Abstract
Background
Few studies have evaluated how to combine dietary and physical activity (PA) interventions to enhance adherence.
Purpose
We tested how sequential vs. simultaneous diet plus PA interventions affected behavior changes.
Methods
200 participants over age 44 years not meeting national PA and dietary recommendations (daily fruit and vegetable servings, percent of calories from saturated fat) were randomized to one of four 12-month telephone interventions: sequential (Exercise-First or Diet-First), Simultaneous, or Attention-Control. At 4 months, the other health behavior was added in the sequential arms.
Results
93% of participants were retained through 12 months. At 4 months, only Exercise-First improved PA, and only the Simultaneous and Diet-First interventions improved dietary variables. At 12 months, mean levels of all behaviors in the Simultaneous arm met recommendations, though not in the Exercise-First and Diet-First arms.
Conclusions
We observed a possible behavioral suppression effect of early dietary intervention on PA that merits investigation.
Keywords: Physical Activity, Dietary Change, Multiple Health Behaviors, Sequential, Simultaneous, Stress
Introduction
Many midlife and older adults fall below nationally recommended guidelines for regular physical activity and healthful eating (1-3). The most effective ways to improve these two key health behaviors remain unclear, particularly with respect to the timing of multiple changes (i.e., whether to promote sequential behavior changes one after the other, or intervene on both behaviors at the same time). Support for sequential change is found in social cognitive theory, which emphasizes the importance of breaking complex behavior patterns into smaller, more realistic steps to enhance initial success and promote mastery and self-efficacy for progressive behavior change (4). The strengths of this approach include building success with one behavior that may transfer to other behaviors (4), i.e., “gateway” or “spill over” effects (5, 6). However, such “spill over” effects, which have been proposed for physical activity behavior change (7), may not occur naturally, and may need to be specifically taught (8).
A sequential approach may be more responsive to motivational readiness for the behaviors being targeted, and may better accommodate differences in the demands underlying different behaviors (9, 10). Changing a more complex behavior in isolation of other behavior changes may produce greater self-efficacy and habit formation. However, the demands exerted by one behavior change could exact a toll on one's motivation and self-control, potentially making it more difficult to add a second behavior change (11-13).
In contrast to the sequential approach, a simultaneous approach focuses on changing multiple health behaviors at the same time (14). The simultaneous approach is based on epidemiological evidence indicating that health behaviors tend to cluster among groups in predicting disease outcomes (5). Certain health behaviors may also share putative social and psychological determinants (5, 6) as well as contextual factors (e.g., social influences, built environments) (15). Finally, the simultaneous approach may tap into more general “healthy living” values or schemas (14) that encompass multiple behaviors, as well as providing early exposure to multiple health behaviors that could be advantageous for those having to leave the program early.
A potential weakness of a simultaneous approach is that chronic stress and similar life circumstances often make it difficult to initiate and maintain changes in one health behavior, let alone several at once (16).
The current evidence on sequential versus simultaneous behavior change is mixed. For example, in treating hypertension, patients instructed to lose weight and follow a low-sodium diet simultaneously were less adherent to either of these regimens compared to patients for whom the two regimens were introduced separately (17). Yet, in another hypertension treatment study, patients given the most dietary and physical activity goals simultaneously were more successful in reaching their goals than those given fewer behavioral goals (18).
The specific health behaviors being targeted may make a difference (5). Researchers have noted that, in particular, diet and physical activity share physiological and behavioral mechanisms that, collectively, can impact energy balance, appetite, and food choices (6, 19). At least two studies have compared the relative effects of sequential versus simultaneous timing of dietary and physical activity instruction on behavior change. In a study by Vandenalotte et al. (20), two 50-minute computer interventions delivered personalized diet and physical activity recommendations sequentially or simultaneously. Between-group differences did not reach statistical significance at either 6 or 24 months. A study by Hyman et al. (21) compared sequential vs. simultaneous interventions for physical activity, dietary change (specifically reducing dietary sodium), and smoking cessation in an African-American hypertensive patient sample, and found no between-arm behavioral differences across the 18-month study period (although a trend favored greater dietary sodium change in the simultaneous arm).
Goals
The Counseling Advice for Lifestyle Management (CALM) trial evaluated the 12-month effects of sequentially versus simultaneously delivered telephone interventions to increase regular physical activity and healthful eating in midlife and older adults. Individuals not currently meeting national guidelines in either behavior and reporting time and stress management complaints were targeted given their particular challenges in making multiple behavior changes (16). Elevated stress levels can deter individuals from attempting health behavior changes (22), as well as drive premature dropout and non-adherence (23, 24).
Dietary targets were aimed at health promotion, as opposed to weight loss (i.e., caloric restriction was not targeted). We hypothesized that: (i) initial behavioral changes in the simultaneous arm would be less robust than in either of the two sequential interventions that began with a single health behavior (either physical activity or dietary change), due to the greater cognitive, behavioral, and motivational demands of a simultaneous behavior change program; but (ii) all 3 interventions would show significantly greater 12-month improvements in both physical activity and dietary behaviors relative to an attention-control (stress management) intervention; and (iii) given prior evidence that physical activity may be a ‘gateway’ to other health behavior changes (5, 6), any differences among the two sequential interventions in changing multiple behaviors would favor the arm receiving physical activity instruction first.
Method
Participants and Experimental Design
The study clinical trial registration number is NCT00131105. Study eligibility criteria were: ages 45 years and older; not engaged in more than 60 minutes per week of moderate-to-vigorous physical activity (MVPA) over the previous 6 months, based on self-reported items drawn from the National Health Interview Survey (25); intake of less than 5 fruit and vegetable servings per day (26); intake of more than 10 percent of total daily calories from saturated fats (26); a score of >2 (i.e., “occurring fairly or very often”) on at least one item of the Cohen 3-item perceived stress scale (which contained items on inability to control stress and control one's time) (27); free of any medical condition that would limit participation in moderate-intensity exercise; body mass index (BMI) ≤ 40; free of uncontrolled hypertension and type II diabetes requiring oral medications; able to speak and understand English sufficiently to participate in study procedures; regular access to a telephone; not planning to move from the area; and willing to be randomized to any of the four study arms. Recruitment strategies included targeted mass mailings, newspaper ads, and promotion through informal family caregiving organizations, given that family caregivers typically report increased stress and time constraints along with challenges maintaining healthy lifestyles (24). The study was described as a “healthy lifestyle” study (as opposed to a weight management study), and individuals interested primarily in weight loss were discouraged from enrolling. Individuals who met eligibility requirements during a telephone screen underwent baseline assessment.
Following stratification by gender and family caregiving status (yes/no), subjects were randomly assigned, using the Efron procedure (28), to one of four 12-month telephone interventions delivered by 4 trained health educators (who were trained to deliver all programs): 1) a sequential arm with exercise advice delivered first, then nutrition advice added at 4 months (Exercise-1st); 2) a sequential arm with nutrition advice first, then exercise advice added at 4 months (Diet-1st); 3) a simultaneous arm of exercise plus nutrition advice (Simultaneous); or 4) a stress management attention-control arm (Control). Four months was chosen as the time point to introduce the second behavior in the sequential arms given extensive evidence that significant improvements in the two behaviors occur by four months (29, 30). Stanford University Institutional Review Board approved the study protocol, and individuals signed IRB-approved study informed consent forms in-person prior to engaging in baseline assessment activities.
Interventions
The interventions were based on the evidence-based Active Choices program (31-33). Active Choices has been effective in a range of populations, including improvements in physical activity and dietary quality among chronically stressed adults (24). It draws on principles and strategies derived from Social Cognitive Theory (SCT) (4) and the Transtheoretical Model (34), including facilitating mastery through self-regulatory skill-building (e.g., harnessing social support for behavior change, structuring realistic outcome expectations, learning cognitive and behavioral processes of change, enacting active problem-solving to overcome barriers to change) (4).
All arms began with one individual in-person introductory session. Table 1 summarizes the telephone schedule and average minutes of advice delivered in each arm. Total volume of contact was equal across study arms. During the 12-month period, the 3 experimental arms were scheduled for 15 health advisor telephone contacts that included physical activity advice (15-20 minutes per call) and 15 that included dietary advice (15-20 minutes per call). In the two sequential arms, the first 4 months focused on only one behavior (exercise or nutrition), followed by longer (30-40 minutes) telephone contacts during the remaining 8 months which included both exercise and nutrition advice. The two sequential arms included six additional ‘booster’ calls for the second behavior in the sequence to ensure equivalent exposure to each behavior.
Table 1.
Intervention Telephone Contact and Call Volume (total minutes), by Study Arm
| Week | Exercise – 1st | Diet – 1st | Simultaneous | Control | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Call # | Content | Minutes | Call # | Content | Minutes | Call # | Minutes | Call # | Minutes | |
| 2 | 1 | Exercise | 15-20 | 1 | Nutrition | 15-20 | 1 | 30-40 | 1 | 30-40 |
| 4 | 2 | Exercise | 15-20 | 2 | Nutrition | 15-20 | 2 | 30-40 | 2 | 30-40 |
| 6 | 3 | Exercise | 15-20 | 3 | Nutrition | 15-20 | 3 | 30-40 | 3 | 30-40 |
| 8 | 4 | Exercise | 15-20 | 4 | Nutrition | 15-20 | 4 | 30-40 | 4 | 30-40 |
| 10 | 5 | 30-40 | 5 | 30-40 | ||||||
| 12 | 5 | Exercise | 15-20 | 5 | Nutrition | 15-20 | ||||
| 14 | 6 | 30-40 | 6 | 30-40 | ||||||
| 16 | 6 | Exercise | 15-20 | 6 | Nutrition | 15-20 | ||||
| 18 | 7 | Nutrition | 15-20 | 7 | Exercise | 15-20 | 7 | 30-40 | 7 | 30-40 |
| 20 | 8 | Ex & Nutr. | 30-40 | 8 | Ex & Nutr. | 30-40 | ||||
| 22 | 9 | Nutrition | 15-20 | 9 | Exercise | 15-20 | 8 | 30-40 | 8 | 30-40 |
| 24 | 10 | Ex & Nutr. | 30-40 | 10 | Ex & Nutr. | 30-40 | ||||
| 26 | 11 | Nutrition | 15-20 | 11 | Exercise | 15-20 | 9 | 30-40 | 9 | 30-40 |
| 28 | 12 | Ex & Nutr. | 30-40 | 12 | Ex & Nutr. | 30-40 | ||||
| 30 | 13 | Nutrition | 15-20 | 13 | Exercise | 15-20 | 10 | 30-40 | 10 | 30-40 |
| 32 | 14 | Ex & Nutr. | 30-40 | 14 | Ex & Nutr. | 30-40 | ||||
| 34 | 15 | Nutrition | 15-20 | 15 | Exercise | 15-20 | 11 | 30-40 | 11 | 30-40 |
| 36 | 16 | Ex & Nutr. | 30-40 | 16 | Ex & Nutr. | 30-40 | ||||
| 38 | 17 | Nutrition | 15-20 | 17 | Exercise | 15-20 | 12 | 30-40 | 12 | 30-40 |
| 40 | 18 | Ex & Nutr. | 30-40 | 18 | Ex & Nutr. | 30-40 | ||||
| 42 | 19 | Ex & Nutr. | 30-40 | 19 | Ex & Nutr. | 30-40 | 13 | 30-40 | 13 | 30-40 |
| 44 | ||||||||||
| 46 | 20 | Ex & Nutr. | 30-40 | 20 | Ex & Nutr. | 30-40 | 14 | 30-40 | 14 | 30-40 |
| 48 | ||||||||||
| 50 | 21 | Ex & Nutr. | 30-40 | 21 | Ex & Nutr. | 30-40 | 15 | 30-40 | 15 | 30-40 |
Note: Minutes in each category represent the range (minimum to maximum) of minutes of telephone advisor-delivered intervention delivery as per study protocol. No significant between-arm differences in total minutes of staff contact were found.
Physical activity intervention
For the three arms receiving physical activity advice, the goal was to meet national guidelines of at least 150 minutes/week of moderate intensity or more vigorous physical activity (MVPA), spread across most days of the week (1). Participants determined the type, location, time of day, and weekly frequency of MVPA. The majority of participants chose to achieve this goal through brisk walking.
Telephone contacts were supplemented with informational mailings and a Yamax Digi-walker pedometer to provide individualized physical activity feedback (AccuSplit, San Jose, CA) (35). Participants recorded their physical activity (e.g., type, frequency, duration, pedometer steps) on simple paper calendars and reported it to their advisor during their regular telephone contacts.
Dietary intervention
Participants randomized to the three experimental arms received a nutrition education program that was matched with the physical activity program on amount and type of staff contact. Content was based on recommendations from the American Heart Association for a healthy diet and focused on nutritional quality rather than quantity (i.e., calorie counting was not a focus) (36). Based on their inclusion across a range of national chronic disease prevention recommendations (e.g., cardiovascular disease, cancer, type 2 diabetes, stroke, hypertension), participants were counseled to reduce total amount of saturated fat and increase fruit and vegetable intake to 5-9 servings per day (2, 3). Participants completed simple paper calendars to track intake and record strategies to reduce saturated fat and increase fruit and vegetable intake, and reported this information during their regular telephone contacts. Different nutrition topics were targeted for monitoring and intervention (e.g., reducing high-fat snacks, reducing fats in food preparation, increasing fruit and vegetable intake). Participants were given homework assignments and detailed reading materials (e.g., recipes).
Stress management control intervention
Individuals in this arm received telephone counseling for skill-building in five stress management areas, based on programs developed and evaluated by King et al. and Luskin et al. (37, 38): progressive muscle relaxation, guided visualization, pleasant events scheduling, sleep hygiene, and time management. Participants were instructed to practice these behaviors throughout the week as a control for the home-based physical activity and dietary behavioral strategies being practiced in the other three arms.
Intervention fidelity
The four intervention arms included weekly evaluation of the number, content, and length of telephone advising sessions (31). Advisor summary forms of each contact were reviewed in case management sessions, along with independent review of audiotaped telephone contacts for appropriate content (approximately one-third of telephone contacts were randomly selected for review by study investigators and doctoral level clinical psychologists).
Measures
Major study assessments occurred at baseline, 4, and 12 months by trained, blinded staff. Upon completion of each assessment, subjects received inexpensive incentive items commensurate with the intervention content they were receiving (e.g., exercise towels for the nutrition/exercise arms; hand-held massagers for the stress management arm).
Physical activity
Elicitation research (39), undertaken in the formative phase of study methods finalization with the stressed population under study, revealed that collection of accelerometry data was overly burdensome to participants and, therefore, not included. We sought to improve the overall stability and accuracy of self-reported physical activity through using a combination of ‘point-prevalence’ recall and ‘usual participation’ frequency measures. The recall measure was the Stanford 7-Day Physical Activity Recall (PAR), a validated structured interview in which the participant estimates the amount of time spent each day during the past seven days in four intensity categories of activity: sleep, and moderate, hard, and very hard physical activity (40). Inter-rater and test-retest reliabilities are in the .69–.86 range and concurrent validity in the .75–.84 range (40, 41).
Given that recalls such as the PAR are vulnerable to ‘unusual week’ effects (i.e., the 7 days being reported on may not reflect participant's typical activities over a longer time period), the CHAMPS physical activity questionnaire was also collected. This instrument has been found to provide a valid and reliable estimate of usual physical activity behavior in middle- and older-aged adults (24, 42). Three-month stability coefficients are in the .70–.84 range in community samples of older adults (42). The instrument has also been shown to have concurrent validity when compared with interviewer-collected physical activity data (42), as well as sensitivity to change in a number of community samples of midlife and older women and men (24, 32, 33).
Combining these two standard, widely accepted measures promotes more reliable outcomes through stabilizing the random measurement errors of these measures and reducing the chance of Type I error (43). Based on national physical activity guidelines (1), average minutes/week in MVPA was the primary physical activity outcome. MVPA values from each measure were determined to have met relevant distributional assumptions prior to combining them. To combine MVPA from the two instruments (for which between-instrument Spearman r coefficient=.50), data from each instrument were first standardized using z scores and then averaged. For the percentage of participants for whom information from one or the other instrument was missing, data from the available instrument was used. For descriptive purposes, the z scores have been converted into mean minutes/week of MVPA in tables and figures.
Dietary intake
Based on current recommendations for a healthy diet (2), the two primary targets for dietary change were daily fruit and vegetable intake and daily percentage of calorie intake from saturated fats. These dietary behaviors were assessed using the Block98 Food Frequency questionnaire, a revised version of the Health Habits and History questionnaire (44). Participants self-reported the average frequency of consumption of 90 foods and average serving sizes over the past 4 months. Correlations between versions of this questionnaire and 4-day dietary records range from 0.42 to 0.71, and correlations range from .41-.73 for essential macronutrients between the questionnaire and unannounced 24-hour recalls (44, 45). It has been shown to be sensitive to change with dietary interventions (24). The National Cancer Institute's DIETSYS program was used to estimate dietary nutrients and quantity of various food groups (46). A food frequency questionnaire was chosen rather than alternative instruments (e.g., dietary records or repeat 24- hour dietary recalls), due to subject burden and cost issues.
Intervention quality assurance
Intervention variables, recorded by intervention staff throughout the trial, included number of telephone call attempts for each scheduled contact, number of successful telephone contacts, and minutes per contact.
Process variables
Parallel forms of Sallis et al.'s Social Support Scales for diet and exercise (47), which included health advisor support, the Stanford self-efficacy questionnaires for physical activity and for dietary behaviors (48), and an outcome expectations/realizations scale (49) were collected at baseline, 4, and 12 months.
Sample Size and Statistical Analysis
Based on previous literature of similar interventions in similarly-aged samples (24, 31, 32), a sample size of 45 participants completing the study per cell was judged to be adequate for detecting a 30 minute per week difference in MVPA at 80% power with 2-sided alpha set at .05 (50). A similar sample size was judged to be adequate for detecting a 1.5% difference in percentage of daily calories from saturated fats and a difference of 1 daily fruit or vegetable (24). To compensate for potential dropout, an additional 20 participants beyond the a priori powered sample (N=180) were included, resulting in a final recruitment sample target of 200. Analysis of variance (general linear models) (51) was used to evaluate between-arm differences at baseline.
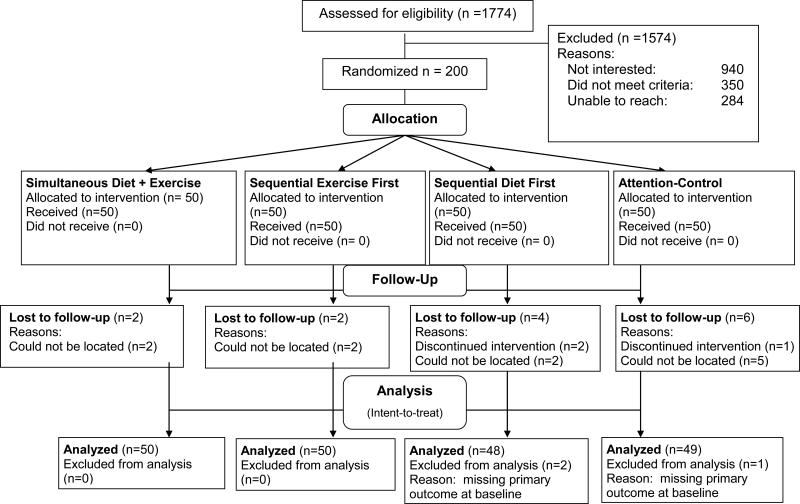
Intent-to-treat principles were used for the primary outcomes, i.e., physical activity, percent of daily calories from saturated fat, and number of daily fruit and vegetable servings. Three participants completed only a portion of the baseline dietary questionnaire (two from the Diet-1st arm and one from the Control arm) and were therefore not included in analyses given the primary focus on dietary and physical activity changes across arms (see Figure 1). For participants with missing or incomplete data at either 4 or 12 months, baseline values were used. This decision was based on evidence indicating that when loss to follow-up is modest and not differential by study arm (see Results), such carry-forward procedures produce more conservative estimates relative to multiple imputation procedures (52). For physical activity, this resulted in baseline imputation for three participants at 4 months (1.5% of sample) and 18 participants at 12 months (9% of sample). For the dietary outcomes (which were more burdensome to complete, requiring about 90 minutes to complete at home just prior to the assessment visit relative to 30 minutes for the combined physical activity instruments, which were completed at the assessment visit), this resulted in baseline imputation for 40 participants at 4 months (20.3% of sample) and 30 participants at 12 months (15.2% of sample). There were no between-arm differences for percent of participants with missing or incomplete data at either 4 months (Exercise-First=41/50 [82%]; Diet-First=42/50 [84%]; Simultaneous= 40/50 [80%]; Control=37/50 [74%]; X2= 1.82, p=.61) or 12 months (Exercise-First=42/50 [84%]; Diet-First=44/50 [88%]; Simultaneous=44/50 [88%]; Control=40/50 [80%]; X2= 1.72, p= .63).
Figure 1.
Study Consort Flow Chart
Given as described in the introduction, the distinct questions of interest related to the 4-month and 12-month time points, analysis of covariance (ANCOVA, general linear model) (53) was used to assess changes in MVPA and the two dietary targets from baseline to 4 months and baseline to 12 months. Given only three time points, within-person random effects incorporated into random regression models (RRM) become relatively unstable (54). However, for completeness, we conducted RRM analysis (Proc Mixed, SAS) (53), and found results similar to those using ANCOVA. The more straightforward ANCOVA results are reported. The main effects for arm assignment were modeled with baseline levels of the dependent variable, gender, and family caregiving status (yes vs. no) entered as covariates. As described earlier, z scores representing the MVPA scores averaged across the PAR and CHAMPS measures were used in the analyses to improve the stability of that outcome. The least-squares adjusted means method was used to compare group means for all significant effects (53).
Results
Descriptive Statistics
Participant flow and retention is shown in Figure 1. Of the 200 participants enrolled, 186 (93%) were retained through 12 months. Retention was not significantly different across the 4 arms (Exercise First= 48/50, or 96%; Diet First= 46/50, or 92%; Simultaneous= 48/50, or 96%; Control= 44/50, or 88%; p= .33). Those with 12-month data were comparable to those without 12-month data (n= 14) on all major baseline demographic, health, and behavioral variables (p values > .14). Descriptive baseline data are shown in Table 2 for selected variables. Raw data are shown to allow for comparisons with other studies and to provide clinically meaningful information. Participants were similar across study arms on the major baseline variables of interest (all p values ≥ .27). Similar to their age group, the average number of chronic health conditions for the sample was 1.4 ± 1.1. Across arms, approximately 46% of participants (no between-arm differences) were on medications for chronic conditions, including hypertension, hyperglycemia, hypercholesterolemia, and asthma. The sample also reported baseline stress levels similar to a sample of chronically stressed family caregivers in the same locale (24). As expected given the intervention focus, there were no significant weight changes across arms.
Table 2.
Descriptive, Process, and Outcome Variables by Study Arm
| Exercise-1st (50) | Diet-1st (48) | Simultaneous (50) | Control (49) | |||||
|---|---|---|---|---|---|---|---|---|
| Demographics: | N | % | N | % | N | % | N | % |
| Women | 23 | 46.0 | 26 | 54.2 | 26 | 52.0 | 26 | 53.1 |
| Household Income≤ $59,999 | 12 | 24.0 | 11 | 22.9 | 8 | 16.0 | 10 | 20.4 |
| Married | 39 | 78.0 | 39 | 81.3 | 41 | 82.0 | 39 | 79.6 |
| Family caregiver | 10 | 20.0 | 11 | 22.9 | 8 | 16.0 | 9 | 18.4 |
| White | 35 | 70.0 | 38 | 79.2 | 35 | 70.0 | 31 | 63.3 |
| Employed Full time | 34 | 68.0 | 34 | 70.8 | 34 | 68.0 | 32 | 65.3 |
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | |
|---|---|---|---|---|---|---|---|---|
| Age (years) | 55.0 | 6.4 | 55.8 | 5.6 | 55.1 | 6.2 | 55.0 | 4.5 |
| Education (years) | 15.2 | 2.2 | 16.0 | 2.0 | 15.9 | 2.0 | 16.1 | 1.7 |
| Baseline body mass index | 29.0 | 4.4 | 30.0 | 4.6 | 28.6 | 4.3 | 29.4 | 4.8 |
| Baseline perceived stress | 28.1 | 7.9 | 28.1 | 9.5 | 27.3 | 7.5 | 26.4 | 6.8 |
| # of chronic conditions | 1.2 | 1.0 | 1.5 | 1.1 | 1.3 | 1.0 | 1.5 | 1.5 |
| Intervention Process: | ||||||||
|---|---|---|---|---|---|---|---|---|
| # Call attempts | 1.5 | 0.4 | 1.5 | 0.4 | 1.6 | 0.6 | 1.7 | 0.5 |
| # Phone contacts | 15.1ae | 3.8 | 15.5ae | 4.7 | 11.6 | 3.5 | 10.2 | 3.7 |
| Call Length (minutes) | 19.1ae | 4.4 | 18.6ae | 4.6 | 21.6 | 4.5 | 24.6 | 7.6 |
| Total minutes of contact | 269.8 | 120.8 | 278.0 | 125.8 | 243.7 | 99.3 | 221.9 | 151.5 |
| Advisor support for exercise, 4months | 22.1af | 8.1 | 12.6 | 4.2 | 23.3af | 10.6 | 14.4 | 7.6 |
| Advisor support for exercise, 12months | 27.1b | 12.9 | 25.9b | 10.0 | 23.8c | 11.0 | 16.9 | 8.6 |
| Advisor support for nutrition, 4months | 29.4 | 10.9 | 39.7ag | 6.1 | 37.7ag | 8.1 | 32.0 | 6.6 |
| Advisor support for nutrition, 12months | 40.8b | 7.5 | 41.1b | 5.4 | 40.0c | 8.6 | 35.4 | 8.3 |
| Behavioral Outcomes (N) | Exercise-1st (50) | Diet-1st (48) | Simultaneous (50) | Control (49) | ||||
|---|---|---|---|---|---|---|---|---|
| MVPA, minutes/week, baseline | 49.7 | 85.8 | 26.9 | 33.5 | 30.1 | 40.6 | 24.9 | 40.1 |
| MVPA, minutes/week, 4months | 190.9 | 159.1 | 99.2g | 110.7 | 130.1h | 105.0 | 100.4i | 112.6 |
| MVPA, minutes/week, 12months | 182.9b | 164.1 | 133.2 | 100.3 | 169.6c | 143.0 | 100.6 | 123.4 |
| F&V servings, baseline | 3.6 | 2.1 | 3.9 | 2.1 | 3.5 | 1.9 | 3.5 | 1.6 |
| F&V servings, 4months | 4.2 | 2.6 | 7.1ag | 3.1 | 6.7ag | 4.4 | 4.0 | 2.3 |
| F&V servings, 12months | 6.6a | 3.5 | 7.6a | 3.5 | 6.3a | 3.2 | 4.2 | 2.2 |
| % Calories from saturated fat, baseline | 12.2 | 3.3 | 12.0 | 1.8 | 12.0 | 2.8 | 12.6 | 3.4 |
| % Calories from saturated fat, 4months | 11.8 | 2.5 | 9.7ag | 1.9 | 9.9ag | 2.4 | 11.5 | 2.4 |
| % Calories from saturated fat, 12months | 10.4d | 2.8 | 9.7a | 2.1 | 9.5a | 2.1 | 11.4 | 2.6 |
Notes: Raw data are presented. MVPA= Moderate-to-Vigorous Physical Activity. F & V= Fruit and Vegetable.
Intervention vs. Control < .001
Intervention vs. Control < .01
Intervention vs. Control < .05
Intervention vs. Control = .056
Intervention vs. Simult <.001
Intervention vs. Diet-1st <.001
Intervention vs. Exercise-1st <.001
Intervention vs. Exercise-1st <.05
Control vs. Exercise-1st <.01.
Overall volume of staff phone contacts (minutes per contact X number of contacts) did not differ significantly across the 4 arms (F(3,196) = 2.0, p = .11) (see Table 2). Average number of call attempts per scheduled telephone contact also did not differ (range= 1.5-1.7 attempts, p= .19). As planned in matching total volume of staff contact, the mean length of calls in the Simultaneous arm was longer, by a modest amount, compared to the sequential arms (F(3,196) = 12.5, p = .001; see Table 2). The sequential arms did not differ significantly from each other on mean call length (p = .54), or number of contacts received (p = .58); both received a greater number of phone contacts (as planned to balance overall contact volume) compared to the Simultaneous arm (F(3,196) = 21.2, p < .0001).
Rated advisor support at 12 months did not differ significantly across the three experimental arms, but all three arms were higher than Controls for support for diet and MVPA (p values ≤ .03; see Table 2). As expected at 4 months, the Exercise-1st and Simultaneous arms did not differ significantly from each other, but reported significantly increased advisor support for physical activity compared to the Diet-1st and Control arms (p values ≤ .001; see Table 2). Similarly, the Diet-1st and Simultaneous arms did not differ significantly from one another, but reported significantly increased 4-month advisor support for dietary change relative to the Exercise-1st and Control arms (p values < .0001).
Dietary Change by Arm Assignment
Daily servings of fruits and vegetables
At 4 months, The Diet-1st and Simultaneous arms did not differ significantly from one another on daily fruit and vegetable servings, and had higher consumption relative to Exercise-1st and Control (F(3, 196) = 13.9, p < .0001; see Table 2). Exercise-1st did not differ from Control (p = 0.86). Main effects for gender and caregiver status were not significant (p values > 0.51).
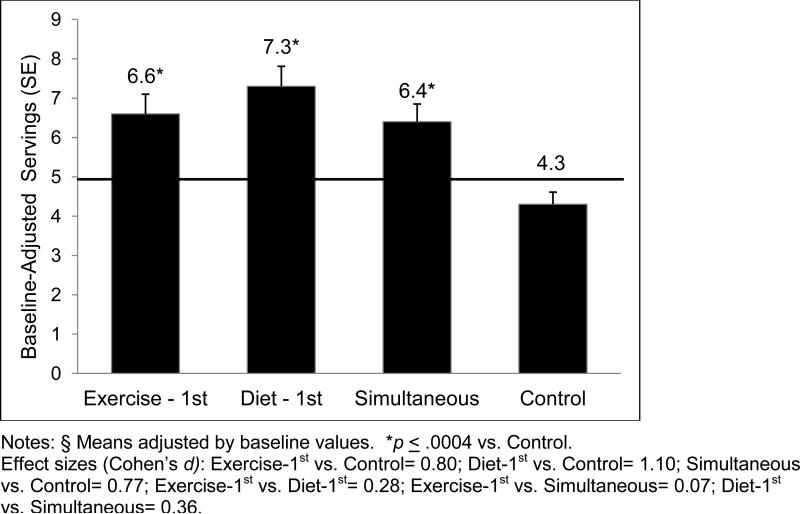
By 12 months, all three experimental arms (Exercise-1st, Diet-1st, Simultaneous) did not differ significantly from one another, and had higher daily servings of fruits and vegetables relative to Control (F(3, 196) = 10.6, p < .0001; see Figure 2, including effect size information). Main effects for gender and caregiver status did not achieve statistical significance (p values ≥ .58).
Figure 2.
Mean Daily Servings of Fruits and Vegetables at 12 Months, by Arm
At 12 months, a greater percentage of subjects in Diet-1st (78%) achieved the national recommendation of 5-9 fruit and vegetable servings relative to Control (33%), X2(1) = 18.2, p < .0001 and Exercise-First (54%), X2(1) = 5.1, p = .02. Other between-arm differences reaching significance were comparisons between Exercise-1st and Control, X2(1) = 3.8, p = .05, and Simultaneous (59%) and Control, X2(1) =5.9, p = .02.
Percent daily calories from saturated fat
At 4 months, The Diet-1st and Simultaneous arms did not differ significantly from one another, and had significantly lower percentages of daily calories from saturated fat relative to Exercise-1st and Control (F(3, 193) = 13.2, p < .0001; Table 2). Exercise-1st did not differ significantly from Control (p = .18). No significant main effects were observed for gender or caregivers status (p values ≥ .11).
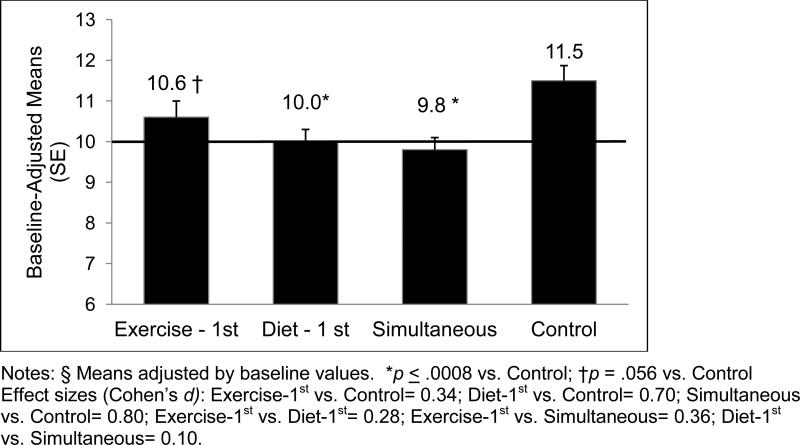
By 12 months, the three experimental arms did not differ from one another, and had generally lower percentages of daily calories from saturated fat relative to Controls (F(3, 196) = 6.0, p = .0006; see Figure 3, including effect size information), although the least-squares adjusted means test indicated that the difference between Exercise-1st and Control was marginally significant (p = .056) (p values for other two arms vs. Control ≤ .0008). No significant main effects for either gender or caregiver status were observed.
Figure 3.
Mean Daily Percent Calories from Saturated Fat at 12 Months, by Arm
At 12 months, a significantly greater percentage of subjects in the Diet-1st (57%) and Simultaneous arms (57%) achieved the national recommendation of less than 10% of daily calories from saturated fat relative to Control (31%), X2(1) = 6.0, p = .02. All other between-arm differences did not reach statistical significance (Exercise-First arm = 40%). As expected, no significant weight loss was observed across arms at either 4 or 12 months (p values > .20).
Physical Activity Change by Arm Assignment
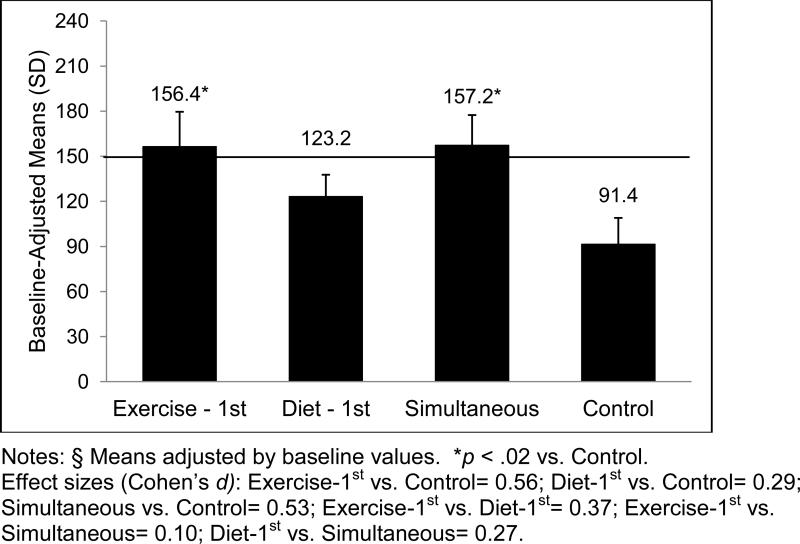
At 4 months, Exercise-1st had significantly greater mean MVPA minutes per week than the other three arms, which did not differ significantly from one another (F(3, 196) = 5.0, p = .002; see Table 2). By 12 months, both the Exercise-1st and Simultaneous arms did not differ significantly from one another, but had significantly greater MVPA minutes/week than Control (F(3, 196) = 2.8, p = .04; see Figure 4, which contains effect size information.) For these two arms, the mean MVPA minutes/week was above national recommendations (1). The Diet-1st mean MVPA minutes/week fell between those of the other arms and Control, and did not differ significantly from the other arms (see Figure 2). While there was no significant main effect for caregiver status, the gender main effect did achieve significance (p = .028), with men reporting an imputed mean[SE] of 192.6 [36.9] MVPA minutes/week while women reported an imputed mean[SE] of 118.3 [11.5] MVPA minutes/week.
Figure 4.
Moderate to Vigorous Physical Activity, Mean Minutes per Week at 12 Months, by Arm
At 12 months, a greater percentage of subjects in Exercise-1st (48%) achieved the nationally recommended guidelines relative to Control (22%), X2(1) = 6.3, p = .01. Other between-arm comparisons were not statistically significant (Diet-1st = 38%; Simultaneous = 40%).
Additional Analyses
Evaluation of 12-month multiple behavior change index scores (formed by summing z scores for each health behavior) (43) indicated a significantly higher index score for each of the three experimental arms relative to Control, with the experimental arms not differing significantly from each other (F(3, 196) = 6.7, p < .001). (Data not shown.)
Exploration of between-arm changes in social cognitive theory process variables (self-efficacy, outcome expectations/realization, social support) yielded few between-arm differences or insights pertaining to the pattern of between-arm outcomes observed. (Data not shown.)
As captured on the outcome realizations scale, at 12 months, Stress Management participants reported significantly greater improvements in stress than the Diet-1st and Simultaneous arms, though not the Exercise-1st arm (F(3, 160) = 2.7, p < .045). The Stress Management arm also reported significantly greater 12-month improvements in coping with stress relative to the other 3 arms (F(3, 161) = 3.8, p < .01). No other between-arm differences were found on these variables. Paired-comparison t tests showed that all 4 arms had significant decreases in perceived stress levels from baseline to 12 months (p values < .003).
Discussion
While interest in enhancing multiple health behavior change has proliferated, few systematic investigations have evaluated the effects of intervention timing, i.e., sequential vs. simultaneous delivery, on successful health behavior adoption and maintenance (20, 21). Strengths of the current study included the matching of overall volume of intervention across the 4 study arms and a high 12-month retention rate (93%). We found partial support for the first hypothesis that health behavior changes would be more robust during the initial 4-month period for the two sequential arms relative to the simultaneous arm. While this hypothesis was supported for physical activity (i.e., the Exercise-1st arm had significantly higher 4-month physical activity than the Simultaneous arm), it was not supported for dietary change (i.e., there were no 4-month differences between Diet-1st and Simultaneous arms; both improved relative to Control).
The second hypothesis postulating 12-month improvements in diet and physical activity across all three experimental arms relative to Control also was partially supported. While 12-month improvements for the Simultaneous arm were statistically different from Control for all three target behaviors (indicating that statistical power was sufficient for all three outcomes), the Exercise-1st arm attained marginal improvement in percent of daily calories from saturated fat relative to Control (at p = .056), and the Diet-1st arm did not significantly improve MVPA relative to Control. By 12 months, mean levels in only the Simultaneous arm met national recommendations for saturated fat, fruits and vegetables, and MVPA. Meanwhile, at 12 months the two sequential arms had the largest proportions of participants reaching national recommendations in the behavior area targeted first (i.e., diet for Dietary-1st; MVPA for Exercise-1st). This pattern suggests that changing one health behavior first may help to ensure maintenance of that health behavior, but may prove detrimental (at least for some people) for the second health behavior. It is possible that the shorter 8-month advice period for the second behavior in the sequence could have disadvantaged participants. However, the fact that Simultaneous arm participants showed little physical activity increase at 4 months, yet were able to reach recommended physical activity levels by 12 months, argues against this possibility.
The poorer performance of Diet-1st participants in achieving physical activity goals, coupled with the poorer 4-month physical activity performance in the Simultaneous arm, suggest that physical activity change may be particularly difficult to achieve when dietary change is already underway. In contrast, Exercise-1st achieved significant improvements in daily fruit and vegetable intake and marginally significant improvements in saturated fat intake, along with significant physical activity improvements. These results provide some support for the premise that initial physical activity change efforts may, at minimum, not unduly impede subsequent dietary change efforts. This may particularly be the case when positive dietary additions (i.e., increasing fruit and vegetable intake), as opposed to restrictions (i.e., reducing saturated fats), are targeted.
An alternative explanation for the differential physical activity results across arms is the intrinsically different demands of changing physical activity versus diet. Given the obligatory nature of eating (i.e., it is already embedded in daily schedules), dietary interventions such as the one targeted in this study typically focus on changes in dietary choices, i.e., substitutions, or additions or deletions to meals. In contrast, increasing physical activity typically means adding a sustained block of activity to people's daily schedules. For the stressed individuals under study, tackling the multi-faceted dietary intervention first (10) may have left less motivational or self-regulatory capacity for adding physical activity, which requires additional time and scheduling adjustments. Such an outcome is commensurate with a “cognitive load” interpretation of self-regulation (12). This interpretation also may help to explain why participants receiving simultaneous instruction in both health behaviors from the beginning had significantly smaller 4-month MVPA increases compared to those assigned to Exercise-1st. While the two behaviors received equal advisor attention from the start in the Simultaneous arm, the dietary change effort may have required more participant attention and cognitive load.
The Active Choices telephone delivery program has demonstrated sustained efficacy for a wide range of adults (31, 32), including chronically stressed persons (24), and has been successfully disseminated in California and other portions of the U.S. (33, 55), as well as through automated technologies (31). As part of this body of evidence, the self-reported increases in physical activity captured via the PAR and CHAMPS have been extensively validated through use of objective assessment procedures (24, 31, 32), although one limitation of the current study was not including objective physical activity assessments due to subject burden constraints. While we specifically chose a population with heightened stress complaints in light of challenges in finding suitable multiple behavior interventions for them, less stressed groups could respond differently to the interventions. It could also be interesting to explore how individual preferences could affect subsequent behavior change success with these types of multiple behavior regimens.
Our interventions were not aimed at weight loss, and, as a result, did not produce significant mean weight loss across the study period. It is possible that, within the context of a weight loss program, other results might be obtained (6). However, given the practice in many weight loss programs of targeting diet earlier and more intensively than physical activity, the Diet-1st arm results reported here may be particularly germane to such programs.
A 4-month time period was used to sequence the health behaviors based on prior studies indicating successful behavior change by 4 months (29, 30). It is possible, however, that either shorter or longer initial time periods could improve the results of sequential interventions.
Finally, while the two health behavior areas were brought together temporally, health advisors did not explicitly seek out ways to more fully integrate the two behaviors in the discussions that occurred with participants. Such proactive methods are recommended.
In summary, the results suggest that, in the current population, delivering physical activity and dietary interventions simultaneously may result in the most positive sustained outcomes across these two health behaviors. In addition, the potential behavioral suppression effects of dietary intervention on physical activity change are noteworthy and deserve further evaluation.
Acknowledgements
The research was supported by Public Health Service Grant R01AG21010 from the National Institute on Aging (King) and Public Health Service Training Grant 5T32HL007034 from the National Heart, Lung, & Blood Institute. The authors thank Carolyn Prosak, Catharine Cassayre, Julia Wu, Arturo Fernandez, Susannah Belding, and Sarah French for implementing the interventions, Dr. Leslie Pruitt and Stephanie Koltiska for their work with respect to study evaluations, and Dr. Judith Prochaska for comments on the manuscript.
Footnotes
Conflict of Interest Statement
The authors have no conflicts of interest to disclose.
Contributor Information
Abby C. King, Department of Health Research & Policy and Stanford Prevention Research Center, Department of Medicine, Stanford University School of Medicine.
Cynthia M. Castro, Stanford Prevention Research Center, Department of Medicine, Stanford University School of Medicine.
Matthew P. Buman, Stanford Prevention Research Center, Department of Medicine, Stanford University School of Medicine.
Eric B. Hekler, Stanford Prevention Research Center, Department of Medicine, Stanford University School of Medicine.
Guido G. Urizar, Jr., Stanford Prevention Research Center, Department of Medicine, Stanford University School of Medicine.
David K. Ahn, Stanford Prevention Research Center, Department of Medicine, Stanford University School of Medicine.
References
- 1.Physical Activity Guidelines Advisory Committee . Report of the Physical Activity Guidelines Advisory Committee, 2008. U.S. Dept. of Health & Human Services; Washington, DC: 2008. [Google Scholar]
- 2.U.S. Department of Health and Human Services and U.S. Department of Agriculture . Dietary Guidelines for Americans, 2005. 6th Ed U.S. Govt. Printing Office; Washington, DC: 2005. [Google Scholar]
- 3.U.S. Department of Health & Human Services . Healthy People 2010, Final Review. US Department of Health and Human Services, Office of Disease Prevention and Health Promotion; Washington, DC: 2011. [Google Scholar]
- 4.Bandura A. Social cognitive theory: an agentic perspective. Ann Rev Psychol. 2001;52:1–26. doi: 10.1146/annurev.psych.52.1.1. [DOI] [PubMed] [Google Scholar]
- 5.Kremers SPJ, de Bruijn GJ, Schaalma H, Brug J. Clustering of energy balance-related behaviours and their intrapersonal determinants. Psychol Health. 2004;19:595–606. [Google Scholar]
- 6.Mata J, Silva MN, Vieira PN, et al. Motivational “spill-over” during weight control: increased self-determination and exercise intrinsic motivation predict eating self-regulation. Health Psychol. 2009;28:709–716. doi: 10.1037/a0016764. [DOI] [PubMed] [Google Scholar]
- 7.Emmons KM, Marcus BH, et al. The relationship between smoking, physical activity, and dietary fat intake among manufacturing workers. Prev Med. 1994;23:481–489. doi: 10.1006/pmed.1994.1066. [DOI] [PubMed] [Google Scholar]
- 8.Wilcox S, King AC, Castro C, Bortz W., II Do changes in physical activity lead to dietary changes in middle and older age? Am J Prev Med. 2000;18:276–283. doi: 10.1016/s0749-3797(00)00117-3. [DOI] [PubMed] [Google Scholar]
- 9.Wansink B, Sobal J. Mindless eating: The 200 daily food decisions we overlook. Environment & Behav. 2007;39:106–123. [Google Scholar]
- 10.King AC, Frey-Hewitt B, Dreon DM, Wood PD. Diet vs exercise in weight maintenance. The effects of minimal intervention strategies on long-term outcomes in men. Arch Intern Med. 1989;149:2741–2746. doi: 10.1001/archinte.149.12.2741. [DOI] [PubMed] [Google Scholar]
- 11.Hagger MS, Wood C, Stiff C, Chatzisarantis NL. Ego depletion and the strength model of self-control: a meta-analysis. Psychol Bull. 2010;136:495–525. doi: 10.1037/a0019486. [DOI] [PubMed] [Google Scholar]
- 12.Baumeister RF, Vohs KD, Tice DM. The strength model of self-control. Current Directions in Psychological Science. 2007;16:351–355. [Google Scholar]
- 13.Kiernan M, King AC, Stefanick M, Kraemer HC. Characteristics of successful and unsuccessful dieters: An application of signal detection methodology. Ann Behav Med. 1998;20:1–6. doi: 10.1007/BF02893802. [DOI] [PubMed] [Google Scholar]
- 14.Kendzierski D. A self-schema approach to healthy eating. J Am Psychiatr Nurses Assoc. 2007;12:350–357. [Google Scholar]
- 15.Wood W, Neal DT. A new look at habits and the habit-goal interface. Psychol Rev. 2007;114:843–863. doi: 10.1037/0033-295X.114.4.843. [DOI] [PubMed] [Google Scholar]
- 16.Oman RF, King AC. the effect of life events and exercise program format on the adoption and maintenance of exercise behavior. Health Psychol. 2000;19:605–612. doi: 10.1037//0278-6133.19.6.605. [DOI] [PubMed] [Google Scholar]
- 17.Hypertension Prevention Trial Research Group The Hypertension Prevention Trial: Three-year effects of dietary changes on blood pressure. Arch Intern Med. 1990;150:153–162. [PubMed] [Google Scholar]
- 18.Young DR, Vollmer WM, King AC, et al. Can individuals meet multiple physical activity and dietary behavior goals? Am J Health Behav. 2009;33:277–286. doi: 10.5993/ajhb.33.3.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lluch A, King NA, Blundell JE. Exercise in dietary restrained women: no effect on energy intake but change in hedonic ratings. Eur J Clin Nutr. 1998;52:300–307. doi: 10.1038/sj.ejcn.1600555. [DOI] [PubMed] [Google Scholar]
- 20.Vandelanotte C, Bourdeaudhuij ID, Brug J. Two-year follow-up of sequential and simultaneous interactive computer-tailored interventions for increasing physical activity and decreasing fat intake. Ann Behav Med. 2007;33:213–219. doi: 10.1007/BF02879903. [DOI] [PubMed] [Google Scholar]
- 21.Hyman DJ, Pavlik VN, Taylor WC, Goodrick GK, Moye L. Simultaneous vs sequential counseling for multiple behavior change. Arch Intern Med. 2007;167:1152–1158. doi: 10.1001/archinte.167.11.1152. [DOI] [PubMed] [Google Scholar]
- 22.Aspinwall LG, Taylor SE. A stitch in time: self-regulation and proactive coping. Psychol Bull. 1997;121:417–436. doi: 10.1037/0033-2909.121.3.417. [DOI] [PubMed] [Google Scholar]
- 23.King AC, Kiernan M, Oman RF, et al. Can we identify who will adhere to long-term physical activity? Signal detection methodology as a potential aid to clinical decision making. Health Psychol. 1997;16:380–389. doi: 10.1037//0278-6133.16.4.380. [DOI] [PubMed] [Google Scholar]
- 24.King AC, Baumann K, O'Sullivan P, Wilcox S, Castro C. Effects of moderate-intensity exercise on physiological, behavioral, and emotional responses to family caregiving: a randomized controlled trial. J Gerontol: Med Sci. 2002;57A:M26–M36. doi: 10.1093/gerona/57.1.m26. [DOI] [PubMed] [Google Scholar]
- 25.National Center for Health Statistics . National Health Interview Survey. National Center for Health Statistics; Washington, DC: 2002. [Google Scholar]
- 26.Block G, Gillespie C, Rosenbaum EH, Jenson C. A rapid food screener to assess fat and fruit and vegetable intake. Am J Prev Med. 2000;18:284–288. doi: 10.1016/s0749-3797(00)00119-7. [DOI] [PubMed] [Google Scholar]
- 27.Cohen S, Williamson G. Perceived stress in a probability sample of the United States. In: Spacapam S, Oskamp S, editors. The social psychology of health: Claremont Symposium on applied social psychology. Sage; Newbury Park, CA: 1988. pp. 31–67. [Google Scholar]
- 28.Efron B. Forcing a sequential experiment to be balanced. Biometrika. 1971;58:403–417. [Google Scholar]
- 29.King AC, Oman RF, Brassington GS, Bliwise DL, Haskell WL. Moderate-intensity exercise and self-rated quality of sleep in older adults. A randomized controlled trial. JAMA. 1997;277:32–37. [PubMed] [Google Scholar]
- 30.Eakin EG, Lawler SP, Vandelanotte C, Owen N. Telephone interventions for physical activity and dietary behavior change: a systematic review. Am J Prev Med. 2007;32:419–434. doi: 10.1016/j.amepre.2007.01.004. [DOI] [PubMed] [Google Scholar]
- 31.King AC, Friedman RM, Marcus BH, et al. Ongoing physical activity advice by humans versus computers: The Community Health Advice by Telephone (CHAT) Trial. Health Psychol. 2007;26:718–727. doi: 10.1037/0278-6133.26.6.718. [DOI] [PubMed] [Google Scholar]
- 32.Castro CM, Pruitt LA, Buman MP, King AC. Physical activity program delivery by professionals vs volunteers: The TEAM randomized trial. Health Psychol. 2011;30:285–294. doi: 10.1037/a0021980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wilcox S, Dowda M, Leviton LC, et al. Active for Life: final results from the translation of two physical activity programs. Am J Prev Med. 2008;35:340–351. doi: 10.1016/j.amepre.2008.07.001. [DOI] [PubMed] [Google Scholar]
- 34.Marcus BH, Simkin LR. The transtheoretical model: applications to exercise behavior. Med Sci Sports Exerc. 1994;26:1400–1404. [PubMed] [Google Scholar]
- 35.Bassett DR, Jr., Ainsworth BE, Leggett SR, et al. Accuracy of five electronic pedometers for measuring distance walked. Med Sci Sports Exerc. 1996;28:1071–1077. doi: 10.1097/00005768-199608000-00019. [DOI] [PubMed] [Google Scholar]
- 36.Pearson TA, Bazzarre TL, Daniels SR, et al. American Heart Association guide for improving cardiovascular health at the community level: a statement for public health practitioners, healthcare providers, and health policy makers from the American Heart Association Expert Panel on Population and Prevention Science. Circulation. 2003;107:645–651. doi: 10.1161/01.cir.0000054482.38437.13. [DOI] [PubMed] [Google Scholar]
- 37.King AC, Winett RA. Tailoring stress-reduction strategies to populations at risk: Comparisons between women from dual-career and dual-working families. Family Commun Health. 1986;9:42–50. [Google Scholar]
- 38.Luskin F, Pelletier K. Stress Free for Good: 10 Scientifically Proven Life Skills for Health and Happiness. Harper Collins; New York, NY: 2005. [Google Scholar]
- 39.Fisher JD, Fisher WA. Changing AIDS-risk behavior. Psychol Bull. 1992;111:455–474. doi: 10.1037/0033-2909.111.3.455. [DOI] [PubMed] [Google Scholar]
- 40.Sallis JF, Haskell WL, Wood PD, et al. Physical activity assessment methodology in the Five-City Project. Am J Epidemiol. 1985;121:91–106. doi: 10.1093/oxfordjournals.aje.a113987. [DOI] [PubMed] [Google Scholar]
- 41.Pruitt LA, King AC, Obarzanek E, et al. Reliability of the 7-Day Physical Activity Recall in a biracial group of inactive and active adults. J Physical Act Health. 2006;3:423–438. doi: 10.1123/jpah.3.4.423. [DOI] [PubMed] [Google Scholar]
- 42.Stewart AL, Mills KM, King AC, et al. CHAMPS physical activity questionnaire for older adults: outcomes for interventions. Med Sci Sports Exerc. 2001;33:1126–1141. doi: 10.1097/00005768-200107000-00010. [DOI] [PubMed] [Google Scholar]
- 43.Prochaska JJ, Velicer WF, Nigg CR, Prochaska JO. Methods of quantifying change in multiple risk factor interventions. Prev Med. 2008;46:260–265. doi: 10.1016/j.ypmed.2007.07.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Block G, Thompson FE, Hartman AM, Larkin FA, Guire KE. Comparison of two dietary questionnaires validated against multiple dietary records collected during a 1-year period. J Am Dietetics Assoc. 1992;92:686–693. [PubMed] [Google Scholar]
- 45.Boucher B, Cotterchio M, Kreiger N, et al. Validity and reliability of the Block98 food-frequency questionnaire in a sample of Canadian women. Public Health Nutr. 2006;9:84–93. doi: 10.1079/phn2005763. [DOI] [PubMed] [Google Scholar]
- 46.Block G, Coyle LM, Hartman AM, Scoppa SM. Revision of dietary analysis software for the Health Habits and History Questionnaire. Am J Epidemiol. 1994;139:1190–1196. doi: 10.1093/oxfordjournals.aje.a116965. [DOI] [PubMed] [Google Scholar]
- 47.Sallis JF, Grossman RM, Pinski RB, Patterson TL, Nader PR. The development of scales to measure social support for diet and exercise behaviors. Prev Med. 1987;16:825–836. doi: 10.1016/0091-7435(87)90022-3. [DOI] [PubMed] [Google Scholar]
- 48.Garcia AW, King AC. Predicting long-term adherence to aerobic exercise: a comparison of two models. J Sport Exerc Psychol. 1991;13:394–410. [Google Scholar]
- 49.Wilcox S, Castro C, King AC. Outcome expectations and physical activity participation in caregiving and non-caregiving women. J Health Psychol. 2006;11:65–77. doi: 10.1177/1359105306058850. [DOI] [PubMed] [Google Scholar]
- 50.Kraemer HC, Thiemann S. How many subjects? Statistical power analysis in research. Sage; Newbury Park: 1987. [Google Scholar]
- 51.Spector PC, Goodnight JH, Sall JP, Sarle WS. SAS user's guide: Statistics. Verson 5. SAS Institute Inc.; Cary, NC: 1985. The GLM procedure. pp. 433–506. [Google Scholar]
- 52.Wood AM, White IR, Hillsdon M, Carpenter J. Comparison of imputation and modelling methods in the analysis of a physical activity trial with missing outcomes. Int J Epidemiol. 2005;34:89–99. doi: 10.1093/ije/dyh297. [DOI] [PubMed] [Google Scholar]
- 53.SAS Institute Inc. SAS/STAT 9.2 User's Guide. 2nd edition SAS Institute; Cary, NC: 2009. [Google Scholar]
- 54.Singer JD, Willett JB. Applied Longitudinal Data Analysis: Modeling Change and Event Occurrence. Oxford University Press; New York: 2003. [Google Scholar]
- 55.Hooker SP, Seavey W, Weidmer CE, et al. The California active aging community grant program: translating science into practice to promote physical activity in older adults. Ann Behav Med. 2005;29:155–165. doi: 10.1207/s15324796abm2903_1. [DOI] [PubMed] [Google Scholar]