decrease in local wound expression of DPPIV can reduce the formation of pro-angiogenic form of NPY and thus affect wound-healing. These data once again suggest that diabetes and neuroischemia can seriously affect angiogenesis through the dysregulation of the NPY pathway in these rabbits. This is an important finding because DPPIV is known to breakdown incretins and hence DPPIV inhibitors are clinically used for glycemic control.
The goal of this present study was to use a wound-healing model that most closely resembles the human pathophysiology to understand the role of cytokines and neuropeptides in wound-healing. Although the rabbit vascular ischemia model is commonly used, there are few animal models of diabetic peripheral neuropathy, most of which are rodents 21-23. The neuropathy/denervation model that we have used, although not perfect, has been used to study the effect of sensory denervation on hypertrophic scarring in absence of diabetes and vascular ischemia 24. Due to the bidirectional connection between nerves and inflammatory cells, in a model of ‘denervation only’, we could expect partial wound-healing impairment most likely through dysregulation of inflammation and deficiency of neuropeptides. We acknowledge that at this time more work needs to be done to fully decipher the mechanisms underlying the wound impairment in our model. Using in vitro models, our group is also investigating the effect of neuropeptides on the individual cellular components that are involved in wound-healing. We also acknowledge that the study has other limitations. Our model is a type I diabetes model and although commonly used for wound-healing studies, this may not model precisely the complexities of type II diabetes. Therefore it will be interesting to use a high fat diet rabbit model of diabetes and perform similar investigations. In the present study we used a 10 day time-point post-surgery to evaluate healing. However, it could be possible that healing is impaired to the extent that it is delayed at that time point but not abrogated. Currently our group is conducting studies where time to wound closure is being evaluated along with an early post-surgery euthanasia time-point to investigate differences in inflammation. Also, this model does not allow for investigation of the effect of pressure on wound-healing. Pressure points associated with deformities in insensate feet are a common problem in diabetes that eludes a perfect animal model at this time. Other limitations include discrepancy in the gene and protein expression. Thus, for some genes that were affected, we did not see a corresponding change in their respective protein expression. This could be explained by the fact that some of these genes, especially the neuropeptides and the cytokines undergo post-transcriptional and -translational modifications. Moreover, there could be a delay in translation of the gene expression changes to manifest in the protein expression changes. Another limitation is the lack of specific antibodies in rabbits such including PGP 9.5, and hence we could not monitor this critical neuronal marker. It should also be emphasized that most antibodies that achieved successful staining were specific for humans and required time-consuming optimization.
In conclusion, this study suggests that neuroischemia and diabetes have the greatest impact on the wound-healing outcome. Although there were no differences in wound-healing between non-diabetic and diabetic neuroischemic wounds, there are clear differences at the molecular level. This model can be very helpful in investigating the pathophysiology of diabetic neuroischemic wound-healing and for testing new therapeutics for the treatment of diabetic neuroischemic wounds.
Supplementary Material
CLINICAL RELEVANCE.
Although, diabetic foot ulceration (DFU) is a major clinical problem and the most common cause for hospital admissions in diabetic patients, there is limited basic and translational research. One of the main reasons is the lack of appropriate animal models that control for factors related to the major complications of diabetes, namely, neuropathy and ischemia The aim of this study was to use a clinically relevant animal model of neuroischemic wound-healing that is representative of human DFU and to examine the contribution of neuropeptides and inflammation. These data, showing a link between neuropeptide and cytokine function, diabetes, and neuroischemia, provide both a basis and a model for studies of wound –healing therapies.
Acknowledgments
The authors would like to acknowledge the help of Dr. Charalambos Gnardellis for his assistance in statistical analysis.
This work was funded in part by: 1) NIH R01 1R01NS066205-01 (MPI) to A. Veves and L. Pradhan Nabzdyk
2) William J. von Liebig Foundation award to F.W. LoGerfo
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.ADA. Diabetes Statistics. American Diabetes Associaiton; 2011. [Google Scholar]
- 2.Boulton AJ. The diabetic foot: grand overview, epidemiology and pathogenesis. Diabetes Metab Res Rev. 2008;24(Suppl 1):S3–6. doi: 10.1002/dmrr.833. [DOI] [PubMed] [Google Scholar]
- 3.Pavlovic S, Daniltchenko M, Tobin DJ, Hagen E, Hunt SP, Klapp BF, et al. Further exploring the brain-skin connection: stress worsens dermatitis via substance P-dependent neurogenic inflammation in mice. J Invest Dermatol. 2008;128(2):434–46. doi: 10.1038/sj.jid.5701079. [DOI] [PubMed] [Google Scholar]
- 4.Pradhan L, Nabzdyk C, Andersen ND, LoGerfo FW, Veves A. Inflammation and neuropeptides: the connection in diabetic wound healing. Expert Rev Mol Med. 2009;11:e2. doi: 10.1017/S1462399409000945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jain M, LoGerfo FW, Guthrie P, Pradhan L. Effect of hyperglycemia and neuropeptides on interleukin-8 expression and angiogenesis in dermal microvascular endothelial cells. J Vasc Surg. 2011;53(6):1654–60 e2. doi: 10.1016/j.jvs.2011.02.019. [DOI] [PubMed] [Google Scholar]
- 6.Dinh T, Tecilazich F, Kafanas A, Doupis J, Gnardellis C, Leal E, et al. Mechanisms Involved in the Development and Healing of Diabetic Foot Ulceration. Diabetes. 2012 doi: 10.2337/db12-0227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Harada E, Kanno T. Rabbit’s ear in cold acclimation studied on the change in ear temperature. J Appl Physiol. 1975;38(3):389–94. doi: 10.1152/jappl.1975.38.3.389. [DOI] [PubMed] [Google Scholar]
- 8.Hill RW, Veghte JH. Jackrabbit ears: surface temperatures and vascular responses. Science. 1976;194(4263):436–8. doi: 10.1126/science.982027. [DOI] [PubMed] [Google Scholar]
- 9.Slepchuk NA, Rumiantsev GV. Role of a decrease in the body’s heat content on the thermoregulatory reaction of the vessels of the external ear. Fiziol Zh SSSR Im I M Sechenova. 1978;64(6):843–9. [PubMed] [Google Scholar]
- 10.Golfert F, Witt M, Scheele K, Hofer A, Kasper M, Funk RH. Hints of a functional connection between the neuropeptidergic innervation of arteriovenous anastomoses and the appearance of epithelioid cells in the rabbit ear. Histochem J. 1998;30(6):435–45. doi: 10.1023/a:1003276310649. [DOI] [PubMed] [Google Scholar]
- 11.Pradhan L, Cai X, Wu S, Andersen ND, Martin M, Malek J, et al. Gene expression of pro-inflammatory cytokines and neuropeptides in diabetic wound healing. J Surg Res. 2011;167(2):336–42. doi: 10.1016/j.jss.2009.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chien S, Wilhelmi BJ. A simplified technique for producing an ischemic wound model. J Vis Exp. 2012;(63):e3341. doi: 10.3791/3341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chien S. Intracellular ATP delivery using highly fusogenic liposomes. Methods Mol Biol. 2012;605:377–91. doi: 10.1007/978-1-60327-360-2_26. [DOI] [PubMed] [Google Scholar]
- 14.Zagon IS, Sassani JW, Carroll MA, McLaughlin PJ. Topical application of naltrexone facilitates reepithelialization of the cornea in diabetic rabbits. Brain Res Bull. 81(2-3):248–55. doi: 10.1016/j.brainresbull.2009.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Davidson JM. Animal models for wound repair. Arch Dermatol Res. 1998;290:S1–S11. doi: 10.1007/pl00007448. [DOI] [PubMed] [Google Scholar]
- 16.Antezana M, Sullivan SR, Usui M, Gibran N, Spenny M, Larsen J, et al. Neutral endopeptidase activity is increased in the skin of subjects with diabetic ulcers. J Invest Dermatol. 2002;119(6):1400–4. doi: 10.1046/j.1523-1747.2002.19618.x. [DOI] [PubMed] [Google Scholar]
- 17.Spenny ML, Muangman P, Sullivan SR, Bunnett NW, Ansel JC, Olerud JE, et al. Neutral endopeptidase inhibition in diabetic wound repair. Wound Repair Regen. 2002;10(5):295–301. doi: 10.1046/j.1524-475x.2002.10504.x. [DOI] [PubMed] [Google Scholar]
- 18.Boer PA, Rossi Cde L, Mesquita FF, Gontijo JA. Early potential impairment of renal sensory nerves in streptozotocin-induced diabetic rats: role of neurokinin receptors. Nephrol Dial Transplant. 26(3):823–32. doi: 10.1093/ndt/gfq512. [DOI] [PubMed] [Google Scholar]
- 19.Ejaz A, LoGerfo F, Khabbaz K, Pradhan L. Expression of Neuropeptide Y, Substance P, and their receptors in the right atrium of diabetic patients. Clinical and Translational Science. 2011 doi: 10.1111/j.1752-8062.2011.00318.x. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Matyal R, Mahmood F, Robich M, Glazer H, Khabbaz K, Hess P, et al. Chronic type II diabetes mellitus leads to changes in neuropeptide Y receptor expression and distribution in human myocardial tissue. Eur J Pharmacol. 2011;665(1-3):19–28. doi: 10.1016/j.ejphar.2011.04.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Obrosova IG, Ilnytska O, Lyzogubov VV, Pavlov IA, Mashtalir N, Nadler JL, et al. High-fat diet induced neuropathy of pre-diabetes and obesity: effects of “healthy” diet and aldose reductase inhibition. Diabetes. 2007;56(10):2598–608. doi: 10.2337/db06-1176. [DOI] [PubMed] [Google Scholar]
- 22.Yamaguchi T, Sasase T, Mera Y, Tomimoto D, Tadaki H, Kemmochi Y, et al. Diabetic Peripheral Neuropathy in Spontaneously Diabetic Torii-Lepr(fa) (SDT Fatty) Rats. J Vet Med Sci. 2012 doi: 10.1292/jvms.12-0149. [DOI] [PubMed] [Google Scholar]
- 23.Hoke A. Animal models of peripheral neuropathies. Neurotherapeutics. 2012;9(2):262–9. doi: 10.1007/s13311-012-0116-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yagmur C, Guneren E, Kefeli M, Ogawa R. The effect of surgical denervation on prevention of excessive dermal scarring: a study on rabbit ear hypertrophic scar model. J Plast Reconstr Aesthet Surg. 64(10):1359–65. doi: 10.1016/j.bjps.2011.04.028. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


