Abstract
Glioblastoma multiforme (GBM) is the most common primary brain tumor in the USA with a median survival of approximately 14 months. Low survival rates are attributable to the aggressiveness of GBM and a lack of understanding of the molecular mechanisms underlying GBM. The disruption of signaling pathways regulated either directly or indirectly by protein kinases is frequently observed in cancer cells and thus the development of inhibitors of specific kinases has become a major focus of drug discovery in oncology. To identify protein kinases required for the survival of GBM we performed a siRNA-based RNAi screen focused on the human kinome in GBM. Inhibition of the polo-like kinase 1 (PLK1) induced a reduction in the viability in two different GBM cell lines. To assess the potential of inhibiting PLK1 as a treatment strategy for GBM we examined the effects of a small molecule inhibitor of PLK1, GSK461364A, on the growth of GBM cells. PLK1 inhibition arrested cells in the mitotic phase of the cell cycle and induced cell kill by mitotic catastrophe. GBM engrafts treated with GSK461364A showed statistically significant inhibition of tumor growth. Further, exposure of different GBM cells to RNAi or GSK461364A prior to radiation resulted in an increase in their radiosensitivity with dose enhancement factor ranging from 1.40 to 1.53 with no effect on normal cells. As a measure of DNA double strand breaks, γH2AX levels were significantly higher in the combined modality as compared to the individual treatments. This study suggests that PLK1 is an important therapeutic target for GBM and can enhance radiosensitivity in GBM.
Keywords: Glioblastoma multiforme, PLK1, GSK461364A, siRNA, radiation
1. Introduction
Glioblastoma multiforme (GBM) is the most common type of malignant primary brain tumor (1). The standard of care for newly diagnosed GBM is maximal surgical resection followed by radiation and chemotherapy. The median progression-free and overall survival times for patients treated with the current standard chemo-radiotherapy are approximately seven and 15 months, respectively (2, 3). On the other hand there is no standard chemotherapy for recurrent or progressive GBM. Hence, identification of novel molecularly targeted therapies for GBM would be of great interest.
Cellular kinases are a family of proteins critical to all signal transduction pathways involved in cell proliferation, growth, survival, adhesion, motility, and differentiation (4). Deregulation of kinase-mediated signal transduction is implied in GBM tumorigenesis [reviewed in (5, 6)]. Therefore, the analysis of all kinases (the kinome) may yield information on aberrant cell signaling pathways in GBM. Several strategies can be used to study the kinome, including Western blot analysis, ELISA assays, mass spectrometry and phosphor-proteome identification by tyrosine specific antibodies. However, these methods are either not amenable to highthroughput analysis or are labor intensive. An alternative strategy is the application of loss of function (LOF) RNA interference (RNAi) screens using chemically synthesized siRNAs or plasmid-encoded short hairpin RNAs (shRNAs). This unbiased functional genomic approach has the potential to identify tumor cell specific signaling pathways and thus novel drug targets (7–10).
In this study we conducted a siRNA-based screen focused on the human kinome in the well-established GBM cell line U87-MG and identified PLK1 as the most robust putative target, which can radiosensitize GBM tumors to radiation therapy.
2. Materials and Methods
2.1 Cell lines and drug treatment
The LN18 and U87-MG (ATCC, (Manassas, VA) and the U251 (National Cancer Institute Frederick Tumor Repository) human GBM cell lines were grown in DMEM (Invitrogen, Carlsbad, CA) with 10% FBS, and maintained at 37°C, 5% CO2. GBAM1, GBM stem cells were established from patient resections as previously described (11) and grown in DMEM/F12 (Invitrogen) containing B27 supplement (1×, Invitrogen), basic fibroblast growth factor and epidermal growth factor (50ng/ml each, Sigma-Aldrich, St. Louis, MO). MRC9 (normal lung fibroblasts) were obtained from ATCC and maintained in minimum essential medium supplemented with 10% FBS, glutamine, sodium pyruvate and non-essential amino acids. GBM stem cells and normal cells were used between passages 3–7. GSK461364A was purchased from MedChem Express (MedChem Express, China), was reconstituted in DMSO and stored at −80°C. Cells were plated 24 hours prior to drug treatment and were treated with GSK461364A at the concentrations indicated in each experiment.
2.2 siRNA based analysis
All siRNAs were arrayed from the Human Druggable Genome siRNA Set Version 2.0 (Qiagen Inc., Germantown, MD). RNAi screens were conducted using synthetic siRNAs corresponding to 691 genes annotated at purchase as associated with kinase activity (Table S1). A detailed protocol is given in the supplementary methods.
The sequences for the PLK1 siRNAs used in this study are as follows siPLK1.2: 5’ CAACGGCAGCGTGCAGATCAA 3’ (SI00071624), siPLK1.3: 5’ CACCATATG AATTGTACAGAA 3’ (S100071631), siPLK1.4: 5’ CCCGAGGTGCTGAGCAAGAAA 3’ (S100071638) and siPLK1.7: 5’ CGCGGGCAAGATTGTGCCTAA 3’ (SI02223844) (Qiagen Inc, Germantown, MD).
2.3 Western blot analysis
Cell pellets were lysed on ice in RIPA buffer (Pierce, Rockford, IL), electrophoresed and transferred to a nitrocellulose membrane. Membranes were blocked, incubated with primary antibody, followed by a HRP-coupled secondary antibody and developed with Visualizer Western Blot Detection Kit (Millipore, Billerica, MA). The following antibodies were utilized: human anti-PLK1 rabbit (1:1000) (Cell Signaling, Danvers, MA); mouse anti-actin (1:2500) (Millipore); goat anti-rabbit-HRP (1:10000), and goat anti-mouse-HRP (1:10000) (Santa Cruz Biotechnology, Santa Cruz, CA).
2.4 Clonogenic assay
Cells were seeded into six-well tissue culture plates and allowed to attach for six hours. GSK461364A or DMSO control was added to the culture media for two hours. For combination treatment, GSK461364A or DMSO control was added to the culture media for two hours followed by treatment with ionizing radiation. A detailed protocol is given in the supplementary methods. For clonogenic assays with RNAi, cells were transfected, trypsinized 48 hours post-transfection and plated as described. The seeding density ranged from 200 cells to 6000 cells for GSK461364A treatment and 200 cells to 3200 cells involving RNAi clonogenics.
2.5 Cell cycle analysis and Apoptotic cell death
Cell cycle phase distribution and G2-checkpoint integrity were evaluated by flow cytometry as described earlier (12). The % double-positive annexin-V/7-AAD cells, representing early/late apoptosis respectively, were used to calculate the apoptotic indices.
2.6 Mitotic catastrophe
Cells were grown in 4-well chamber slides, fixed with methanol, and stained overnight at 4°C with mouse anti-α-tubulin antibody (Sigma). A detailed protocol is given in the supplementary methods.
2.7 Histone H2A.X phosphorylation cellular ELISA
We performed cell based ELISA assay for measuring phosphorylation of histone H2A.X according to the manufacturer’s protocol (MBL International, Woburn, Massachusetts). A detailed protocol is given in the supplementary methods.
2.8 In vivo tumor growth
All animal studies were conducted in accordance with the principles and procedures outlined in the NIH Guide for the Care and Use of Animals. Four to eight week old, female, athymic NCr nu/nu, nude mice (NCI Animal Production Program, Frederick, MD) were used for all in vivo studies as described in the supplementary methods.
2.9 Statistical analysis
Data presented are the mean ± the standard deviation from three independent experiments unless indicated otherwise. All statistical tests were two-sided. For comparisons between groups, a Student’s t test was used. All analyses were completed using GraphPad Prism software (GraphPad Prism Inc., San Diego, CA).
3. Results
3.1 RNAi screen of the human kinome identifies PLK1 as an essential kinase for the viability of GBM cells
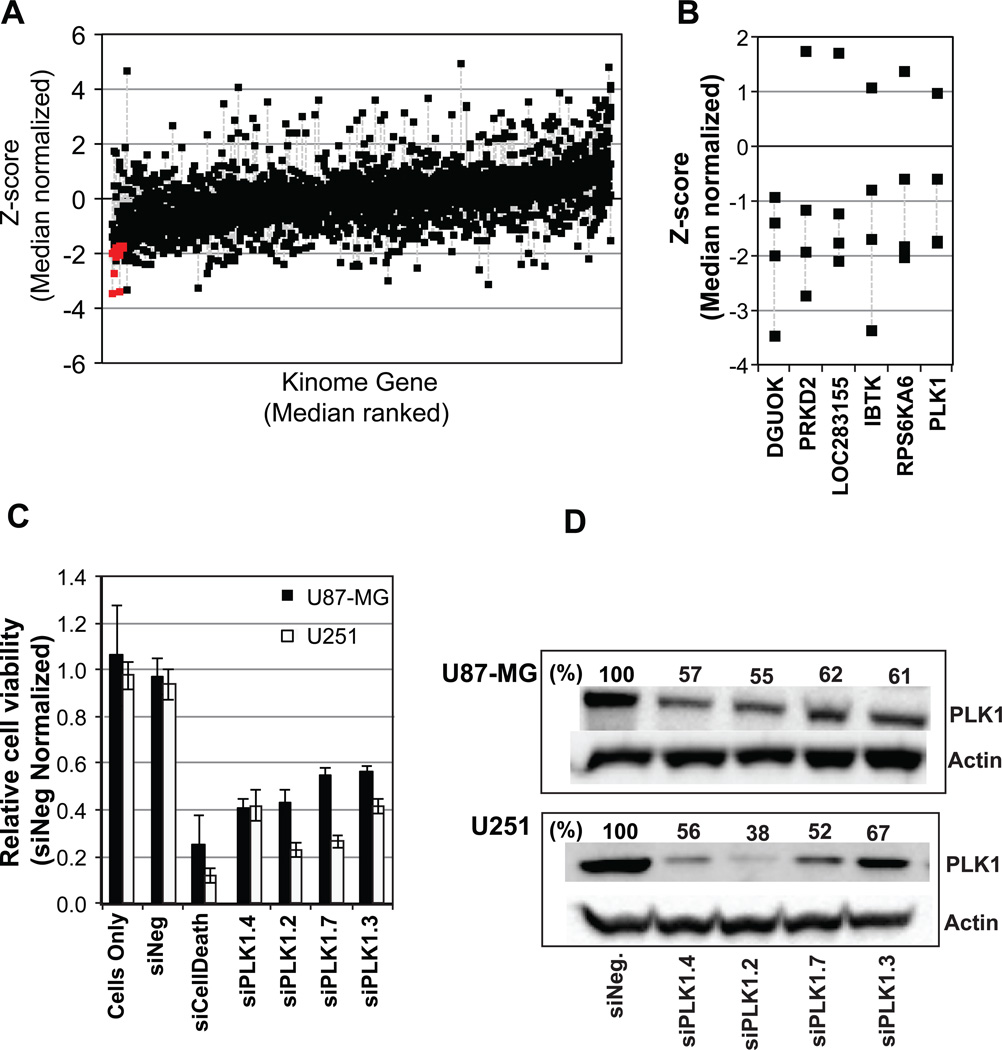
To determine if glioma cells are dependent on specific kinases to maintain survival we performed a synthetic siRNA based RNAi screen of the human kinome (four siRNAs per gene; 691 genes) in the GBM cell line U87-MG (Table S1). The quality control data for the screen is shown in Figure S1 and summary of the screen is shown in Figure 1A, where those genes for which two or more siRNAs induced a significant reduction in cell viability is indicated by the red squares. Five annotated kinase proteins, DGUOK, PRKD2, IBTK, RPS6KA6 and PLK1 were identified for which at least two of four siRNAs induced a significant decrease in cell growth (a z score of ≤ −1.45883) (Figure 1B; the annotated gene LOC283155 similar to ALK-3 has been discontinued). Of these kinase genes we were most interested by the effects induced following silencing of PLK1. Expression of the polo-like kinase 1 (PLK1) gene has been observed in a variety of cancer histologies including breast, pancreas, lung, and prostate (13), and moreover, PLK1 activity can be pharmacologically inhibited.
Figure 1. A siRNA screen of the human kinome in the GBM cell line U87-MG identifies PLK1 as potential molecular target.
(A) The human GBM cell line U87-MG was transfected with siRNAs corresponding 691 kinase genes (four siRNAs per gene) and 96 hours later cell viability was assessed. Data is shown as the z-score of the median normalized data for each siRNA. The data is ranked according to the median value for the four siRNAs corresponding to each gene, with the four siRNA corresponding to each gene aligned vertically and linked by a grey dotted line. Those genes for which, two or more siRNAs mediated a significant decrease (a z-score of ≤ −1.4483, p≤ 0.05) in cell viability are indicated by the red squares. (B) The four individual z-scores for those kinases for which, two or more siRNAs mediated a significant decrease (a z-score of ≤ −1.4483, p≤ 0.05) in cell viability. Those siRNAs inducing a reduction in cell viability are linked by a grey dotted line. (C) The human GBM cell lines U87-MG and U251 were transfected with four different siRNAs corresponding to PLK1, siNegative, or siCelldeath, (negative and positive control siRNAs) in triplicate. Cell viability was assessed 96 hours post siRNA transfection. (D) Seventy-two hours post siRNA transfection of U251 cells, cell lysates were measured for PLK1 protein levels by Western blot analysis using actin as a loading control.
To validate the effects of inhibiting PLK1, cell kill was measured using cell titer glow assay using multiple siRNAs corresponding to PLK1 in U87-MG cells and a second GBM line U251 (Figure 1C) and also confirmed inhibition of PLK1 protein levels in U251 cells (Figure 1D). Inhibition of PLK1 expression in both U87-MG and U251 cells reduced cell viability with U251 cells exhibiting the greater sensitivity to PLK1 loss of function. We also validated DGUOK, PRKD2, IBTK and RPS6KA6 in U251 cells to confirm the validity of the screen (Figure S2). Downregulation of these genes induced a significant reduction in the cell viability (Figure S2 A) and done so by downregulation of mRNA levels (Figure S2 B).
3.2 Pharmacologic PLK1 inhibition affects the survival ability of GBM cells
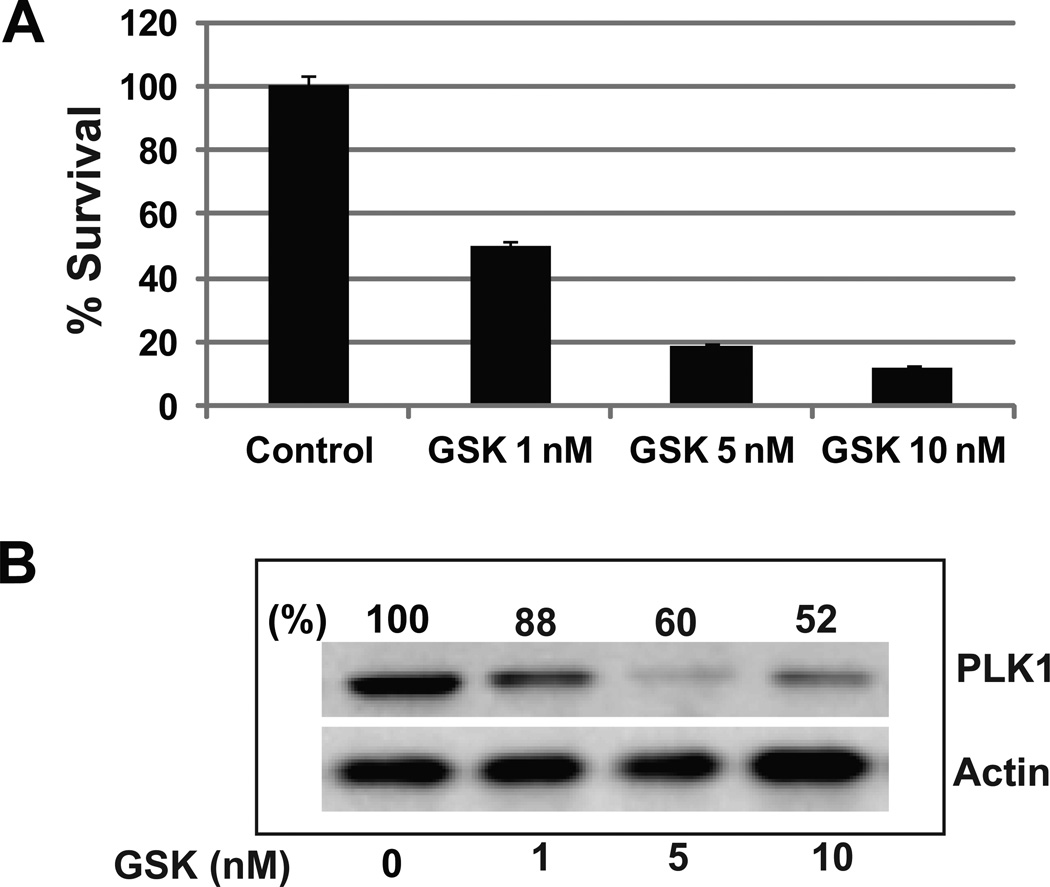
GSK461364A is an ATP-competitive inhibitor of polo-like kinase 1 (PLK1) that has recently been the subject of a phase I clinical trial in patients with advanced solid tumors. To determine if GSK461364A can alter the ability of GBM cells to proliferate we used a clonogenic cell survival assay. U251 cells were treated with three different concentrations of GSK461364A in triplicate for 2 hours and colony-forming efficiency was determined 12 days later. We observed a dose dependent inhibition of colony formation with 50% inhibition at 1 nM of GSK461364A (Figure 2A). These data indicate that inhibition of PLK1 results in the abrogation of the proliferative capacity of GBM cells and is correlated with PLK1 protein down-regulation as shown in Figure 2B. For example, 1 nM of GSK461364A induces a 12% decrease in PLK1 protein levels compared to the control, and 10 nM induces about 50% decrease.
Figure 2. Pharmacologic inhibition of PLK1 reduces GBM cell growth.
(A) U251 cells were exposed to GSK461364A or DMSO control for two hours. Colony-forming efficiency was determined 10–14 days later and % survival generated after normalizing to DMSO control. (B), Western blot analysis of PLK1 protein levels from GSK461364A treated U251 cells; actin levels were measured as a loading control.
3.3 PLK1 inhibition alters the cell cycle distribution of GBM cells and arrests them under mitosis and induces mitotic catastrophe in GBM cells
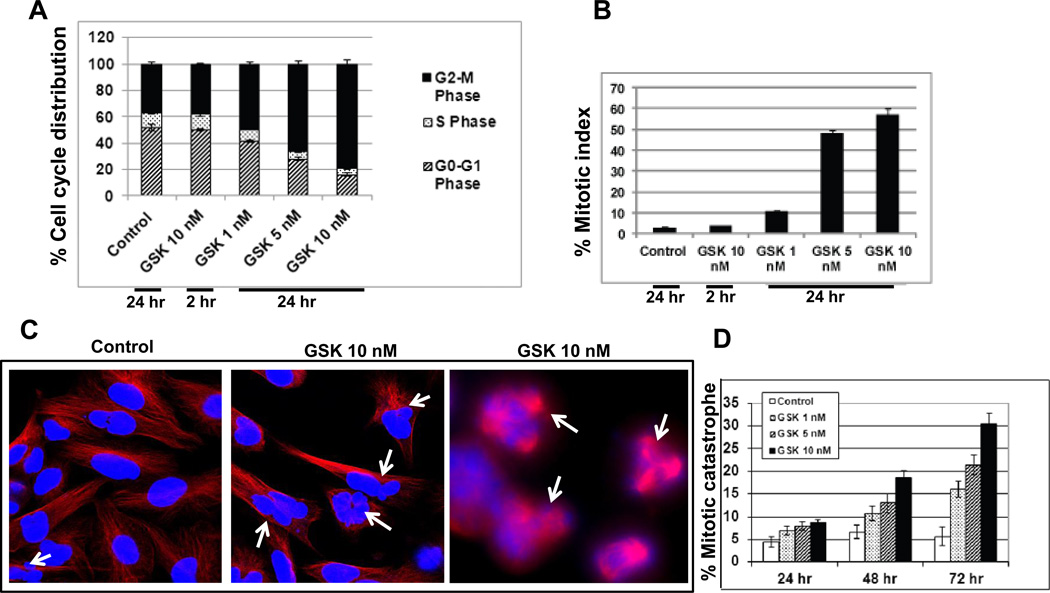
PLK1 is a critical regulator of cell cycle progression. To investigate the effects GSK461364A on GBM cells we pre-treated U251 cells with GSK461364A for two hours. We observed dose dependent changes in the cell cycle phase distribution of GSK461364A treated cells compared to DMSO treated cells (Figure 3A), with increasing doses of GSK461364A inducing an increase in cells in G2-M phase of cell cycle compared to control cells. We next investigated those cells arrested in G2-M phase by distinguishing G2-versus M-phase cells in the 4N cell population following GSK461364A treatment by staining for the phospho-H3 histone as an M-phase marker. We observed both a dose- and time-dependent alteration in the mitotic index of GSK461364A U251 cells compared to control cells (Figure 3B). The high mitotic index observed in drug treated cells indicates an abrogation in mitosis. Taken together, these data indicate that GSK461364A induces cell cycle redistribution and G2-M checkpoint abrogation in GBM cells.
Figure 3. PLK1 inhibition induces mitotic arrest and mitotic catastrophe in glioblastoma cells.
(A) To assess cell cycle phase distribution GBM U251 cells were exposed to GSK461364A or DMSO control for two hours and then stained with propidium iodide 24 hours later for flow cytometry analysis. (B) To evaluate the activation of G2 cell cycle checkpoint mitotic cells were distinguished from G2 cells. The mitotic index was determined according to the expression of phosphorylated histone H3 as detected in the 4N DNA content population by the flow cytometric analysis of GBM U251 cells exposed to GSK461364A or DMSO control for two hours and assayed 24 hours later. (C) To assess mitotic catastrophe represented as the number of cells with multilobulated giant nuclei (left two panels) and cells with abnormal mitoses (right panel) (white arrows), U251 cells stained with anti-tubulin antibody (red) and nuclei were visualized with DAPI (blue) staining and (D) nuclear fragmentation was evaluated in 150 cells per treatment per experiment and plotted against the drug concentration and time intervals.
As our data showed that inhibition of PLK1 arrests cells in the mitotic phase of cell cycle, we next studied whether this arrest results into mitotic catastrophe. U251 cells were treated with GSK461364A for two hours and then analyzed for mitotic catastrophe, represented as the number of cells with multilobulated giant nuclei and cells with abnormal mitoses as a function of time after GSK461364A treatment. Cells treated with GSK461364A resulted in a significant increase in cells undergoing mitotic catastrophe at 48 and 72 hours as compared with untreated cells (Figure 3C and 3D). As shown in the representative photomicrograph (Figure 3C), cells undergoing mitotic catastrophe could be clearly distinguished form their normal counterparts. Overall, there was a dose and time-dependent increase in the number of cells undergoing mitotic catastrophe with increasing doses of GSK461364A (Figure 3D).
3.4 PLK1 abrogation inhibits GBM tumor growth in-vivo
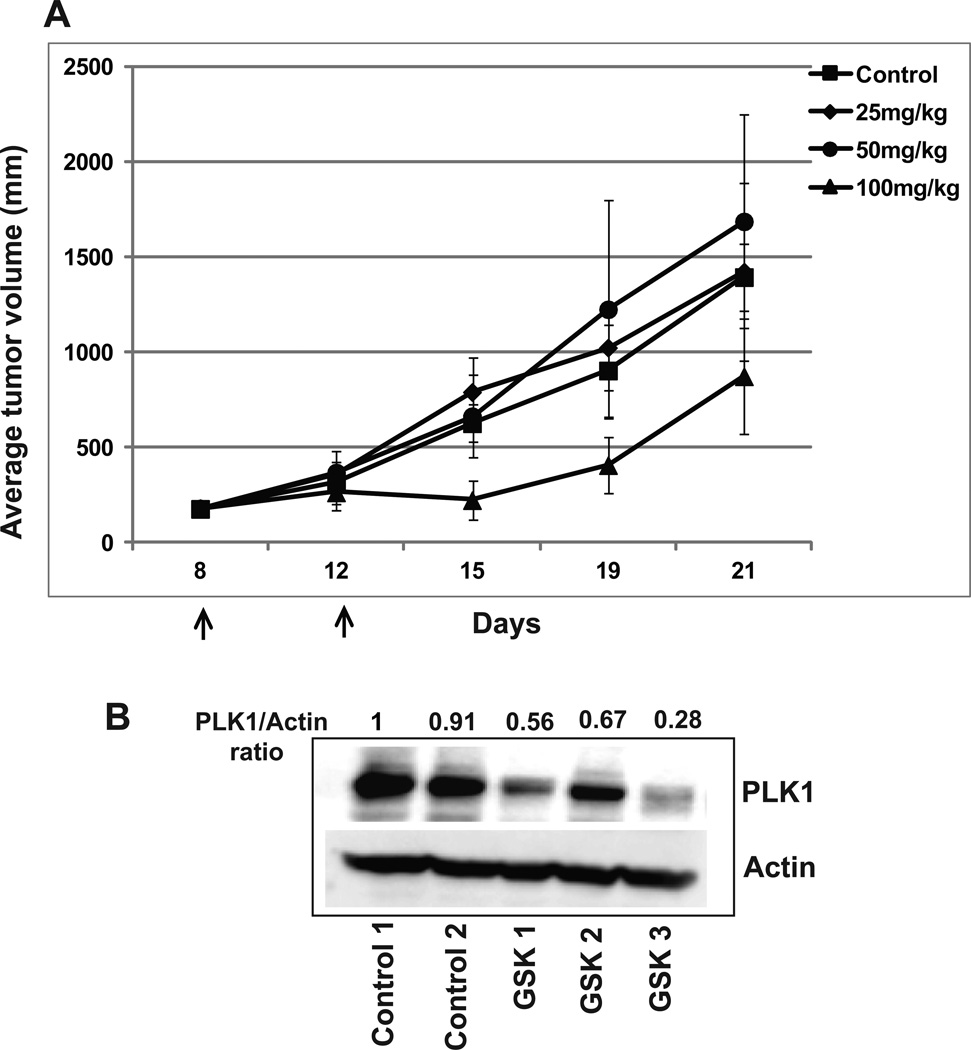
To determine whether the inhibitory activities of PLK1 abrogation would translate to an in vivo model, U251 cells were implanted subcutaneously into nude mice and tumor growth was monitored over time. Tumors grown to ≈172 mm3 (range 116 – 228 mm3) were randomized to one of four treatment arms: vehicle treated control, GSK461364A (25 mg/kg), GSK461364A (50 mg/kg), or GSK461364A (100 mg/kg). Average growth rates are shown in Figure 4A. There was a tumor growth delay of -0.1 day, -0.9 day and 3 days for 25, 50 and 100mg/kg of GSK461364A. The in-vivo efficacy of GSK461364A treatment correlated with PLK1 protein down-regulation in treated tumors as shown in Figure 4B.
Figure 4. PLK1 abrogation inhibits GBM tumor growth in-vivo.
(A) U251 cells were implanted subcutaneously into nude mice and tumor growth was monitored over time. Tumors grown to ≈172 mm3 were randomized to one of four treatment arms: vehicle treated control, GSK461364A (25 mg/kg), GSK461364A (50 mg/kg), or GSK461364A (100 mg/kg). Mice were treated with two doses of GSK461364A or DMSO control four days apart. Tumor volume plotted as the mean volume ± SD. (B) Cell lysates from control and GSK461364A treated tumors were measured for PLK1 protein levels by Western blot analysis using actin as a loading control. PLK1/actin ratio is shown.
3.5 PLK1 inhibition enhances the radiosensitivity of GBM cells with no effect on normal cells
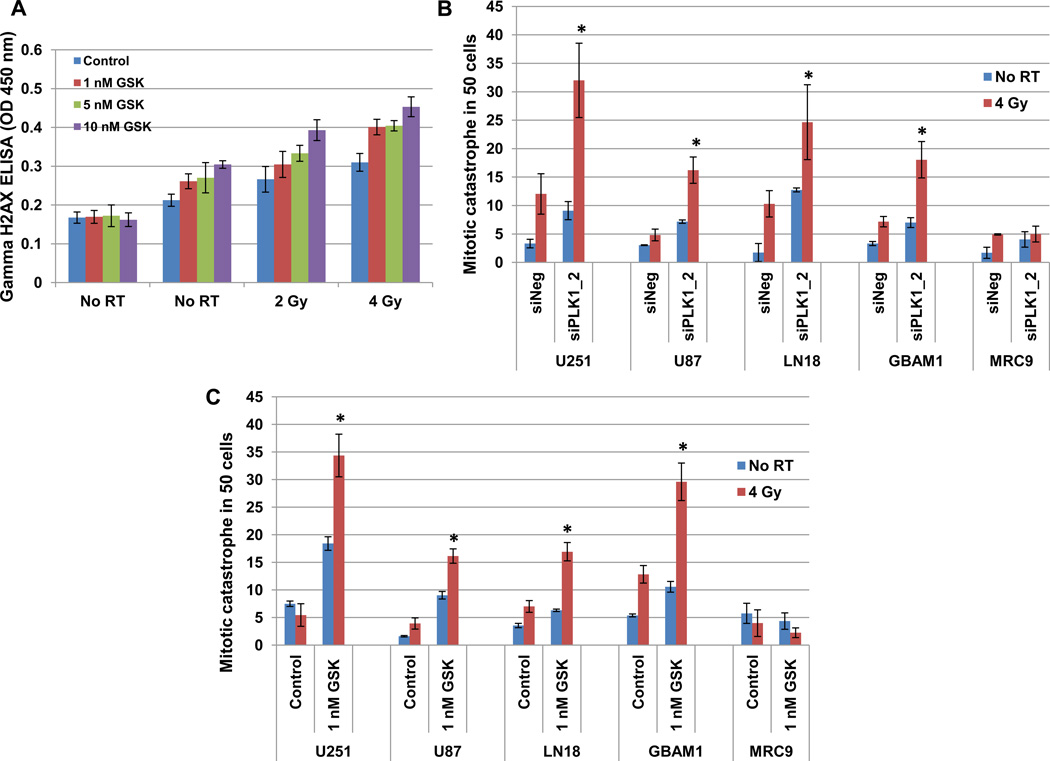
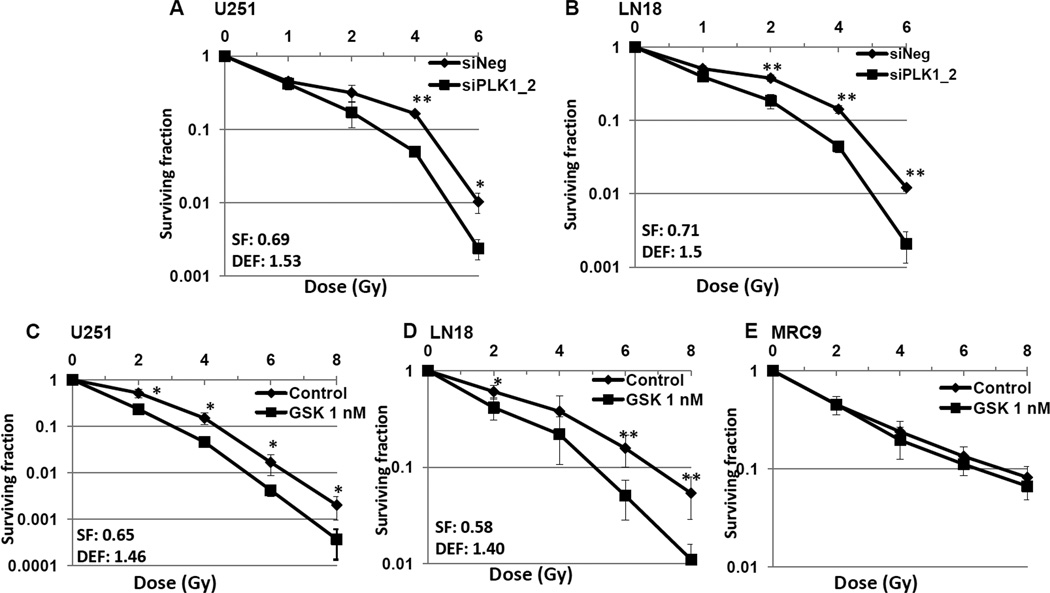
To determine if PLK1 inhibition could increase the sensitivity of GBM cells to radiation, we analyzed levels of phosphorylated histone H2A.X (H2AX) after GSK461364A and radiation treated cells (Figure 5A). H2AX levels were significantly higher at 24 hours following combined modality treatment when compared to individual treatments or to vehicle treated controls. This data is suggestive of non-repaired DNA DSBs and an enhancement of radiation sensitivity. To confirm this, mitotic catastrophe and clonogenic survival assays were used (Figures 5B, 5C and 6). Three GBM tumor lines (U251, LN18 and U87-MG), one each GBM stem cell line (GBAM1) and normal cell line (MRC9) were assessed using siPLK1 and PLK1 inhibitor, GSK461364A. Cells were either transfected (with siNeg or siPLK1) or treated with GSK461364A prior to IR and mitotic catastrophe and colony-forming efficiency was determined. U251, U87-MG, LN18 and GBAM1 all showed significant increase in post-radiation mitotic catastrophe in combination with PLK1 downregulation, moreover, PLK1 downregulation had no effect on the radiosensitivity of the normal cell line tested (Figure 5). On clonogenic survival, the survival efficiencies ranged from 58% to 71% and PLK1 inhibition increased radiosensitivity of U251 and LN18 cells with the dose enhancement factor of 1.40 – 1.53 at a surviving fraction of 10% (Figure 6). However, GSK461364A and radiation treatment had no effects on normal cell survival (Figure 6). These results suggest that PLK1 contributes to survival after irradiation of tumor cells and stem cells but not normal cells.
Figure 5. PLK1 inhibition enhances the radiosensitivity by inducing mitotic catastrophe in glioblastoma cells.
(A) U251 cells were exposed to GSK461364A or DMSO control for 2 hours before irradiation. H2AX levels measured at 24 hours and optical density at 450 nm was plotted. (B and C) Three GBM tumor lines (U251, LN18 and U87-MG), one each GBM stem cell line (GBAM1) and normal fibroblast cell line (MRC9) were either transfected (siNegative/siPLK1-2) (B) or exposed to 1 nM GSK461364A or DMSO control (C). Cells stained with anti-tubulin antibody and nuclei were visualized with DAPI staining. The dose of radiation used is 4 Gy. Mitotic catastrophe represented as the number of cells with multilobulated giant nuclei and cells with abnormal mitoses, was evaluated in 100 cells per treatment per experiment and plotted. Values represent the mean ± SD for 3 independent experiments, *, p < 0.05 according to the Student t test.
Figure 6. PLK1 inhibition enhances the radiosensitivity of glioblastoma cells.
Cells were either transfected (siNegative/siPLK1-2) (A and B) or exposed to 1 nM GSK461364A or DMSO control (C – E) and clonogenic survival was assessed. Colony-forming efficiency was determined in two GBM tumor lines (U251 and LN18) and one each GBM stem cell line (GBAM1) and normal fibroblast cell line (MRC9) 10 to 14 days later and survival curves were generated after normalizing for the cytotoxicity induced by siNegative/GSK461364A alone and surviving fraction and dose enhancement factor were calculated. Values represent the mean standard deviation for 3 independent experiments, *, p < 0.05; **, p < 0.01 according to the Student t test.
4. Discussion
Kinases are known to be involved in the deregulated signal transduction associated with GBM development and progression. Small interfering RNA (siRNAs), the main effectors of RNA interference (RNAi), are now routinely used to identify potential drug targets (10, 14–16). Based on our screening of the kinome we identified five annotated kinases that fit our screening criteria and chose to focus on PLK1. Polo-like kinases (PLKs) are a conserved family of serine–threonine kinases. The PLKs appear to regulate the G1/S transition of the cell cycle, entry of cells into mitosis, cytokinesis and the cellular response to DNA damage (17). The best-characterized member of the PLK family is PLK1 whose overexpression has been associated with poor prognosis in various cancers and more recently in glioblastomas (18–21). Elevated PLK1 levels have been found in many cancers as compared to their normal counterparts (19, 22–24). In GBM, PLK1 expression is associated with higher tumor grade and the mesenchymal/proliferative subtypes of GBM (19). Depletion of PLK1 has been shown to inhibit cell proliferation and tumor growth in number of cancer histologies (25–27). Also, PLK1 transcript levels correlate well with PLK1 protein levels, thus measurement of PLK1 mRNA could be predictive of its functional activity in cancer (26, 28). However, there is no data available for inhibition of PLK1 in GBM. Therefore the objective of this study was to determine the preclinical effects of targeting PLK1 in GBM in-vitro and in-vivo models.
Pharmacological inhibition of cell proliferation and clonogenicity in the GBM cell lines demonstrated in this study is consistent with published reports in other cancers (25) (29, 30). The inhibition of cell growth observed was due to arrest in G2 phase of cell cycle leading to mitotic catastrophe. These findings are not surprising as PLK1 plays role in all the aspects of mitosis (13). Proper control of entry into and progression through mitosis is essential for normal cell proliferation and the maintenance of genome stability. Targeting key components of the mitotic machinery has been envisaged as a possible strategy in cancer treatment. Recently, Liu and coworkers showed that elevated PLK1 is critical for PTEN-depleted cells to adapt to mitotic stress for survival and confers the tumorigenic competence to PTEN-deleted prostate cancer cells (31). Loss of PTEN, which occurs in 40–70% of GBM, results in uncontrolled cell proliferation and immortality and has been associated with poorer survival outcomes in GBM (32, 33). Thus, PLK1 might be a promising target for GBM patients with inactivating PTEN mutations. Here we took advantage of the high potency and selectivity of GSK461364A to inhibit cell growth of both PTEN mutated (U251) and PTEN wild type (LN18) GBM tumor cells (34). Concentrations as low as 1–10 nmol were sufficient to inhibit PLK1, cause cell cycle arrest and mitotic cell death. However, both PTEN mutated and PTEN wild type cells showed similar sensitivity to PLK1 abrogation. Importantly, untransformed normal cells were less sensitive to PLK1 inhibition at these concentrations as shown here as well as published by others (35). PLK1 play a critical role in neural stem cell survival, self-renewal and its down regulation can cause stem cell growth arrest, apoptosis and inhibition of tumorigenicity. (36). In the present study, nanomolar concentrations of GSK461364A induced mitotic catastrophe in GBM stem cells, but had no effects on normal cells at this concentration. Similar results have been shown with another PLK1 inhibitor, BI2536 on neuroblastoma initiating cells (19). Thus, PLK1 inhibition can target both tumor and stem cells without harming surrounding normal cells. We next examined the ability of GSK461364A given by oral injections, to block the growth of human GBM xenografts. Our schedule was well-tolerated, showed effective tumor growth delay and did so by downregulation of PLK1.
As radiation therapy (RT) plays an important role in GBM treatment, we next explored whether inhibition of PLK1 can sensitize GBM tumors to RT. We observed decrease potential of DNA repair and enhanced inhibition of clonogenic potential in radiation and drug treated cells. The observed radiosensitization could be attributed to arrest of cells in radiosensitive G2/M phase after PLK1 inactivation. Similar radiosensitization has been shown in head and neck carcinoma and rectal carcinoma (27, 37). PLK1 plays role in the resumption of cancer cell proliferation after a checkpoint arrest overriding DNA damage induced checkpoint arrest (38). Thus, it is not surprising that combination of PLK1 inactivation and RT could radiosensitize GBM cells. Whereas downregulation of PLK1 could induce radiosensitization of tumor cells and GBM stem cells, it had no effect on the radiosensitivity of normal cells. The tumor selectivity may involve the increased dependence of tumor cells on PLK1 activity to complete mitosis.
Recently, GSK461364A has been prospectively evaluated in a phase I clinical trial to provide pharmacodynamic endpoints alongside tumor response evaluation (39). The results show that GSK461364A effectively targets PLK1, and crosses blood brain barrier to induce a therapeutic effect (30, 39). These studies along with our preclinical results presented here demonstrate that, PLK1 inhibitors hold great promise and might work well in combination with radiation therapy to treat human GBM.
Supplementary Material
Acknowledgements
This research was supported by the Intramural Research Program of the National Institutes of Health, National Cancer Institute.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest Statement
We have no financial or other interests that might be construed as a conflict of interest with regard to this manuscript.
References
- 1.Preusser M, de Ribaupierre S, Wohrer A, Erridge SC, Hegi M, Weller M, et al. Current concepts and management of glioblastoma. Ann Neurol. 2011;70(1):9–21. doi: 10.1002/ana.22425. [DOI] [PubMed] [Google Scholar]
- 2.Stupp R, Mason WP, van den Bent MJ, Weller M, Fisher B, Taphoorn MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352(10):987–996. doi: 10.1056/NEJMoa043330. [DOI] [PubMed] [Google Scholar]
- 3.Stupp R, Hegi ME, Mason WP, van den Bent MJ, Taphoorn MJ, Janzer RC, et al. Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet Oncol. 2009;10(5):459–466. doi: 10.1016/S1470-2045(09)70025-7. [DOI] [PubMed] [Google Scholar]
- 4.Futreal PA, Kasprzyk A, Birney E, Mullikin JC, Wooster R, Stratton MR. Cancer and genomics. Nature. 2001;409(6822):850–852. doi: 10.1038/35057046. [DOI] [PubMed] [Google Scholar]
- 5.Quant EC, Wen PY. Novel medical therapeutics in glioblastomas, including targeted molecular therapies, current and future clinical trials. Neuroimaging Clin N Am. 2010;20(3):425–448. doi: 10.1016/j.nic.2010.04.007. [DOI] [PubMed] [Google Scholar]
- 6.Holand K, Salm F, Arcaro A. The Phosphoinositide 3-Kinase Signaling Pathway as a Therapeutic Target in Grade IV Brain Tumors. Curr Cancer Drug Targets. 2011;ll:894-18. doi: 10.2174/156800911797264743. [DOI] [PubMed] [Google Scholar]
- 7.MacKeigan JP, Murphy LO, Blenis J. Sensitized RNAi screen of human kinases and phosphatases identifies new regulators of apoptosis and chemoresistance. Nat Cell Biol. 2005;7(6):591–600. doi: 10.1038/ncb1258. [DOI] [PubMed] [Google Scholar]
- 8.Grueneberg DA, Li W, Davies JE, Sawyer J, Pearlberg J, Harlow E. Kinase requirements in human cells: IV. Differential kinase requirements in cervical and renal human tumor cell lines. Proc Natl Acad Sci U S A. 2008;105(43):16490–16495. doi: 10.1073/pnas.0806578105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Manning BD. Challenges and opportunities in defining the essential cancer kinome. Sci Signal. 2009;2(63):pel5. doi: 10.1126/scisignal.263pe15. [DOI] [PubMed] [Google Scholar]
- 10.Murrow LM, Garimella SV, Jones TL, Caplen NJ, Lipkowitz S. Identification of WEE1 as a potential molecular target in cancer cells by RNAi screening of the human tyrosine kinome. Breast Cancer Res Treat. 2010;122(2):347–357. doi: 10.1007/s10549-009-0571-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McCord AM, Jamal M, Williams ES, Camphausen K, Tofilon PJ. CD133+ glioblastoma stem-like cells are radiosensitive with a defective DNA damage response compared with established cell lines. Clin Cancer Res. 2009;15(16):5145–5153. doi: 10.1158/1078-0432.CCR-09-0263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Buchanan IM, Scott T, Tandle AT, Burgan WE, Burgess TL, Tofilon PJ, et al. Radiosensitization of glioma cells by modulation of Met signalling with the hepatocyte growth factor neutralizing antibody, AMG102. J Cell Mol Med. 2011;15(9):1999–2006. doi: 10.1111/j.1582-4934.2010.01122.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Strebhardt K. Multifaceted polo-like kinases: drug targets and antitargets for cancer therapy. Nat Rev Drug Discov. 2010;9(8):643–660. doi: 10.1038/nrd3184. [DOI] [PubMed] [Google Scholar]
- 14.Sigoillot FD, King RW. Vigilance and validation: Keys to success in RNAi screening. ACS Chem Biol. 2011;6(1):47–60. doi: 10.1021/cb100358f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sioud M. Promises and challenges in developing RNAi as a research tool and therapy. Methods Mol Biol. 2011;703:173–187. doi: 10.1007/978-1-59745-248-9_12. [DOI] [PubMed] [Google Scholar]
- 16.Martin SE, Wu ZH, Gehlhaus K, Jones TL, Zhang YW, Guha R, et al. RNAi Screening Identifies TAK1 as a Potential Target for the Enhanced Efficacy of Topoisomerase Inhibitors. Curr Cancer Drug Targets. 2011;ll(8):976–986. doi: 10.2174/156800911797264734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bahassi el M. Polo-like kinases and DNA damage checkpoint: beyond the traditional mitotic functions. Exp Biol Med (Maywood) 2011;236(6):648–657. doi: 10.1258/ebm.2011.011011. [DOI] [PubMed] [Google Scholar]
- 18.Degenhardt Y, Lampkin T. Targeting Polo-like kinase in cancer therapy. Clin Cancer Res. 2010;16(2):384–389. doi: 10.1158/1078-0432.CCR-09-1380. [DOI] [PubMed] [Google Scholar]
- 19.Foong CS, Sandanaraj E, Brooks HB, Campbell RM, Ang BT, Chong YK, et al. Glioma-Propagating Cells as an In Vitro Screening Platform: PLK1 as a Case Study. J Biomol Screen. 2012;17(9):1136–1150. doi: 10.1177/1087057112457820. [DOI] [PubMed] [Google Scholar]
- 20.Lee C, Fotovati A, Triscott J, Chen J, Venugopal C, Singhal A, et al. Polo-like kinase 1 inhibition kills glioblastoma multiforme brain tumor cells in part through loss of SOX2 and delays tumor progression in mice. Stem Cells. 2012;30(6):1064–1075. doi: 10.1002/stem.1081. [DOI] [PubMed] [Google Scholar]
- 21.Takai N, Hamanaka R, Yoshimatsu J, Miyakawa I. Polo-like kinases (Plks) and cancer. Oncogene. 2005;24(2):287–291. doi: 10.1038/sj.onc.1208272. [DOI] [PubMed] [Google Scholar]
- 22.Grinshtein N, Datti A, Fujitani M, Uehling D, Prakesch M, Isaac M, et al. Small molecule kinase inhibitor screen identifies polo-like kinase 1 as a target for neuroblastoma tumor-initiating cells. Cancer Res. 2011;71(4):1385–1395. doi: 10.1158/0008-5472.CAN-10-2484. [DOI] [PubMed] [Google Scholar]
- 23.Eckerdt F, Yuan J, Strebhardt K. Polo-like kinases and oncogenesis. Oncogene. 2005;24(2):267–276. doi: 10.1038/sj.onc.1208273. [DOI] [PubMed] [Google Scholar]
- 24.Yamada S, Ohira M, Horie H, Ando K, Takayasu H, Suzuki Y, et al. Expression profiling and differential screening between hepatoblastomas and the corresponding normal livers: identification of high expression of the PLK1 oncogene as a poor-prognostic indicator of hepatoblastomas. Oncogene. 2004;23(35):5901–59ll. doi: 10.1038/sj.onc.1207782. [DOI] [PubMed] [Google Scholar]
- 25.Steegmaier M, Hoffmann M, Baum A, Lenart P, Petronczki M, Krssak M, et al. BI 2536, a potent and selective inhibitor of polo-like kinase 1, inhibits tumor growth in vivo. Curr Biol. 2007;17(4):316–322. doi: 10.1016/j.cub.2006.12.037. [DOI] [PubMed] [Google Scholar]
- 26.Ackermann S, Goeser F, Schulte JH, Schramm A, Ehemann V, Hero B, et al. Polo-like kinase 1 is a therapeutic target in high-risk neuroblastoma. Clin Cancer Res. 2011;17(4):731–741. doi: 10.1158/1078-0432.CCR-10-1129. [DOI] [PubMed] [Google Scholar]
- 27.Gerster K, Shi W, Ng B, Yue S, Ito E, Waldron J, et al. Targeting polo-like kinase 1 enhances radiation efficacy for head-and-neck squamous cell carcinoma. Int J Radiat Oncol Biol Phys. 2010;77(1):253–260. doi: 10.1016/j.ijrobp.2009.11.027. [DOI] [PubMed] [Google Scholar]
- 28.Rodel F, Keppner S, Capalbo G, Bashary R, Kaufmann M, Rodel C, et al. Polo-like kinase 1 as predictive marker and therapeutic target for radiotherapy in rectal cancer. Am J Pathol. 2010;177(2):918–929. doi: 10.2353/ajpath.2010.100040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Morales AG, Brassesco MS, Pezuk JA, Oliveira JC, Montaldi AP, Sakamoto-Hojo ET, et al. BI 2536-mediated PLK1 inhibition suppresses HOS and MG-63 osteosarcoma cell line growth and clonogenicity. Anticancer Drugs. 2011;22(10):995–1001. doi: 10.1097/CAD.0b013e32834a16d4. [DOI] [PubMed] [Google Scholar]
- 30.Qian Y, Hua E, Bisht K, Woditschka S, Skordos KW, Liewehr DJ, et al. Inhibition of Polo-like kinase 1 prevents the growth of metastatic breast cancer cells in the brain. Clin Exp Metastasis. 2011;28(8):899–908. doi: 10.1007/s10585-011-9421-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liu XS, Song B, Elzey BD, Ratliff TL, Konieczny SF, Cheng L, et al. Polo-like kinase 1 facilitates loss of Pten tumor suppressor-induced prostate cancer formation. J Biol Chem. 2011;286(41):35795–35800. doi: 10.1074/jbc.C111.269050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ang C, Guiot MC, Ramanakumar AV, Roberge D, Kavan P. Clinical significance of molecular biomarkers in glioblastoma. Can J Neurol Sci. 2010;37(5):625–630. doi: 10.1017/s0317167100010805. [DOI] [PubMed] [Google Scholar]
- 33.Koul D. PTEN signaling pathways in glioblastoma. Cancer Biol Ther. 2008;7(9):1321–1325. doi: 10.4161/cbt.7.9.6954. [DOI] [PubMed] [Google Scholar]
- 34.Lee JJ, Kim BC, Park MJ, Lee YS, Kim YN, Lee BL, et al. PTEN status switches cell fate between premature senescence and apoptosis in glioma exposed to ionizing radiation. Cell Death Differ. 2011;18(4):666–677. doi: 10.1038/cdd.2010.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nappi TC, Salerno P, Zitzelsberger H, Carlomagno F, Salvatore G, Santoro M. Identification of Polo-like kinase 1 as a potential therapeutic target in anaplastic thyroid carcinoma. Cancer Res. 2009;69(5):1916–1923. doi: 10.1158/0008-5472.CAN-08-1693. [DOI] [PubMed] [Google Scholar]
- 36.Grinshtein N, Datti A, Fujitani M, Uehling D, Prakesch M, Isaac M, et al. Small molecule kinase inhibitor screen identifies polo-like kinase 1 as a target for neuroblastoma tumor-initiating cells. Cancer Res. 2011;71(4):1385–1395. doi: 10.1158/0008-5472.CAN-10-2484. [DOI] [PubMed] [Google Scholar]
- 37.Rodel F, Keppner S, Capalbo G, Bashary R, Kaufmann M, Rodel C, et al. Polo-like kinase 1 as predictive marker and therapeutic target for radiotherapy in rectal cancer. Am J Pathol. 2010;177(2):918–929. doi: 10.2353/ajpath.2010.100040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Smits VA, Klompmaker R, Arnaud L, Rijksen G, Nigg EA, Medema RH. Polo-like kinase-1 is a target of the DNA damage checkpoint. Nat Cell Biol. 2000;2(9):672–676. doi: 10.1038/35023629. [DOI] [PubMed] [Google Scholar]
- 39.Olmos D, Barker D, Sharma R, Brunetto AT, Yap TA, Taegtmeyer AB, et al. Phase I study of GSK461364, a specific and competitive Polo-like kinase 1 inhibitor, in patients with advanced solid malignancies. Clin Cancer Res. 2011;17(10):3420–3430. doi: 10.1158/1078-0432.CCR-10-2946. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.