Abstract
The continuous multiplication of Plasmodium parasites in red blood cells leads to a rapid increase in parasite numbers and is responsible for the disease symptoms of malaria. Survival and virulence of the parasite are linked to parasite-induced changes of the host red blood cells. These alterations require export of a large number of parasite proteins that are trafficked across multiple membranes to reach the host cell. Two classes of exported proteins are known, those with a conserved Plasmodium export element (PEXEL/HT) or those without this motif (PNEPs). Recent work has revealed new aspects of the determinants required for export of these 2 protein classes, shedding new light on the mode of trafficking during the different transport steps en route to the host cell.
Introduction
Many intracellular pathogens are taken up by host cells through endocytic or phagocytic mechanisms and subsequently establish themselves in modified host-derived vacuoles. In contrast, Apicomplexan parasites, including malaria parasites, actively penetrate their host cell to end up in a special non-fusogenic vacuole. This entry mechanism may have evolved precisely to avoid the endosomal/lysosomal pathway of the host cell. In addition, some highly specialized host cells are incapable of endocytosis or phagocytosis. For example, malaria parasites invade and replicate within red blood cells (RBCs) that provide dissemination of the parasite through the blood circulation but lack many basic features of eukaryotic cells. Malaria parasites are well adapted to this environment. They induce extensive host cell remodeling to install organelles, reinforce the host cell cytoskeleton, change the RBC surface to induce cell adhesive properties, and change the RBC permeability to nutrients. These profound alterations of the host cell require the export of a large set of parasite proteins.
As malaria parasites reside in a permanent parasitophorous vacuole (PV), exported proteins must enter the parasite secretory pathway and subsequently cross the parasite plasma membrane and the PV membrane (PVM) in order to reach the host cell. Furthermore, parasite-derived organelles in the host cell termed Maurer’s clefts in Plasmodium falciparum are thought to be required for export of proteins to the host cell surface. One of these exported proteins is the major virulence factor PfEMP1 that localizes to particular sub-regions on the infected RBC surface termed knobs [1]. Most exported proteins contain a simple motif termed Plasmodium export element (PEXEL) or host targeting signal (HT) with the consensus sequence RxLxE/Q/D [2,3] (Figure 1a). This motif, if located in the appropriate position after a signal peptide, mediates protein export. However, an increasing number of PEXEL negative exported proteins (PNEPs) [4] (Figure 1a) suggest alternative signal requirements. Here we will discuss recent advances in our understanding of the signals and the individual steps of protein export in P. falciparum, the causative agent of the severest form of human malaria.
Figure 1.
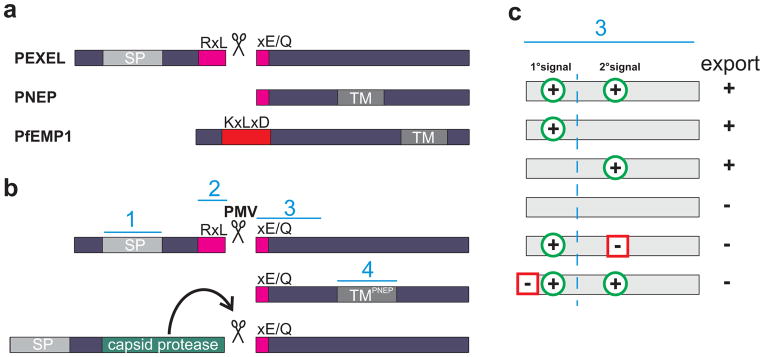
Export motifs in context. (a) Types of exported proteins with PfEMP1 representing a potential subtype of PNEPs. (b) Strategies to uncouple export from plasmepsin V (PM5) cleavage. Top, PEXEL proteins; middle, construct with a PNEP TM containing the mature PEXEL N-terminus only; bottom, insertion of a self-cleaving protease domain similarly exposes a mature PEXEL N-terminus. Blue lines labeled 1–4 represent signals influencing export. (c) Export determinants in the mature N-terminus. The consensus from the strategies used in (b) to test export without the full PEXEL [14,15] suggests multiple determinants influencing export. Green circles with a plus indicate export signals, squares with a minus export inhibitory signals. The column labeled ‘export’ indicates whether the combination of signals leads to export of a reporter (+) or not (−). The primary (1°) signal consists of the PEXEL residues remaining in the exposed mature N-terminus.
Right here, right now: Protein targeting to the host cell is determined at the parasite endoplasmic reticulum (ER)
The PEXEL/HT motif is reported to be a binding site for phosphatidylinositol 3-phosphate (PI3P) [5] and a proteolytic cleavage site for an aspartic protease [6,7], Plasmepsin V (PM5), a finding based on the initial surprising discovery that the PEXEL is processed [8]. Both, PI3P binding and processing, occur in the parasite endoplasmic reticulum and are proposed to be essential for export, suggesting that the decision for export is made early in the secretory pathway [6–9]. How PI3P binding and PEXEL/HT cleavage trigger export and their relative contributions to export are topics of current debate [5,6,10]. For example, it has been proposed that PI3P binding segregates a protein into specific export competent ER sub-compartments or vesicles, where PM5 is responsible for the correctly timed release from the membrane [5]. It is currently unclear how PI3P, which normally is only found on the cytoplasmic leaflet of membranes, is transferred into the ER, and what would generate and maintain its asymmetric distribution to function in sorting PEXEL/HT proteins to ER export sites. Parallels based on function and evolutionary history have also been drawn between PI3P binding in host cell targeting of proteins in Plasmodium and Oomycete parasites [5,11,12], but it should be noted that the Oomycete data is at present a matter of dispute [13].
Despite the demonstrated requirement of the native PEXEL/HT motif for PI3P binding and PM5 cleavage, recent data indicate additional levels in the control of PEXEL/HT protein export. Cleavage of the PEXEL/HT occurs between position 3 and 4 of the motif. The nascent N-terminus is then acetylated, leading to a protein starting with Ac-XE/Q/D [8]. Two studies have now shown that this mature N-terminus, when exposed by other means than PM5 cleavage of a PEXEL motif, can drive protein export [14,15] (Figure 1b). Specifically, one study demonstrated that the mature PEXEL N-terminus is sufficient to mediate export of a reporter in combination with the transmembrane (TM) domain of a PNEP [14] (Figure 1b). The second study used a self-cleaving protease domain between the N-terminal signal peptide and the processed PEXEL motif, leading to the exposure of the mature PEXEL N-terminus [15]. This cleaved protein was exported, suggesting that the full PEXEL motif is not necessary as long as the mature N-terminus is presented correctly after ER entry. Together these studies argue that PI3P binding is not strictly necessary for export and that the mature N-terminus is sufficient to drive export, either upon cleavage by a protease or by direct exposure in combination with a TM domain. Notably export was abolished in a construct where a correct mature PEXEL N-terminus was created by signal peptidase cleavage [6]. The reason for this discrepancy is currently unclear. The existence of PI3P- independent export was also inferred from constructs where a surprising level of export was achieved with mutants in supposedly essential PEXEL positions that also abrogated PI3P binding [10].
Several recent studies have demonstrated that the newly exposed amino acid positions P4 and P5 in the mature PEXEL N-terminus are important for efficient protein export [14–16], confirming earlier results that P5 holds important trafficking information independent of PM5 cleavage [17]. Moreover additional redundant export signals downstream of the PEXEL that can compensate for P5 [14,15] and some regions from non-exported proteins can prevent export despite presence of a bona fide PEXEL [14,16] (Figure 1c). It has been proposed that the mature PEXEL N-terminus may also function through binding to PI3P [11]. However, as most export mediating residues appear to be negatively charged and can function on a scrambled or alanine background, this seems unlikely [14–16,18].
PNEPs represent a second class of exported proteins that consist of a growing number of proteins with structural similarities but no shared primary sequence features. The PNEP N-terminus lacks a signal sequence and is interchangeable with mature PEXEL N-termini in its ability to promote protein export [14]. PNEPs do not appear to be N-terminally processed, with the exception of REX2 [19]. PNEPs harbor an internal TM domain that can functionally complement the N-terminal signal sequence when combined with a mature PEXEL N-terminus [14]. PfEMP1 is also not cleaved at the PEXEL-like sequence KxLxD by PM5 and may therefore be considered a PNEP [16]. Other large antigens with similar structural features, such as Pf332 and members of the SURFIN family [20–21], appear to contain similar trafficking determinants to PNEPs [22] and therefore may all belong to this group of exported proteins. This may indicate different systems for the initial phases of export of PNEPs and PEXEL proteins that depend on a TM or N-terminal processing, respectively, to render the protein export-competent.
It is noteworthy that signal peptide cleavage seems to be inefficient in some PEXEL proteins, indicating that membrane association itself could play an important role for export [17]. Alternative membrane association through the TM may therefore have led to loss of the signal peptide and the full PEXEL motif in proteins where the TM was able to replace this function. This may have been the case for PfEMP1, as the addition of its TM seemed to reduce the strict requirement for the PEXEL residues in a reporter construct [10]. In contrast, all PEXEL residues seem to be essential in the TM protein STEVOR, which would explain why it retained the full PEXEL [23].
Insane in the membrane: Protein unfolding and translocation at the parasite host cell interface
It is not clear yet whether the different classes of exported proteins are trafficked separately or within the same cargo environment through the parasite secretory pathway. However, they all must pass the parasite host cell interface in order to reach the host cell (Figure 2a,b). After fusion of cargo vesicles with the parasite plasma membrane (PPM), soluble proteins are released into the surrounding parasitophorous vacuole, where they appear to be pumped into the host cell through a PVM-localized translocon. This hypothesis is based on a classical study analyzing transfer of an exported protein across the PVM [24], and on more recent observations demonstrating that exported proteins fused to mDHFR are blocked in the parasitophorous vacuole if the mDHFR domain is arrested in its folded state [25]. This is a well-established indicator for unfolding-dependent membrane translocation [26]. For TM proteins this concept is mechanistically more problematic because, after fusion of secretory vesicles with the PPM, they would remain embedded in this membrane. Hence, continued vesicular trafficking was considered a strong possibility [27,28]. However, fusion of exported TM proteins to mDHFR resulted in a folding-induced block of the protein at the PPM [14], suggesting presence of a second translocation activity in this membrane. Such a hypothetical PPM-localized translocon would extract TM proteins out of the PPM, after which they would become competent for translocation through the PVM-localized translocon. In support of this hypothesis, multiple chaperones that could provide the energy for this process have been identified in the PV [29]. Extraction of TM proteins out of the membrane is already known from the ER-associated degradation pathway [30].
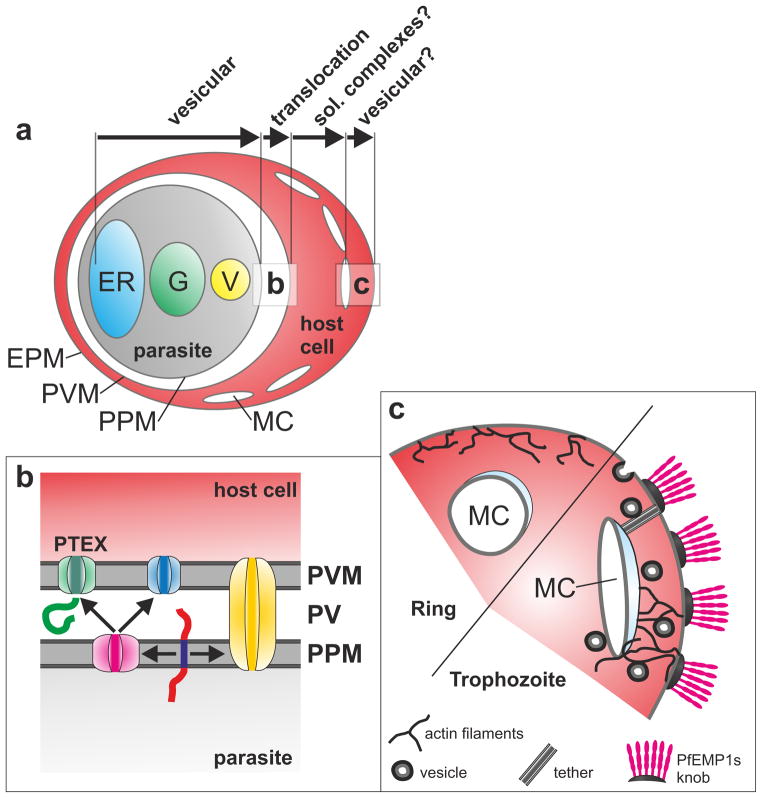
Figure 2.
General transport pathway into the host cell. (a) Overview of an infected RBC. Modes of transport for particular steps are indicated with labeled arrows. EPM, erythrocyte plasma membrane; ER, endoplasmic reticulum; G, Golgi, MC, Maurer’s clefts; V, secretory vesicle. Squares indicate particular steps shown in (a) and (c). (b) Possible arrangements for translocons. A soluble (green) and a TM protein (red, with dark blue TM) are shown. The soluble protein can reach the host cell through PTEX (green). TM proteins either pass through a translocon spanning both the PPM and PVM (yellow), or get extracted into the PV (pink) to translocate through PTEX or a different PNEP-specific translocon (blue). (c) Development of Maurer’s clefts. Shown are the different states of Maurer’s clefts in rings (or in RBCs with mutated hemoglobin) and trophozoites (or wild type RBCs); the latter are more permissive for vesicular transport of virulence factors to the host cell surface.
While the identity of the PPM translocon remains obscure, a multimeric protein complex termed PTEX fulfills several criteria qualifying it as a putative PVM translocon [31–33]. Providing formal proof of function for PTEX is however challenging. A recent study used deconvolution microscopy and 3D SIM (three-dimenional structures illumination microscopy) for a detailed co-localization analysis of PTEX components and some of its substrates [34]. The data suggested that PTEX is present at the parasite periphery in clusters that at least partially co-localize with PEXEL proteins or a PEXEL-mDHFR fusion construct arrested in a folded state. In contrast, little co-localization was observed with PTEX and PfEMP1, suggesting that PfEMP1 may take an alternative pathway. This is also supported by a recent study indicating that in early parasite stages PfEMP1 accumulates in specific PV subdomains that do not co-localize with the PTEX component HSP101 [35]. However, as PfEMP1 is a TM protein, it may require translocation at the PPM [14], which might account for the distinct localization pattern. More generally, co-localization with substrates is not sufficient to validate PTEX as a bona fide translocon. It could even be argued that exported proteins may reside only very transiently at translocation sites and accumulate to detectable levels only in regions of the PVM containing inactive PTEX or lacking it altogether. Hence, the finding that in early stage parasites PfEMP1 remains in the parasite periphery [35] could also mean that this protein is stored in inactive/non-export competent areas before moving to the translocon for correctly timed release into the host cell.
Taken together these studies provide a first glimpse at a potentially complex translocation system at the PPM and PVM (Figure 2b) that may mediate precise delivery of proteins between these compartments, possibly on a par with those found in mitochondria and chloroplasts [36], which possess similarly closely adjoined membranes.
A stairway to the surface: trafficking to and from the Maurer’s clefts
Presence of parasite surface proteins in Maurer’s clefts suggested early on that these organelles may act as an intermediate station for proteins en route to the host cell membrane, and this hypothesis still holds [1]. While soluble proteins can reach the Maurer’s clefts by simple diffusion after crossing the PVM, the situation is again more complicated for TM proteins. Although different types of vesicular transport were initially postulated [28,37], there is increasing evidence suggesting that TM proteins are also delivered to the Maurer’s clefts in a soluble state [14,38,39]. This interpretation was based on the detection of soluble TM proteins in the host cell and on a combination of time-lapse imaging and photoconvertible reporters to track pools of exported TM proteins in the host cell cytosol. Maintaining TM proteins in a soluble state requires energy, possibly provided in the form of chaperoned complexes. Candidates for such complexes are the recently identified J dots, mobile foci in the host cell cytosol that contain the exported parasite chaperones HSP40 and HSP70 [40,41]. However, vesicular transport as an alternative route for a subset of TM proteins cannot be excluded at this point.
Another key question is how surface antigens like PfEMP1 are transported from the Maurer’s clefts to the infected RBC surface. Several candidate structures were recently identified, but direct evidence for their involvement in surface transport is currently lacking. Ultrastructural studies identified tether structures that connect Maurer’s clefts to both the cell surface and the PVM [42,43] and often contained electron dense vesicles [44] (Figure 2c). In addition, an extensive network of actin filaments and associated vesicles (some containing PfEMP1) connects the Maurer’s clefts directly with the knob structures on the infected RBC surface [45]. These filaments may therefore provide directed vesicular transport to the surface, and their rearrangement appears to be important to anchor Maurer’s clefts [46], although the data about the latter is conflicting [35]. Interestingly, hemoglobin mutations that protect from severe malaria [47] and cause altered PfEMP1 surface display [48–50] affect rearrangements of these actin filaments and Maurer’s cleft morphology [45], which may be the underlying cause for the observed effect on PfEMP1 surface exposure and clinical protection (Figure 2c).
PfEMP1 reaches the RBC surface around 24 hours post-invasion [51], inducing adherence properties of the infected RBC and coinciding with significant increases in its rigidity [52,53]. Around the same time, Maurer’s clefts change from a mobile to a fixed state within a short time frame of ~30 minutes [39]. Interestingly, this phase of the life cycle also coincides with a mild echinocytosis of the host cell [39], which may reflect the proposed rearrangements of the actin cytoskeleton [45] (Figure 2c). Hence, Maurer’s clefts arrest may represent a prerequisite for vesicular trafficking to the infected RBC surface.
Concluding remarks
The identification of a simple export motif in Plasmodium proteins has provided a rational basis for systematic investigation of the P. falciparum export pathway. Meanwhile other classes of exported proteins have been identified. Based on our current knowledge, it appears that these are all trafficked through the parasite vesicular pathway, unfolded at the PPM, translocated through the PVM and released into the host cell cytoplasm (Figure 2). Thereafter the pathway of surface antigens is less clear, but current evidence suggests that they remain soluble until reaching the Maurer’s clefts and are then trafficked in vesicles to the surface. The recent results also support the existence of multiple checkpoints and multiple partially redundant signals to ensure selective export of proteins destined to the host cell. Whether the signals all act consecutively or to what extent there are parallel pathways remains to be determined. Future studies will provide answers to the many remaining questions: Is PM5 cleavage co-translational and in place of signal sequence processing, and how does it facilitate export? Are exported proteins segregated into separate vesicles at the ER level, similarly to GPI-anchored proteins in yeast [54]? The full PEXEL is recognized in the ER, but where does the mature PEXEL N-terminus act? What is the identity of the translocation machines in the parasite periphery? How are TM proteins unfolded at the PPM, and how is this state maintained in the host cell cytosol? Are there other exported proteins, such as multi TM proteins, that use only vesicular trafficking based pathways?
Highlights.
Malaria parasites export a large number of proteins into their host cell
Multiple determinants in a protein can control export
Different groups of exported proteins follow similar export pathways
Soluble and transmembrane proteins reach the host cell through translocation
Maurer’s clefts are important for antigens targeted to the host cell surface
Acknowledgments
The authors wish to thank the members from the Marti and Spielmann labs for critically reading the manuscript. Work in the Marti lab is funded by grants 5R01AI077558 and 1R21AI105328 from the National Institutes of Health. Work in the Spielmann lab is funded by DFG grant SP1209/1, the GRK1459 and Graduate School ‘Model Systems of Infectious Diseases’ of the Leibniz Center for Research on Infectious Diseases.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Maier AG, Cooke BM, Cowman AF, Tilley L. Malaria parasite proteins that remodel the host erythrocyte. Nat Rev Microbiol. 2009;7:341–54. doi: 10.1038/nrmicro2110. [DOI] [PubMed] [Google Scholar]
- 2.Marti M, Good RT, Rug M, Knuepfer E, Cowman AF. Targeting malaria virulence and remodeling proteins to the host erythrocyte. Science. 2004;306:1930–3. doi: 10.1126/science.1102452. [DOI] [PubMed] [Google Scholar]
- 3.Hiller NL, Bhattacharjee S, van Ooij C, Liolios K, Harrison T, Lopez-Estrano C, Haldar K. A host-targeting signal in virulence proteins reveals a secretome in malarial infection. Science. 2004;306:1934–7. doi: 10.1126/science.1102737. [DOI] [PubMed] [Google Scholar]
- 4.Spielmann T, Gilberger T. Protein export in malaria parasites: do multiple export motifs add up to multiple export pathways? Trends Parasitol. 2010;26:6–10. doi: 10.1016/j.pt.2009.10.001. [DOI] [PubMed] [Google Scholar]
- 5**.Bhattacharjee S, Stahelin RV, Speicher KD, Speicher DW, Haldar K. Endoplasmic reticulum PI(3)P lipid binding targets malaria proteins to the host cell. Cell. 2012;148:201–12. doi: 10.1016/j.cell.2011.10.051. This study shows binding of the PEXEL to PI3P and proposes the new concept that PI3P binding of the PEXEL in the ER is the key mediator in export. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Boddey JA, Hodder AN, Gunther S, Gilson PR, Patsiouras H, Kapp EA, Pearce JA, de Koning-Ward TF, Simpson RJ, Crabb BS, Cowman AF. An aspartyl protease directs malaria effector proteins to the host cell. Nature. 2010;463:627–31. doi: 10.1038/nature08728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Russo I, Babbitt S, Muralidharan V, Butler T, Oksman A, Goldberg DE. Plasmepsin V licenses Plasmodium proteins for export into the host erythrocyte. Nature. 2010;463:632–6. doi: 10.1038/nature08726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chang HH, Falick AM, Carlton PM, Sedat JW, DeRisi JL, Marletta MA. N-terminal processing of proteins exported by malaria parasites. Mol Biochem Parasitol. 2008;160:107–15. doi: 10.1016/j.molbiopara.2008.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Osborne AR, Speicher KD, Tamez PA, Bhattacharjee S, Speicher DW, Haldar K. The host targeting motif in exported Plasmodium proteins is cleaved in the parasite endoplasmic reticulum. Mol Biochem Parasitol. 2010;171:25–31. doi: 10.1016/j.molbiopara.2010.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bhattacharjee S, Speicher KD, Stahelin RV, Speicher DW, Haldar K. PI(3)P-independent and -dependent pathways function together in a vacuolar translocation sequence to target malarial proteins to the host erythrocyte. Mol Biochem Parasitol. 2012;185:106–13. doi: 10.1016/j.molbiopara.2012.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jiang RH, Stahelin RV, Bhattacharjee S, Haldar K. Eukaryotic virulence determinants utilize phosphoinositides at the ER and host cell surface. Trends Microbiol. 2013;21:145–56. doi: 10.1016/j.tim.2012.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kale SD, Gu B, Capelluto DGS, Dou D, Feldman E, Rumore A, Arredondo FD, Hanlon R, Fudal I, Rouxel T, Lawrence CB, Shan W, Tyler BM. External lipid PI3P mediates entry of eukaryotic pathogen effectors into plant and animal host cells. Cell. 2010;142:284–95. doi: 10.1016/j.cell.2010.06.008. [DOI] [PubMed] [Google Scholar]
- 13.Wawra S, Belmonte R, Lobach L, Saraiva M, Willems A, van West P. Secretion, delivery and function of oomycete effector proteins. Curr Opin Microbiol. 2012;15:685–91. doi: 10.1016/j.mib.2012.10.008. [DOI] [PubMed] [Google Scholar]
- 14**.Grüring C, Heiber A, Kruse F, Flemming S, Franci G, Colombo SF, Fasana E, Schoeler H, Borgese N, Stunnenberg HG, Przyborski JM, Gilberger T, Spielmann T. Uncovering common principles in protein export of malaria parasites. Cell host microbe. 2012;12:717–29. doi: 10.1016/j.chom.2012.09.010. Provides evidence that TM proteins are exported by translocation, suggesting this to be a common theme in malaria protein export. Reveals export properties in mature PEXEL N-termini exchangeable with PNEP N-termini, indicating a shared export domain in these two groups of proteins. [DOI] [PubMed] [Google Scholar]
- 15*.Tarr SJ, Cryar A, Thalassinos K, Haldar K, Osborne AR. The C-terminal portion of the cleaved HT motif is necessary and sufficient to mediate export of proteins from the malaria parasite into its host cell. Mol Microbiol. 2013;87:835–50. doi: 10.1111/mmi.12133. In agreement with [14] shows that the mature PEXEL N-terminus is sufficient to mediate exported independent from plasmepsin V cleavage and dissects the sequence requirements in the new N-terminus. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16**.Boddey JA, Carvalho TG, Hodder AN, Sargeant TJ, Sleebs BE, Marapana D, Lopaticki S, Nebl T, Cowman AF. Role of Plasmepsin V in export of diverse protein families from the Plasmodium falciparum exportome. Traffic. 2013;14:532–50. doi: 10.1111/tra.12053. Shows that PEXELs with K in position 1 are not cleaved by plasmepsin V and not functional when embedded in a PEXEL background. Shows that relaxed PEXEL are functional and analyses flanking residues in export. Uses this data to predict an improved PEXEL exportome lacking the PfEMP1 family. [DOI] [PubMed] [Google Scholar]
- 17.Boddey JA, Moritz RL, Simpson RJ, Cowman AF. Role of the Plasmodium export element in trafficking parasite proteins to the infected erythrocyte. Traffic. 2009;10:285–99. doi: 10.1111/j.1600-0854.2008.00864.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bhattacharjee S, Hiller NL, Liolios K, Win J, Kanneganti T, Young C, Kamoun S, Haldar K. The malarial host-targeting signal is conserved in the Irish potato famine pathogen. PLoS Path. 2006;2:e50. doi: 10.1371/journal.ppat.0020050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Haase S, Herrmann S, Gruring C, Heiber A, Jansen PW, Langer C, Treeck M, Cabrera A, Bruns C, Struck NS, Kono M, Engelberg K, Ruch U, Stunnenberg HG, Gilberger T, Spielmann Sequence requirements for the export of the Plasmodium falciparum Maurer’s clefts protein REX2. Mol Microbiol. 2009;71:1003–17. doi: 10.1111/j.1365-2958.2008.06582.x. [DOI] [PubMed] [Google Scholar]
- 20.Winter G, Kawai S, Haeggstrom M, Kaneko O, von Euler A, Kawazu S, Palm D, Fernandez V, Wahlgren M. SURFIN is a polymorphic antigen expressed on Plasmodium falciparum merozoites and infected erythrocytes. J Exp Med. 2005;201:1853–63. doi: 10.1084/jem.20041392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mattei D, Scherf A. The Pf332 gene of Plasmodium falciparum codes for a giant protein that is translocated from the parasite to the membrane of infected erythrocytes. Gene. 1992;110:71–9. doi: 10.1016/0378-1119(92)90446-v. [DOI] [PubMed] [Google Scholar]
- 22.Zhu X, Yahata K, Alexandre JSF, Tsuboi T, Kaneko O. The N-terminal segment of Plasmodium falciparum SURFIN(4. 1) is required for its trafficking to the red blood cell cytosol through the endoplasmic reticulum. Parasitol Int. 2012;62:215–29. doi: 10.1016/j.parint.2012.12.006. [DOI] [PubMed] [Google Scholar]
- 23.Przyborski JM, Miller SK, Pfahler JM, Henrich PP, Rohrbach P, Crabb BS, Lanzer M. Trafficking of STEVOR to the Maurer’s clefts in Plasmodium falciparum-infected erythrocytes. The EMBO J. 2005;24:2306–17. doi: 10.1038/sj.emboj.7600720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ansorge I, Benting J, Bhakdi S, Lingelbach K. Protein sorting in Plasmodium falciparum-infected red blood cells permeabilized with the pore-forming protein streptolysin O. Biochem J. 1996;315:307–14. doi: 10.1042/bj3150307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gehde N, Hinrichs C, Montilla I, Charpian S, Lingelbach K, Przyborski JM. Protein unfolding is an essential requirement for transport across the parasitophorous vacuolar membrane of Plasmodium falciparum. Mol Microbiol. 2009;71:613–28. doi: 10.1111/j.1365-2958.2008.06552.x. [DOI] [PubMed] [Google Scholar]
- 26.Eilers M, Schatz G. Binding of a specific ligand inhibits import of a purified precursor protein into mitochondria. Nature. 1986;322:228–232. doi: 10.1038/322228a0. [DOI] [PubMed] [Google Scholar]
- 27.Tilley L, Sougrat R, Lithgow T, Hanssen H. The twists and turns of Maurer’s cleft trafficking in P. falciparum-infected erythrocytes. Traffic. 2008;9:187–97. doi: 10.1111/j.1600-0854.2007.00684.x. [DOI] [PubMed] [Google Scholar]
- 28.Trelka DP, Schneider TG, Reeder JC, Taraschi TF. Evidence for vesicle-mediated trafficking of parasite proteins to the host cell cytosol and erythrocyte surface membrane in Plasmodium falciparum infected erythrocytes. Mol Biochem Parasitol. 2000;106:131–45. doi: 10.1016/s0166-6851(99)00207-8. [DOI] [PubMed] [Google Scholar]
- 29.Nyalwidhe J, Lingelbach K. Proteases and chaperones are the most abundant proteins in the parasitophorous vacuole of Plasmodium falciparum-infected erythrocytes. Proteomics. 2006;6:1563–73. doi: 10.1002/pmic.200500379. [DOI] [PubMed] [Google Scholar]
- 30.Smith MH, Ploegh HL, Weissman JS. Road to ruin: targeting proteins for degradation in the endoplasmic reticulum. Science. 2011;334:1086–90. doi: 10.1126/science.1209235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.de Koning-Ward TF, Gilson PR, Boddey JA, Rug M, Smith BJ, Papenfuss AT, Sanders PR, Lundie RJ, Maier AG, Cowman AF, Crabb BS. A newly discovered protein export machine in malaria parasites. Nature. 2009;459:945–9. doi: 10.1038/nature08104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bullen HE, Charnaud SC, Kalanon M, Riglar DT, Dekiwadia C, Kangwanrangsan N, Torii M, Tsuboi T, Baum J, Ralph SA, Cowman AF, de Koning-Ward TF, Crabb BS, Gilson PR. Biosynthesis, localization, and macromolecular arrangement of the Plasmodium falciparum translocon of exported proteins (PTEX) J Biol Chem. 2012;287:7871–84. doi: 10.1074/jbc.M111.328591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bullen HE, Crabb BS, Gilson PR. Recent insights into the export of PEXEL/HTS-motif containing proteins in Plasmodium parasites. Curr Opin Microbiol. 2012;15:699–704. doi: 10.1016/j.mib.2012.09.008. [DOI] [PubMed] [Google Scholar]
- 34**.Riglar DT, Rogers KL, Hanssen E, Turnbull L, Bullen HE, Charnaud SC, Przyborski J, Gilson PR, Whitchurch CB, Crabb BS, Baum J, Cowman AF. Spatial association with PTEX complexes defines regions for effector export into Plasmodium falciparum-infected erythrocytes. Nat Commun. 2013;4:1415. doi: 10.1038/ncomms2449. High resolution microscopy describing the dynamics of translocon candidate PTEX during parasite development and effort at co-localising it with suspected PTEX substrates. Proposes export in translocon positive subdomains of the PVM. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.McMillan PJ, Millet C, Batinovic S, Maiorca M, Hanssen E, Kenny S, Muhle RA, Melcher M, Fidock DA, Smith JD, Dixon MW, Tilley L. Spatial and temporal mapping of the PfEMP1 export pathway in Plasmodium falciparum. Cell Microbiol. 2013 doi: 10.1111/cmi.12125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schleiff E, Becker T. Common ground for protein translocation: access control for mitochondria and chloroplasts. Nat Rev Mol Cell Biol. 2011;12:48–59. doi: 10.1038/nrm3027. [DOI] [PubMed] [Google Scholar]
- 37.Spycher C, Rug M, Klonis N, Ferguson DJ, Cowman AF, Beck HP, Tilley L. Genesis of and trafficking to the Maurer’s clefts of Plasmodium falciparum-infected erythrocytes. Mol Cell Biol. 2006;26:4074–85. doi: 10.1128/MCB.00095-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Papakrivos J, Newbold CI, Lingelbach K. A potential novel mechanism for the insertion of a membrane protein revealed by a biochemical analysis of the Plasmodium falciparum cytoadherence molecule PfEMP-1. Mol Microbiol. 2005;55:1272–84. doi: 10.1111/j.1365-2958.2004.04468.x. [DOI] [PubMed] [Google Scholar]
- 39**.Grüring C, Heiber A, Kruse F, Ungefehr J, Gilberger T, Spielmann T. Development and host cell modifications of Plasmodium falciparum blood stages in four dimensions. Nat Commun. 2011;2:165. doi: 10.1038/ncomms1169. First vizualization of the development of individual asexual blood stage parasites using time lapse imaging. Shows early appearance and parasite-stage dependent mobility of Maurer’s clefts and contradicts the nascent cleft hypothesis of TM protein export. [DOI] [PubMed] [Google Scholar]
- 40.Külzer S, Rug M, Brinkmann K, Cannon P, Cowman A, Lingelbach K, Blatch GL, Maier AG, Przyborski JM. Parasite-encoded Hsp40 proteins define novel mobile structures in the cytosol of the P. falciparum-infected erythrocyte. Cell Microbiol. 2010;12:1398–420. doi: 10.1111/j.1462-5822.2010.01477.x. [DOI] [PubMed] [Google Scholar]
- 41*.Külzer S, Charnaud S, Dagan T, Riedel J, Mandal P, Pesce ER, Blatch GL, Crabb BS, Gilson PR, Przyborski JM. Plasmodium falciparum-encoded exported hsp70/hsp40 chaperone/co-chaperone complexes within the host erythrocyte. Cell Microbiol. 2012;14:1784–95. doi: 10.1111/j.1462-5822.2012.01840.x. Reveals that a parasite HSP70 is a PNEP exported into the host cell where it partially co-localizes with HSP40 in J dots and a proximity ligation assay suggested an interaction with PfEMP1. This provides evidence for export of PfEMP1 in a soluble chaperoned complex. [DOI] [PubMed] [Google Scholar]
- 42.Pachlatko E, Rusch S, Müller A, Hemphill A, Tilley L, Hanssen E, Beck HP. MAHRP2, an exported protein of Plasmodium falciparum, is an essential component of Maurer’s cleft tethers. Mol Microbiol. 2010;77:1136–52. doi: 10.1111/j.1365-2958.2010.07278.x. [DOI] [PubMed] [Google Scholar]
- 43.Hanssen E, Sougrat R, Frankland S, Deed S, Klonis N, Lippincott-Schwartz J, Tilley L. Electron tomography of the Maurer’s cleft organelles of Plasmodium falciparum-infected erythrocytes reveals novel structural features. Mol Microbiol. 2008;67:703–18. doi: 10.1111/j.1365-2958.2007.06063.x. [DOI] [PubMed] [Google Scholar]
- 44.Hanssen E, Carlton P, Deed S, Klonis N, Sedat J, DeRisi J, Tilley L. Whole cell imaging reveals novel modular features of the exomembrane system of the malaria parasite, Plasmodium falciparum. Int J Parasitol. 2010;40:123–34. doi: 10.1016/j.ijpara.2009.09.004. [DOI] [PubMed] [Google Scholar]
- 45**.Cyrklaff M, Sanchez CP, Kilian N, Bisseye C, Simpore J, Frischknecht F, Lanzer M. Hemoglobins S and C interfere with actin remodeling in Plasmodium falciparum-infected erythrocytes. Science. 2011;334:1283–6. doi: 10.1126/science.1213775. Shows actin filaments with PfEMP1 positive vesicles between Maurer’s clefts and surface knobs, suggesting parasite-induced remodeling of host cell actin. In host cells with hemoglobin mutations remodeling is reduced, suggesting that this leads to the impaired surface antigen trafficking responsible for the disease-protecting mechanism in hemoglobinopathies. [DOI] [PubMed] [Google Scholar]
- 46.Kilian N, Dittmer M, Cyrklaff M, Ouermi D, Bisseye C, Simpore J, Frischknecht F, Sanchez CP, Lanzer M. Haemoglobin S and C affect the motion of Maurer’s clefts in Plasmodium falciparum-infected erythrocytes. Cell Microbiol. 2013 doi: 10.1111/cmi.12102. [DOI] [PubMed] [Google Scholar]
- 47.Taylor SM, Parobek CM, Fairhurst RM. Haemoglobinopathies and the clinical epidemiology of malaria: a systematic review and meta-analysis. Lancet Infect Dis. 2012;12:457–68. doi: 10.1016/S1473-3099(12)70055-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Fairhurst RM, Baruch DI, Brittain NJ, Ostera GR, Wallach JS, Hoang HL, Hayton K, Guindo A, Makobongo MO, Schwartz OM, Tounkara A, Doumbo OK, Diallo DA, Fujioka H, Ho M, Wellems TE. Abnormal display of PfEMP-1 on erythrocytes carrying haemoglobin C may protect against malaria. Nature. 2005;435:1117–21. doi: 10.1038/nature03631. [DOI] [PubMed] [Google Scholar]
- 49.Cholera R, Brittain NJ, Gillrie MR, Lopera-Mesa TM, Diakité SA, Arie T, Krause MA, Guindo A, Tubman A, Fujioka H, Diallo DA, Doumbo OK, Ho M, Wellems TE, Fairhurst RM. Impaired cytoadherence of Plasmodium falciparum-infected erythrocytes containing sickle hemoglobin. Proc Natl Acad Sci USA. 2008;105:991–6. doi: 10.1073/pnas.0711401105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Fairhurst RM, Bess CD, Krause MA. Abnormal PfEMP1/knob display on Plasmodium falciparum-infected erythrocytes containing hemoglobin variants: fresh insights into malaria pathogenesis and protection. Microbes Infect. 2012;14:851–62. doi: 10.1016/j.micinf.2012.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kriek N, Tilley L, Horrocks P, Pinches R, Elford BC, Ferguson DJP, Lingelbach K, Newbold CI. Characterization of the pathway for transport of the cytoadherence-mediating protein, PfEMP1, to the host cell surface in malaria parasite-infected erythrocytes. Mol Microbiol. 2003;50:1215–27. doi: 10.1046/j.1365-2958.2003.03784.x. [DOI] [PubMed] [Google Scholar]
- 52.Maier AG, Rug M, O’Neill MT, Brown M, Chakravorty S, Szestak T, Chesson J, Wu Y, Hughes K, Coppel RL, Newbold C, Beeson JG, Craig A, Crabb BS, Cowman AF. Exported proteins required for virulence and rigidity of Plasmodium falciparum-infected human erythrocytes. Cell. 2008;134:48–61. doi: 10.1016/j.cell.2008.04.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Cooke BM, Mohandas N, Coppel RL. Malaria and the red blood cell membrane. Semin Hematol. 2004;41:173–88. doi: 10.1053/j.seminhematol.2004.01.004. [DOI] [PubMed] [Google Scholar]
- 54.Muniz M, Morsomme P, Riezman H. Protein sorting upon exit from the endoplasmic reticulum. Cell. 2001;104:313–20. doi: 10.1016/s0092-8674(01)00215-x. [DOI] [PubMed] [Google Scholar]