Abstract
Interferon regulatory factors IRF-3 and IRF-7 are central to the establishment of the innate antiviral response. This study examines HSV-1 pathogenesis in IRF-3−/−, IRF-7−/− and double-deleted IRF3/7−/− (DKO) mice. Bioluminescence imaging of infection revealed that DKO mice developed visceral infection following corneal inoculation, along with increased viral burdens in all tissues relative to single knockout mice. While all DKO mice synchronously reached endpoint criteria 5 days post infection, the IRF-7−/− mice survived longer, indicating that although IRF-7 is dominant, IRF-3 also plays a role in controlling disease. Higher levels of systemic proinflammatory cytokines were found in IRF7−/− and DKO mice relative to wild-type and IRF-3−/− mice, and IL-6 and G-CSF, indicative of sepsis, were increased in the DKO mice relative to wild-type or single-knockout mice. In addition to controlling viral replication, IRF-3 and −7 therefore play coordinating roles in modulation of inflammation during HSV infection.
Keywords: Herpes simplex virus, IRF-3, IRF-7, Innate immunity
INTRODUCTION
Herpes simplex virus 1 (HSV-1) is highly sero-prevalent in the human population (D. E. Alexander et al., 2007). Its ability to establish latency renders it refractory to clearance by the immune system, allowing it to persist for the lifetime of the host (Whitley et al., 1998). HSV-1 infection most commonly manifests as orofacial lesions (Spruance, 1992), but also causes more serious diseases such as stromal keratitis (Behrens-Baumann, 2010; Hsiao et al., 2009; Inoue, 2008), meningitis, encephalitis (Baringer, 2008; Brochet et al., 1990; McGrath et al., 1997; Sköldenberg, 1996; Kenneth L Tyler, 2004) and hepatitis (Al Midani et al., 2011; Riediger et al., 2009). These can occur during primary infection or reactivation events, particularly in immune-deficient hosts (Guo et al., 2011; Sancho-Shimizu et al., 2011, 2007; S.–Y. Zhang et al., 2007).
Type I interferons (IFNα and IFNβ) play major roles in the host innate immune response to HSV-1 infection, and both humans and mice lacking components of the IFN pathway show increased susceptibility and mortality during infection with HSV-1 (Guo et al., 2011; Ishikawa et al., 2009; Lafaille et al., 2012; Luker et al., 2003; Pasieka et al., 2011, 2008; Sancho-Shimizu et al., 2011; S.-Y. Zhang et al., 2007). The importance of IFN signaling in the containment of HSV-1 infection is also reflected by the array of proteins encoded by HSV-1 that function to subvert establishment of the anti-viral state and facilitate viral replication (Chou et al., 1995; He et al., 1997; Johnson et al., 2008; Rongtuan Lin et al., 2004; Melroe et al., 2004; Mulvey et al., 2004; Orvedahl et al., 2007; Pasieka et al., 2012; Sànchez and Mohr, 2007; Xing et al., 2012). Pattern recognition receptors (PRR) such as toll-like receptors (TLRs) (Lemaitre et al., 1996), melanoma differentiation factor (MDA5) (Kang et al., 2002), retinoic acid inducible gene (RIG-I) (Yoneyama et al., 2004), interferon inducible protein-16 (IFI16) (Unterholzner et al., 2010), absent in melanoma 2 (AIM2) (Bürckstümmer et al., 2009), and DNA dependent activator of IRFs (DAI) (Takaoka et al., 2007) recognize pathogen-associated molecular patterns (PAMPs) such as dsRNA and viral DNA during infection. Once activated, these PRRs initiate signaling pathways that culminate in the activation of the transcription factors IRF-3 and IRF-7, leading to production of type 1 IFN.
IRF-3 is a constitutively expressed protein in most cells, existing as an inactive monomer in the cytoplasm. Upon activation, serine residues at the C terminus of IRF-3 are phosphorylated (J Hiscott et al., 1999; R Lin et al., 1998; Sonia Sharma et al., 2003), IRF-3 assembles into dimers and translocates to the nucleus (M Sato et al., 1998), facilitating transcription of interferon β. Newly-produced IFNβ secreted from virus infected cells interacts with cell surface IFNα/β receptors (IFNARs) on infected and uninfected neighboring cells (Mogensen et al., 1999), activating the JAK-STAT signaling pathway (Levy and García-Sastre, 2001), inducing transcription of IFN responsive genes including IRF-7 (Fu et al., 1992; Reich, 2007; Zimmerer et al., 2007). The C-terminus of newly synthesized IRF-7 is phosphorylated, promoting dimerization and translocation to the nucleus (Mitsuharu Sato et al., 2000), where it induces production of large amounts of IFNα, IFNβ and IFN stimulated genes, thus establishing the antiviral state. IRF-7 has long been recognized as the “master regulator” of interferon signaling (Honda et al., 2005), but it is also accepted that IRF-3 plays an important role in controlling HSV-1 replication - particularly within tissues of the central nervous system (Menachery et al., 2010). In addition to its role in inducing anti-viral responses, recent reports (Tarassishin et al., 2011a, 2011b) have indicated that IRF-3 also plays a role in minimizing immune mediated pathology through modulation of pro-inflammatory cytokine expression. Given the non-regenerative nature of certain cell types within the CNS, this ability to switch from a tissue-damaging pro-inflammatory state to a tissue recovery-promoting, anti-inflammatory state is likely to play a role in successful resolution of infection and recovery of the host. The study herein utilizes single and double knockout mouse models of IRF-3 and IRF-7 to examine in detail the roles played by IRF-3 and IRF-7, both singly and in combination, in controlling HSV-1 replication, and disease pathology.
MATERIALS AND METHODS
Cell culture and viruses
Vero cells, maintained in Dulbecco’s Modification of Eagle Medium (DMEM) supplemented with 10% v/v fetal bovine serum (FBS) and 1% penicillin/streptomycin were used to propagate and titer HSV-1 preparations. Bone marrow derived dendritic cells (BMDCs) were generated from 6–8 week old wild-type, IRF-3−/−, IRF-7−/− and IRF-3/7−/− mice. Briefly, bone marrow was flushed from femurs and cultured in RPMI supplemented with 10 % FBS, glutamax, sodium pyruvate, non-essential amino acids, 1% penicillin/streptomycin and 2% granulocyte macrophage colony stimulating factor (GM-CSF) for 7 days. BMDCs were harvested and divided into aliquots for infection. Virus was added in a minimal volume of medium for 1 h at 37°C. After 1 h adsorption, cells were centrifuged at low speed, washed and re-suspended in fresh medium in 6 well plates for the duration of the experiment.
HSV-1 strain 17 syn+ (S. M. Brown et al., 1973) and HSV-1 McKrae/Dlux were used in this study. McKrae/Dlux was generated through homologous recombination of HSV-1 McKrae strain infectious DNA with a pUIC plasmid containing a pDlux cassette, as previously described (Summers et al., 2001). Recombinant viruses were screened for luciferase expression, plaque purified three times and verified by Southern blot (data not shown). All viral stocks were titered by plaque assay on vero cell monolayers as described previously (D A Leib et al., 1989). Briefly, serial 10-fold dilutions of virus were applied to cell monolayers, adsorbed for 1 h and overlaid with a semi-solid methylcellulose overlay. After 4 days monolayers were stained with neutral red and plaques were counted.
Migration assay
Chemotaxis in response to CCL19 was analyzed by measuring the number of cells migrating through a polycarbonate filter in transwell chambers (2 µm pore size; Corning). 3 × 105 wild-type, IRF-3−/− , IRF-7−/− or DKO BMDCs were seeded into the top chambers of the transwells in culture medium. Culture medium supplemented with 20 ng/ml of CCL19 was added to the bottom chambers. Cultures were incubated for 3 h at 37°C and cells that migrated from the top to the bottom chamber were counted using an automated cell counter.
Mice
Mouse strains used in this study included wild-type C57BL6 mice and IRF-3−/− (Mitsuharu Sato et al., 2000), IRF-7−/− (Honda et al., 2005) and IRF-3/7−/− (generated by crossing IRF-3−/− X IRF-7−/− single knockout mice) knockout, referred to hereafter as DKO (double knockout) strains, all on the C57BL6 background. Mice were genotyped by PCR and housed in the barrier facility in the Center for Comparative Medicine and Research at The Geisel School of Medicine at Dartmouth and were infected in the biohazard facility between the ages of 6–8 weeks. Mice were housed, treated, and euthanized when necessary in accordance with all Federal and University policies.
Animal infection, disease scoring and organ harvest
Mice were anesthetized intraperitoneally with ketamine (87 mg/kg body weight) and xyalazine (13mg/kg). Corneas were bilaterally scarified and mice were inoculated by adding 2 × 106 p.f.u per eye in a 5µl volume (Rader et al., 1993). At indicated times post infection, mice were weighed and scored for disease pathology using the following disease scores: 0 – no visible pathology, 0.5 – minor eyelid swelling, 1.0 – minor eyelid and nasal swelling, 1.5 – moderate eyelid and nasal swelling, 2.0 – severe eyelid swelling, minor periocular hair loss and skin lesions, 2.5 – severe eyelid swelling, moderate periocular hair loss and skin lesions, 3.0 – neurological symptoms. Endpoint criteria for sacrificing mice for mortality include loss of ≥15% of starting body weight, altered locomotion, and labored breathing. Eye swab material was collected for analysis by plaque assay at selected times post infection, as previously described (D A Leib et al., 1989). Mice were sacrificed and trigeminal ganglia, brain stems, brains, livers and spleens were harvested into an appropriate volume of media, mechanically disrupted with either 1 mm or 2.5 mm glass beads, sonicated and titered via standard plaque assay (Menachery et al., 2010).
Bioluminescence imaging
Mice infected with McKrae/Dlux were injected intraperitoneally with 150 µg/g body weight of D-Luciferin potassium salt (GoldBio) in PBS, anaesthetized with 2.5 % isoflurane and imaged (exposure time of 1–60 s, f-stop 1 or 2, field of view 15 or 19.6) with a cooled charge coupled device (CCD) camera based bioluminescence in vitro imaging system (IVIS 100, Caliper Life Sciences, Hopkinton, MA) (Pasieka et al., 2011). For analysis, regions of interest (ROI) were defined manually around eyes, lymph nodes and liver. Background light emission was subtracted from ROIs and photon flux was calculated using Living Image and Igor pro software (Version 2.6).
Cytokine analysis
Blood was harvested from mice at indicated times post infection and plasma was separated by centrifugation. For this experiment, a mouse 32-plex kit (Milipore) was utilized. Samples were diluted 1:1 with assay buffer, and processed as per manufacturers instructions. Cytokines concentrations were determined by comparison to recombinant cytokine standard curves.
Real time reverse transcription PCR of tissue
At the indicated time post infection tissues were harvested into Trizol (Invitrogen). Tissue was homogenized using a tissue blender, RNA was extracted as per manufacturers instructions and DNAse treated with DNA-free (Ambion). cDNA was generated with superscript III reverse transcriptase (Invitrogen) using random hexamers to prime the reaction. Efficiency curves were generated for all primer sets to determine PCR efficiencies of target and reference genes, as described by Pfaffl (Pfaffl, 2001). PCR mixtures were prepared with iQ SYBR green supermix (Biorad), 0.2 – 0.5 µM primers, and 2 µl cDNA. For each amplified PCR product, a single peak was obtained by melting curve analysis, and only a single band of the predicted size was observed by agarose gel electrophoresis. The Pfaffl method (Pfaffl, 2001), which takes PCR efficiencies into account, was used for data analysis.
RESULTS
Bioluminescence imaging of mice infected with HSV-1 McKrae/Dlux
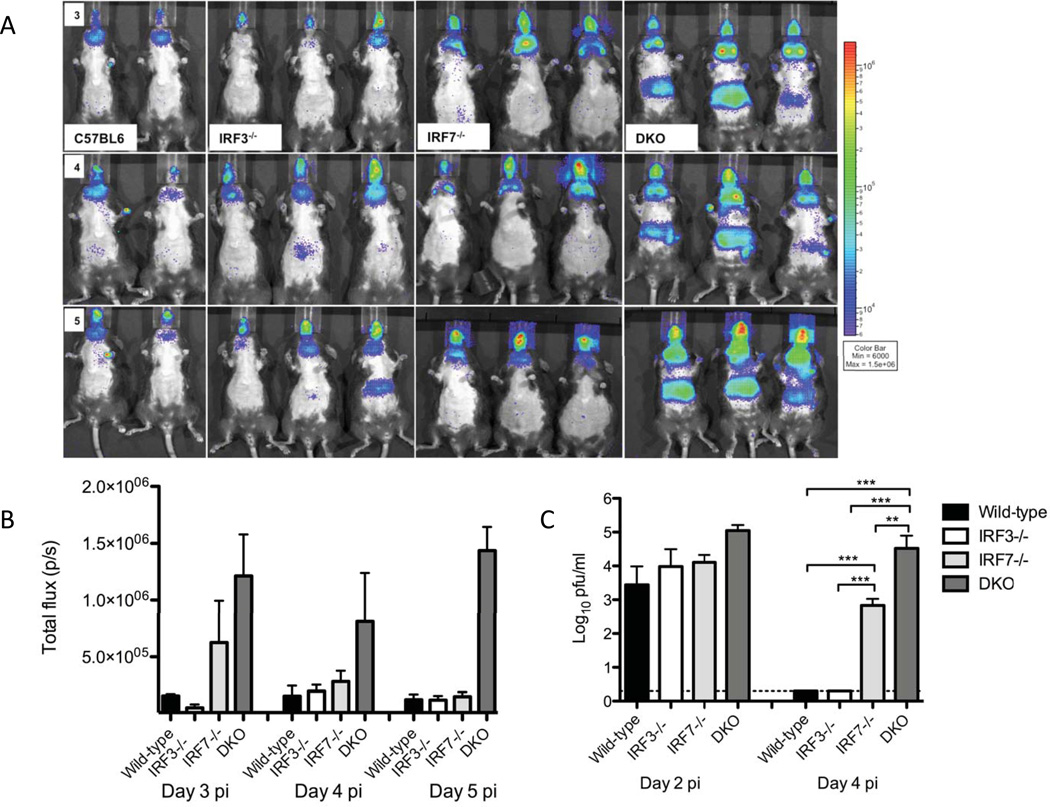
Bioluminescence imaging (BLI) has proved useful in several previous studies (Luker et al., 2003; Pasieka et al., 2011) when attempting to gain an overview of viral pathogenesis in a variety of knockout mice. One issue, however, has been that existing bioluminescent strains in the HSV-1 KOS background are of insufficient virulence to robustly infect mice in the C57BL/6 background. We had, however, observed that strain McKrae had a high rate of lethal infection following corneal challenge of C57BL/6 wild-type and IRF-3−/− mice (Menachery et al., 2010). We wanted to determine the roles played by IRF-3 and IRF-7 when faced with a virulent HSV-1 infection so we created a new luciferase-expressing virus in the McKrae background (McKraeDlux) and performed BLI (Fig. 1A and 1B). Robust photon flux was observed for all mouse strains at 3, 4, and 5 days post-infection, underscoring the utility of McKraeDlux. When taken as a whole, BLI signals were not significantly different between C57BL/6 and the single knockout mice (IRF3−/− , and IRF7−/−)), although there were trends towards higher abdominal signals in the IRF-3−/− mice and higher head/sub-mandibular lymph node signals in the IRF-7−/− mice. In contrast, imaging of the DKO mice revealed significantly higher photon fluxes in lymph node, eye and abdominal regions relative to wild-type and single knockout mice at days 3, 4 and 5 post-infection. Having shown a pattern of increased BLI activity and generalized spread in the DKO we next wished to assess viremia during acute infection (Fig. 1C). All mice showed viremia on day 2, although the DKO mice had almost 10-fold more virus in their serum than wild-type or single knockout mice. Wild-type and IRF-3−/− mice were, however, capable of clearing virus from the bloodstream by day 4. In contrast, the DKO mice were unable to clear virus from the bloodstream with titers remaining relatively constant between days 2 and 4. In this time frame, titers decreased in the blood of IRF-7−/− mice although there was still significant viremia on day 4 post-infection. The widespread viral dissemination observed on day 5 in the DKO mice by BLI, with especially strong signals in the liver, is therefore consistent with their inability to clear virus from the bloodstream.
Figure 1. Pattern of viral spread in wild-type, IRF-3−/−, IRF-7−/− and DKO mice.
(A) In vivo bioluminescent imaging analysis was performed on days 2 through 5 post corneal infection with McKrae/DLux. Daily images for the same mice are shown on identical photon flux scales and are representative of 2 independent experiments. (B) Region of interest analysis of lymph node bioluminescence. ROIs were drawn around the lymph node area using the Living Image and IgorPro software and bioluminescent signal was reported in photons/sec. (C) Viral titers in the blood stream. Serum was isolated from mice two and four days post infection with McKrae/DLux and assayed on vero cell monolayers (n=4 mice per group, 2 independent experiments).
Combined IRF-3 and IRF-7 loss leads to enhanced HSV-1 replication in peripheral tissues
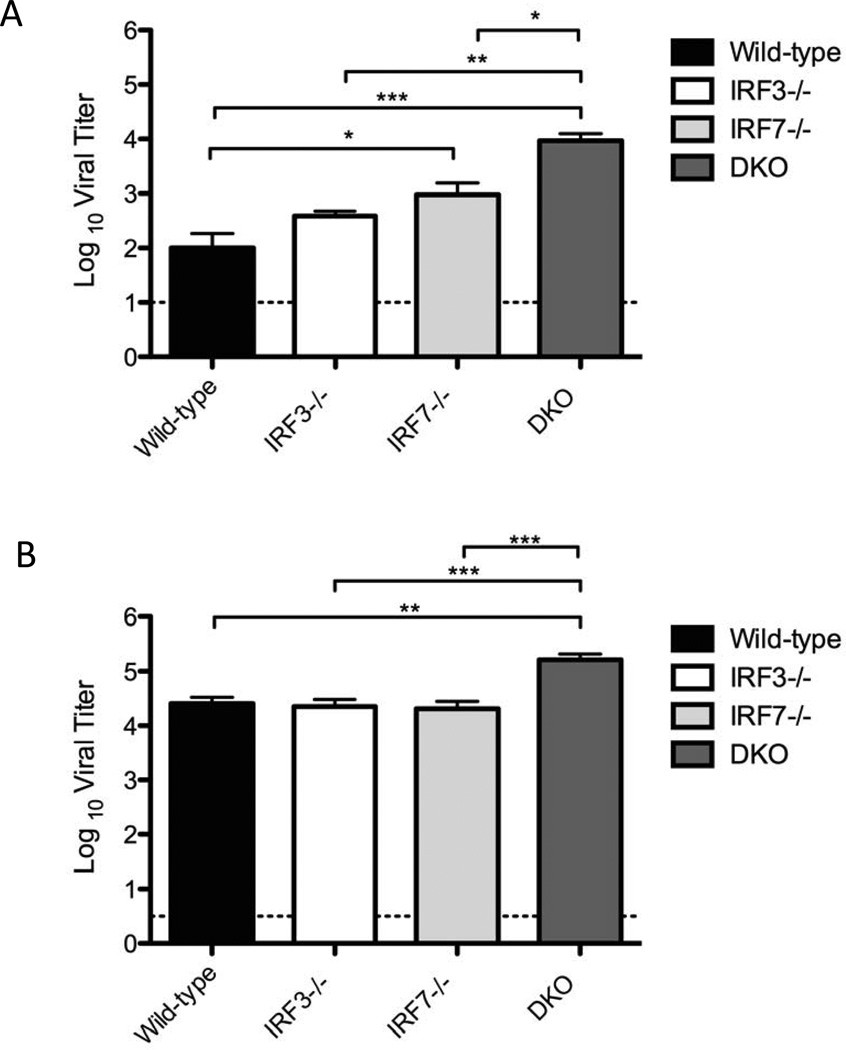
Having established the general patterns of pathogenesis in the DKO mice using BLI, we wished to examine virus replication more specifically in corneas and trigeminal ganglia. For studies which did not require BLI we switched to infection with wild-type McKrae virus, but this led to very rapid death (≤4 days) of the DKO mice (data not shown). We therefore used the less virulent HSV-1 strain 17 for these experiments to allow the study of HSV-1 replication in these tissues over a longer time frame. Analysis of corneal swabs (Fig. 2A) revealed no defect in IRF-3−/− mice for control of HSV-1 replication on either day 2 (data not shown) or day 4 post infection relative to wild-type mice, consistent with previously published data (Menachery et al., 2010). In contrast, HSV replication in corneas of IRF-7−/− mice was significantly increased (p≤0.05) relative to wild-type, and even greater viral loads (p≤0.001) were observed in the corneas of DKO mice. Consistent with this, we found the trigeminal ganglia of DKO infected mice had significantly higher viral loads than wild-type or single knockout mice (Fig. 2B). In contrast to the cornea, IRF-7−/− mice showed no defect in controlling virus replication in the trigeminal ganglia. These data show, consistent with the BLI data, that there is an increase in productive virus infection in corneas and trigeminal ganglia of DKO mice relative to wild-type or single IRF3/7 knock-out mice.
Figure 2. Virus replication in peripheral tissues of wild-type and IRF-3−/−, IRF-7−/−, and IRF3/7−/− (DKO) mice.
Viral titers in (A) cornea, (B) trigeminal ganglia, 4 days following corneal infection with 2 × 106 pfu/eye. Graphs represent averages and standard errors of the mean for several mice from two independent experiments (n ≥ 5 mice per group). The dotted line indicates the threshold of detection. Statistical analysis (ANOVA) was performed using GraphPad Prism 5.0. Asterisks indicate ranges of significance ( * , P ≤ 0.05; ** , P ≤0.01; ***, P ≤0.001).
IRF-3 and IRF-7 are both required for control of disease pathology and lethality
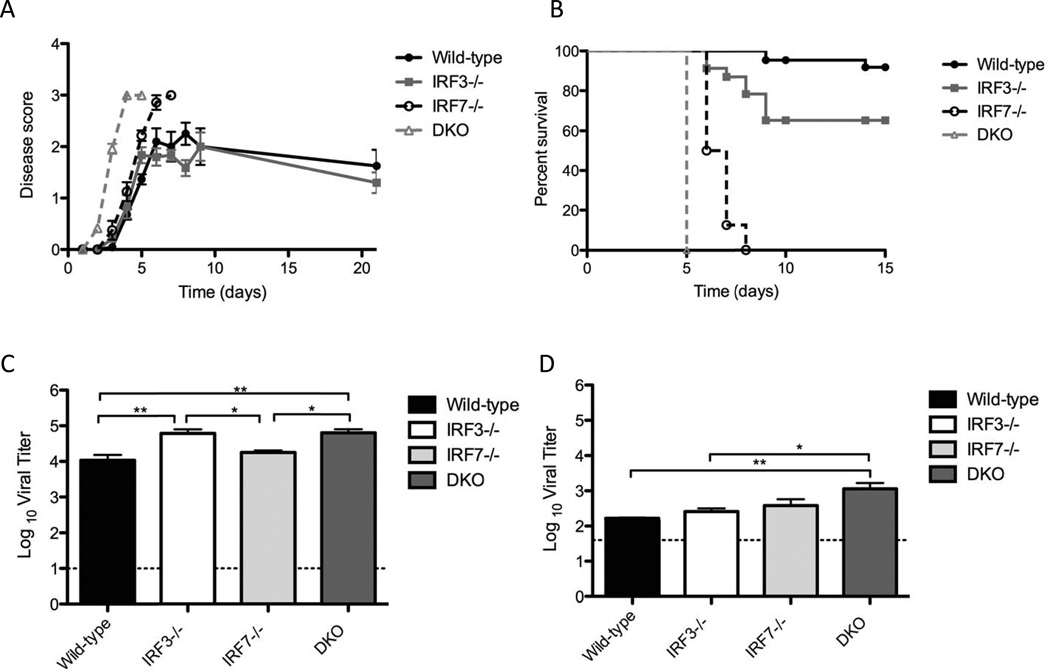
Following infection with HSV-1 strain 17, we monitored mice for development of disease, and for timing to reach endpoint disease criteria (Fig. 3A, 3B). Wild-type and IRF-3−/− mice displayed a similar degree and rate of disease development, with symptoms such as eyelid and nasal swelling reaching maximum levels between 5 and 6 days post infection, and remaining steady, or declining thereafter. IRF-7−/− mice developed symptoms at a similar rate to both the wild-type and IRF-3−/− mice up until 5 days postinfection (Fig 3A). There was, however, a continued increase in disease severity, with all IRF-7−/− mice reaching end-point criteria 6–8 days post-infection (Fig 3B). DKO mice showed an increase in both rate of onset and severity of disease symptoms relative to wild-type or single knockout mice (Fig 3A). Symptoms progressed rapidly and all DKO mice reached end point criteria synchronously on the fifth day postinfection (Fig. 3B). This was 2–3 days earlier than IRF-7 −/− mice, indicating that while IRF-7 may be the critical regulator of IFN responses, IRF-3 becomes critical for controlling HSV-1 disease progression in the absence of IRF-7.
Figure 3. Disease, mortality and CNS replication following HSV-1 replication in C57BL6, IRF-3−/− , IRF-7−/− or IRF-3/7−/− (DKO) mice.
(A) Pathology following HSV-1 infection. Corneas of C57BL6, IRF-3−/− , IRF-7−/− and DKO mice were scarified and inoculated with 2 × 106 pfu/eye. Disease scores were recorded in accordance with parameters detailed in the materials and methods. Data represents disease scores from three independent experiments (n ≥ 12 mice per group). (B) Survival of wild-type, IRF-3−/−, IRF-7−/− and DKO mice following corneal infection with 2 × 106 p.f.u HSV-1 strain 17 per eye. Survival curves were carried out independent of other studies and represent data from three individual experiments. (n ≥ 12 mice per group). Brain stems (C) and brains (D) were harvested at specified days following infection of wild-type, IRF-3−/− , IRF-7−/− and DKO mice with 2 × 10 p.f.u. HSV-1 strain 17 per eye. Graphs represent mean +/− SEM of two independent experiments, (n ≥ 5 mice per group)
We reasoned that the increased mortality in the 1RF7−/− and DKO mice might be due to viral neuroinvasion and encephalitis, as neurological symptoms were present prior to euthanasia. To examine whether death correlated with viral titers within the CNS, we compared viral loads in brain stems and brains of infected mice (Figs. 3C and 3D). Interestingly, we observed small but significant increases in viral loads in the brainstems of IRF-3−/− and DKO mice, relative to wild-type and IRF-7−/− mice. Higher constitutive expression levels of IRF-3 than IRF-7 within CNS tissue (J. E. Christensen et al., 2012; Delhaye et al., 2006; Ousman et al., 2005), supports IRF-3 having a more important role during the early stages of HSV-1 replication within the CNS, prior to induction of IRF-7. Comparison of viral loads within the whole brain revealed that only brains from the DKO mice had significantly higher viral loads than wild-type mice (Fig 3D). These data are consistent with the hypothesis that while IRF-3 may be important in controlling initial entry into the brain stem, IRF-3 and IRF-7 act in concert to limit further virus spread within the brain. That said, it seemed unlikely that loss of IRF-3/7 leading to increased virus replication in the CNS could be the sole explanation for the drastic changes in disease and survival seen in IRF7−/− and DKO mice (Fig 3A, 3B).
Role of IRF-3 and IRF-7 in migration of immune derived cells in vitro and production of cytokines in vivo
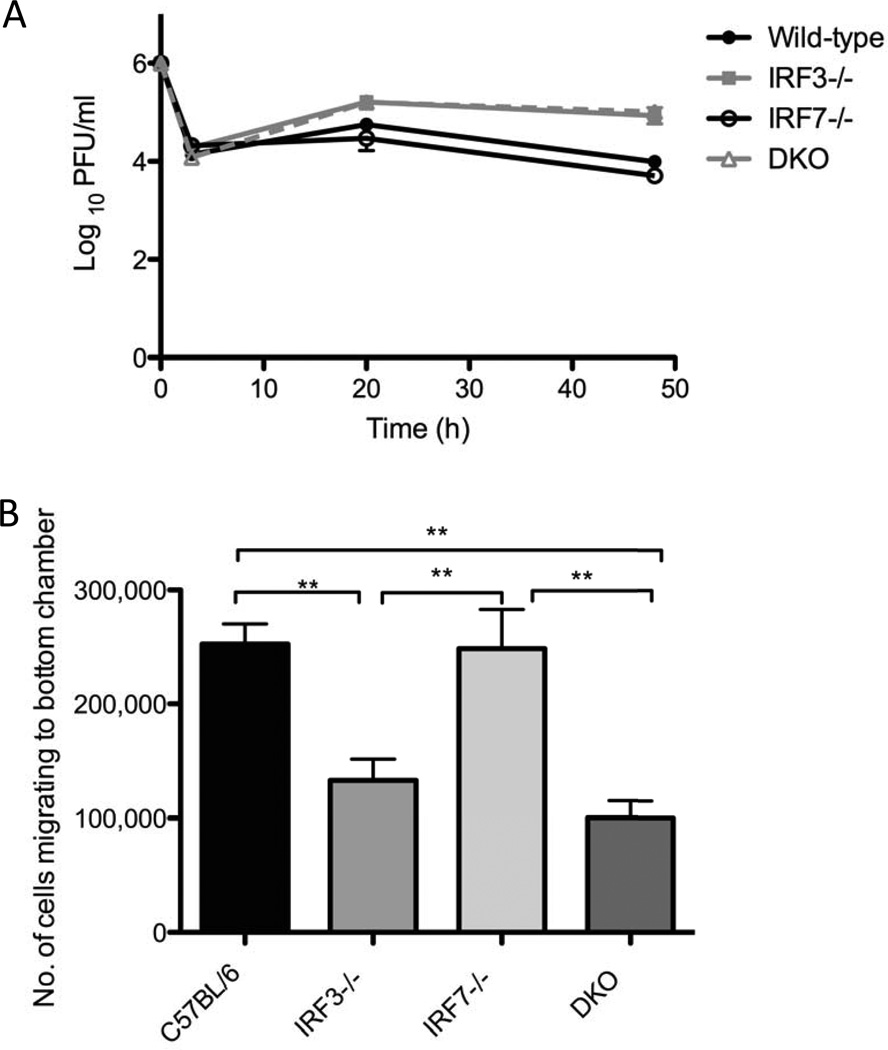
Previous work has demonstrated that while IRF-3 is important for control of HSV-1 replication in immune cells, IRF-7 is dispensable (Menachery and David A. Leib, 2009). Consistent with this, virus replication was increased in IRF-3−/− relative to wild-type and IRF-7−/− BMDCs, and no additional increases in replication were observed in the DKO BMDCs (Fig.4A). In addition to impairments in controlling viral replication, it has been shown that dendritic cells of IRF-3−/− mice are impaired in their ability to migrate to lymph nodes relative to wild-type DCs, possibly leading to sub-optimal priming of the adaptive immune response (Marichal et al., 2010). Using an in vitro transwell system, we investigated the ability of wild-type, IRF-3−/−, IRF-7−/− and DKO BMDCs to migrate towards CCL19, a chemokine expressed abundantly in lymph nodes. As expected, IRF-3−/− BMDCs showed defective migration (Fig. 4B). IRF-7−/− BMDCs exhibited no defect in migration relative to wild-type, and DKO BMDCs had no additional migratory defects above those observed in IRF-3−/− BMDCs, indicating that any defects in migration are due solely to loss of IRF-3.
Figure 4. HSV replication in wild-type and IRF-3−/−IRF-7−/−and IRF3/7−/−(DKO) BMDCs and impact on DC migration.
(A) In vitro replication in bone marrow derived dendritic cells. Primary BMDCs were infected with HSV-1 strain 17 at MOI 1. At indicated times post infection, cell supernatants were harvested and viral titers assayed on vero cell monolayers. Results shown represent the mean +/− SEM of two independent experiments, each carried out in triplicate. (B) Migratory ability of primary BMDCs. The ability of wild-type, single and DKO BMDCs to migrate towards the chemokine CCL19 was determined by counting the number of cells which migrated through a 2 µm pore size polycarbonate filter of a transwell plate. Results shown represent one experiment carried out in duplicate. The experiment was repeated twice with identical results. Statistical analysis (ANOVA) was performed using GraphPad Prism 5.0. (* , P ≤ 0.05; ** , P ≤ 0.01; ***, P ≤ 0.001).
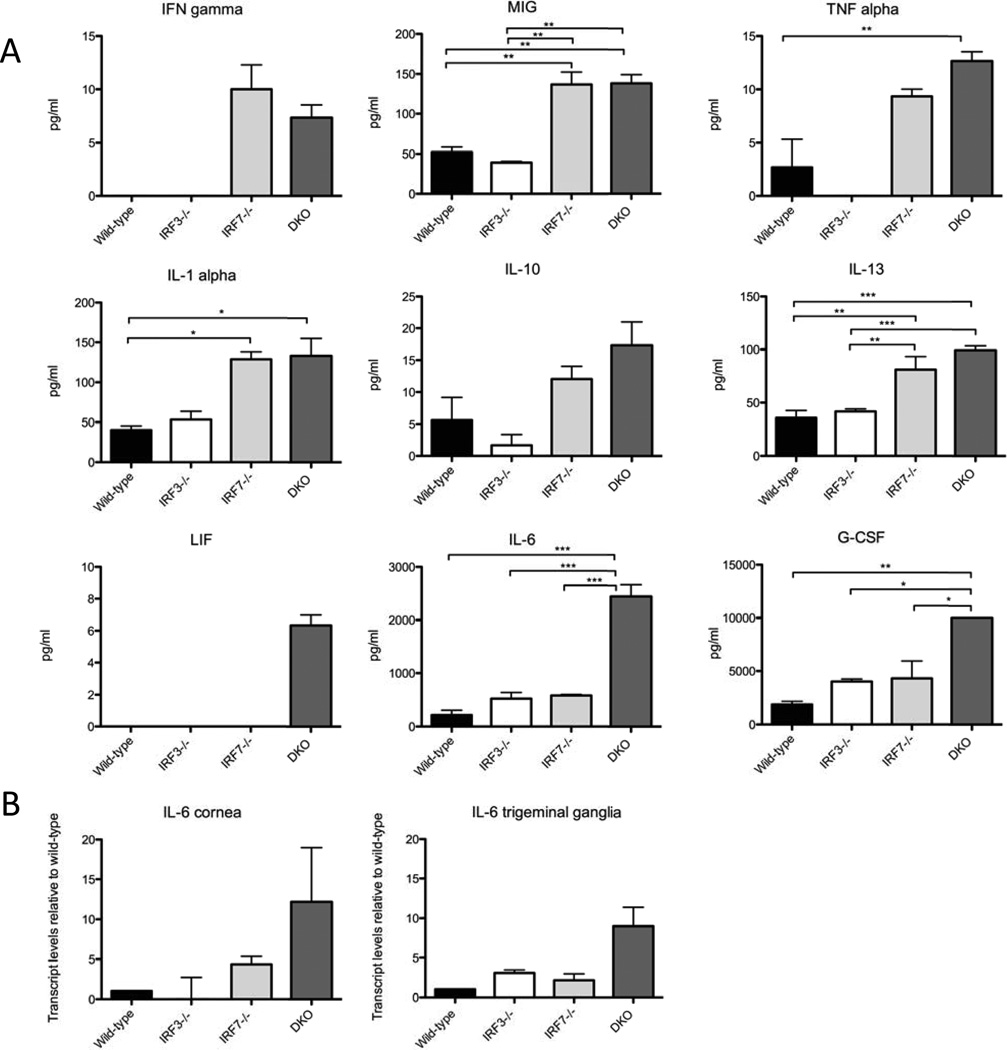
To examine whether increased disease pathology in the DKO mice correlated with altered cytokine levels, we examined plasma of mock and HSV-1 infected wild-type, IRF-3−/−, IRF-7−/− and DKO mice with a BioRad Bio-Plex multiple cytokine assay (Fig. 5). While many cytokines showed elevated expression during infection with HSV-1 we focused on those which were elevated in the IRF-7−/− and DKO populations, compared to control and IRF-3−/− mice. Disruption of interferon signaling can inhibit the IFNγ negative feedback loop, leading to increased expression of the pro-inflammatory cytokine IFNγ (Ben-Asouli et al., 2002; Kaempfer, 2006). In agreement with this, IFNγ was only detectable in IRF-7−/− and DKO mice, and expression of MIG (Farber, 1997), a biomarker for IFNγ activity was also higher in IRF-7−/− and DKO mice (Fig 5A). TNFα and IL-1α, pro-inflammatory cytokines which play roles in development of stromal keratitis (Keadle et al., 2000), were both elevated in IRF-7−/− and DKO relative to wild-type and IRF-3−/− mice. Interestingly, some anti-inflammatory cytokines, IL-10 and IL-13, were also expressed at higher levels in IRF7−/− and DKO mice than wild-type and IRF3−/− mice, although the significance of this is unclear (Fig 5A).
Figure 5. Cytokine analysis from sera of wild-type, IRF-3−/−, IRF-7−/−, and IRF3/7−/− mice following HSV infection.
(A) Multiplex cytokine analysis of plasma from HSV-1 infected wild- type, IRF3−/−IRF7−/− and DKO mice. Mice were infected by corneal scarification and blood was harvested 4 days pi. Average concentrations from groups of 3 mice are reported as pg/ml. Mock samples were omitted from the graphs for clarity. (B) Transcript levels of IL-6 within corneal and trigeminal tissue. RNA was extracted from corneas and trigeminal ganglia of wild-type, IRF-3−/− , IRF-7−/− and DKO mice 4 days post infection with HSV-1 strain 17. After conversion to cDNA, transcript levels of IL-6 in knockout mice were compared to levels in wild-type mice. Graphs represent means (+/− SEM) from three biological replicates. Each biological replicate was subjected to three technical replicates.
While the elevated levels of the above-mentioned cytokines likely contribute to the altered pathology observed in infected mice, their levels were largely equivalent in IRF-7−/− and DKO mice, suggesting that other factors likely contribute to the especially rapid lethality observed in the DKO mice. Leukemia inhibitory factor (LIF), a member of the IL-6 family of proteins with pro- and anti-inflammatory properties (Mathieu et al., 2012), and two pro-inflammatory cytokines, G-CSF and IL-6, had notably high expression levels in DKO mice relative to IRF-7−/− mice: (Fig 5A). Elevated levels of G-CSF mobilize cells from bone marrow and stimulate prolonged survival, leading to increased numbers of circulating neutrophils (Lawlor et al., 2004; Xu et al., 1996), while increased IL-6 production in IFN signaling deficient mice has previously been associated with increased neutrophil infiltration and corneal inflammation during HSV-1 infection (Pasieka et al., 2009). Corneas from DKO mice showed increased opacity post infection with HSV-1, and real time PCR of corneal tissues confirmed that transcript levels of IL-6 mRNA were 12 fold higher in DKO mice relative to wild-type infected mice, while they were only 4 fold higher in IRF-7−/− mice relative to wild-type mice (Fig 5B). IL-6 transcript levels were also higher in trigeminal ganglia of DKO mice relative to wild-type mice, than in trigeminal ganglia of IRF-7−/− mice (Fig. 5B), confirming an elevated IL-6 signaling response in the peripheral tissues of DKO mice.
DISCUSSION
The importance of a timely and measured type 1 IFN response in the control of HSV-1 infection is well-established (C D Conrady et al., 2012; Christopher D Conrady et al., 2011; Ishikawa and Barber, 2008; Lundberg et al., 2008; Pasieka et al., 2009, 2008), and defects in the IFN signaling pathway can lead to life-threatening conditions such as encephalitis (Lafaille et al., 2012; McGrath et al., 1997; Kenneth L Tyler, 2004). Interferon regulatory factors 3 and 7 are central to the establishment of this response (Daffis et al., 2008; Honda et al., 2005; Juang et al., 1998; Marié et al., 1998; M Sato et al., 1998), and this study demonstrates that a combined deficiency of IRF-3 and IRF-7 results in a rapid onset, severity and progression of disease pathology, along with increased mortality following infection with HSV-1. That said, the deletion of IRF-7 alone had a significant impact on pathogenesis, especially on viremia and mortality, and the impact of IRF-7 loss was greater than the impact of loss of IRF-3 alone. This non-redundancy of IRF-3 and 7 is similar to the pattern observed for West Nile virus (Daffis et al., 2008), but distinct from observations with Chikungunya and murine norovirus (Schilte et al., 2012; Thackray et al., 2012) in which significant changes in susceptibility were observed only after deletion of both IRF-3 and −7.
Despite evidence for a dominant role for IRF-7, this study has revealed that IRF-3 and IRF-7 act synergistically to exert control over viral replication, with significantly elevated viral burdens in all tissues examined in DKO relative to single knockout mice. Altered tissue tropism and elevated levels of viremia were also observed, correlating with a previous study characterizing WNV infection of DKO mice (Daffis et al., 2009) . Although 100% mortality was observed in DKO mice in both studies, the earlier invasion of virus into the CNS and the lethal uncontrolled viral replication that was observed during WNV infection was not recapitulated during HSV infection. Based on the BLI study it appears more likely that the susceptibility of the DKO mice is largely due to the profound viscerotropism of HSV-1 in the absence of IRF-3 and −7, consistent with the pattern of HSV infection in IFNR−/− mice (Luker et al., 2003). Such patterns of infection are accompanied with alterations in AST/ALT levels within the livers of both experimental mice and in humans resulting in herpetic hepatitis and fatal liver failure (Minuk and Nicolle, 1986; Norvell et al., 2007) .
Previous studies have demonstrated that loss of critical components of the IFN signaling pathway, such as STAT1, can lead to enhanced inflammation and pathology during infection with HSV-1, and such disease can be independent of changes in viral titers (Lundberg et al., 2008; Pasieka et al., 2009, 2008). Several studies have also reported that IRF-3 has anti-inflammatory properties which may help control immune-mediated pathology (Hua et al., 2002; Suh et al., 2009; Tarassishin et al., 2011a, 2011b). These studies therefore prompted us to investigate in this study whether a dysfunctional immune response was contributing to the observed pathology and mortality. Consistent with this idea, cytokine profiling revealed higher levels of systemic pro-inflammatory cytokines in 1RF7−/− and DKO mice relative to wild-type and IRF-3−/− mice. IL-6 and G-CSF, which lead to increased infiltration of immune cells into tissues and increased pathology (Fielding et al., 2008; Lawlor et al., 2004; Xu et al., 1996; Yong, 1996) have previously been implicated in the development of herpes stromal keratitis (Divito and Hendricks, 2008; Fenton et al., 2002; Inoue, 2008). Consistent with this, we observed high levels of IL-6 transcripts in the corneas of DKO mice, indicating that it likely contributes to the ocular and periocular pathology that occurs during infection in these mice.
Studies have also shown a negative correlation between IL-6 levels and survival of herpes simplex encephalitis (Bociaga-Jasik et al., 2011). Additionally, elevated levels of LIF (Auernhammer and Melmed, 2000; Villers et al., 1995), IL-6 (Damas et al., 1997; Ebong et al., 1999) and G-CSF have all been reported during the development of systemic inflammatory response syndrome, or sepsis. Sepsis is characterized by an initial hyper-inflammatory response to infection, which can be followed by a period of immune suppression (Hotchkiss et al., 2009), and is associated with development of organ dysfunction, hypoperfusion or hypotension and intravascular coagulation (Sriskandan and Altmann, 2008). Anti-inflammatory cytokines are also produced during sepsis as the host attempts to modulate the inflammatory response (Marchant et al., 1994), perhaps explaining our observation of elevated levels of IL-10 and IL-13. Interestingly, the pathophysiology of neonatal HSV infection is typical of a systemic inflammatory response, and it has been reported that neonates, whose immune systems are immature in comparison to the adult immune system, produce higher quantities of pro-inflammatory cytokines such as IL-6 in response to HSV challenge. This correlates well with our observations that deficiencies in the innate immune response lead to uncontrolled inflammation and sepsis, and indicate that this may be a useful model for further study of neonatal HSV pathology.
While our data supports the development of a hyper-inflammatory state in IRF-3/7−/− deficient mice, it contrasts with a previous study which reported increased expression of transcripts encoding multiple inflammatory markers within the CNS of STAT1−/− mice with an accompanying breach of the blood brain barrier (Pasieka et al., 2009). In this study we analyzed expression of cytokines and markers of inflammation in brain stem tissues. Surprisingly, we found no evidence of further elevation of proinflammatory cytokines in the brains of the DKO mice relative to the other mice (data not shown). We also found no evidence of increased cellular infiltrates or breach of the blood-brain barrier (data not shown). These data suggest that the elevated cytokines observed in the DKO mice are causing their damage outside of the CNS, most likely coupled with the significant generalized infection visualized through the BLI experiments. In the absence of IRF-3 and IRF-7, it is possible that the initial hyperinflammatory phase of sepsis is so rapid that death ensues before the development of breach of the BBB.
In summary, our study has revealed that while IRF-7 is the dominant factor in the control of HSV-induced disease and mortality, IRF-3 and IRF-7 act synergistically to control viral replication, with loss of both leading to higher viral loads. We also demonstrate that they play an essential role in modulation of inflammatory responses during infection with HSV, preventing the development of severe systemic inflammation. It is likely that a combination of increased viral replication and markedly increased levels of pro-inflammatory cytokines such as G-CSF and IL-6 in the DKO mice are responsible for the increased disease and mortality observed. These mice will therefore likely prove a useful model for future studies of sepsis and immune pathology.
Highlights.
We compare HSV-1 infection of IRF-3, IRF-7, and IRF-3/7 (DKO)-deficient mice.
DKO develop visceral disease and succumb rapidly to infection.
IRF-7 was more critical than IRF-3 for protection of mice from fatal infection.
Both factors are critical for modulating the inflammatory responses to infection.
ACKNOWLEDGEMENTS
National Institutes of Health grants to D.A.L (RO1 EY09083) and a Hitchcock Foundation award to A.A.M supported this study. Cytokine analysis was carried out at the Geisel School of Medicine at Dartmouth in the Immunoassays and Flow Cytometry Shared Resource, which was established by equipment grants from the Fannie E. Rippel Foundation, the NIH Shared Instrument Program, and the Geisel School of Medicine at Dartmouth and is supported in part by a Core Grant (CA 23108) from the National Cancer Institute to the Norris Cotton Cancer Center and grants from the National Center for Research Resources (5P30RR032136-02) and the National Institute of General Medical Sciences (8 P30 GM103415-02) from the National Institutes of Health to Dartmouth's Center for Molecular, Cellular, and Translational Immunological Research.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Al Midani A, Pinney J, Field N, Atkinson C, Haque T, Harber M. Fulminant hepatitis following primary herpes simplex virus infection. Saudi J Kidney Dis Transpl. 2011;22:107–111. [PubMed] [Google Scholar]
- Alexander DE, Ward SL, Mizushima N, Levine B, Leib David A. Analysis of the Role of Autophagy in Replication of Herpes Simplex Virus in Cell Culture. J. Virol. 2007;81:12128–12134. doi: 10.1128/JVI.01356-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auernhammer CJ, Melmed S. Leukemia-Inhibitory Factor—Neuroimmune Modulator of Endocrine Function. Endocrine Reviews. 2000;21:313–345. doi: 10.1210/edrv.21.3.0400. [DOI] [PubMed] [Google Scholar]
- Baringer JR. Herpes simplex infections of the nervous system. Neurol Clin. 2008;26:657–674. viii. doi: 10.1016/j.ncl.2008.03.005. [DOI] [PubMed] [Google Scholar]
- Behrens-Baumann W. [Herpes simplex keratitis. A short overview of the current therapy] Klin Monbl Augenheilkd. 2010;227:388–392. doi: 10.1055/s-0029-1245289. [DOI] [PubMed] [Google Scholar]
- Ben-Asouli Y, Banai Y, Pel-Or Y, Shir A, Kaempfer R. Human Interferon-γ mRNA Autoregulates Its Translation through a Pseudoknot that Activates the Interferon-Inducible Protein Kinase PKR. Cell. 2002;108:221–232. doi: 10.1016/s0092-8674(02)00616-5. [DOI] [PubMed] [Google Scholar]
- Bociaga-Jasik M, Cieśla A, Kalinowska-Nowak A, Skwara P, Garlicki A, Mach T. Role of IL-6 and neopterin in the pathogenesis of herpetic encephalitis. Pharmacol Rep. 2011;63:1203–1209. doi: 10.1016/s1734-1140(11)70640-5. [DOI] [PubMed] [Google Scholar]
- Brochet B, Henry P, Piquemal-Baluard A, Dupasquier P. Recurrent herpetic encephalitis. Rev. Neurol. (Paris) 1990;146:450–454. [PubMed] [Google Scholar]
- Brown SM, Ritchie DA, Subak-Sharpe JH. Genetic studies with herpes simplex virus type 1. The isolation of temperature-sensitive mutants, their arrangement into complementation groups and recombination analysis leading to a linkage map. J. Gen. Virol. 1973;18:329–346. doi: 10.1099/0022-1317-18-3-329. [DOI] [PubMed] [Google Scholar]
- Bürckstümmer T, Baumann C, Blüml S, Dixit E, Dürnberger G, Jahn H, Planyavsky M, Bilban M, Colinge J, Bennett KL, Superti-Furga G. An orthogonal proteomic-genomic screen identifies AIM2 as a cytoplasmic DNA sensor for the inflammasome. Nat. Immunol. 2009;10:266–272. doi: 10.1038/ni.1702. [DOI] [PubMed] [Google Scholar]
- Chou J, Chen JJ, Gross M, Roizman B. Association of a M(r) 90,000 phosphoprotein with protein kinase PKR in cells exhibiting enhanced phosphorylation of translation initiation factor eIF-2 alpha and premature shutoff of protein synthesis after infection with gamma 134.5- mutants of herpes simplex virus 1. Proc Natl Acad Sci U S A. 1995;92:10516–10520. doi: 10.1073/pnas.92.23.10516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen JE, Fenger C, Issazadeh-Navikas S, Krug A, Liljestrøm P, Goriely S, Paludan Søren Riis, Finsen B, Christensen JP, Thomsen AR. Differential Impact of Interferon Regulatory Factor 7 in Initiation of the Type I Interferon Response in the Lymphocytic Choriomeningitis Virus-Infected Central Nervous System versus the Periphery. J. Virol. 2012;86:7384–7392. doi: 10.1128/JVI.07090-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conrady CD, Zheng M, Fitzgerald KA, Liu C, Carr DJJ. Resistance to HSV-1 infection in the epithelium resides with the novel innate sensor, IFI-16. Mucosal Immunol. 2012;5:173–183. doi: 10.1038/mi.2011.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conrady Christopher D, Jones H, Zheng Min, Carr Daniel JJ. A Functional Type I Interferon Pathway Drives Resistance to Cornea Herpes Simplex Virus Type 1 Infection by Recruitment of Leukocytes. J Biomed Res. 2011;25:111–119. doi: 10.1016/S1674-8301(11)60014-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daffis S, Samuel MA, Suthar MS, Keller BC, Gale M, Diamond MS. Interferon Regulatory Factor IRF-7 Induces the Antiviral Alpha Interferon Response and Protects against Lethal West Nile Virus Infection. J Virol. 2008;82:8465–8475. doi: 10.1128/JVI.00918-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daffis S, Suthar MS, Szretter KJ, Gale M, Diamond MS. Induction of IFN-β and the Innate Antiviral Response in Myeloid Cells Occurs through an IPS-1-Dependent Signal That Does Not Require IRF-3 and IRF-7. PLoS Pathog. 2009:5. doi: 10.1371/journal.ppat.1000607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damas P, Canivet JL, de Groote D, Vrindts Y, Albert A, Franchimont P, Lamy M. Sepsis and serum cytokine concentrations. Crit. Care Med. 1997;25:405–412. doi: 10.1097/00003246-199703000-00006. [DOI] [PubMed] [Google Scholar]
- Delhaye S, Paul S, Blakqori G, Minet M, Weber F, Staeheli P, Michiels T. Neurons produce type I interferon during viral encephalitis. Proc Natl Acad Sci U S A. 2006;103:7835–7840. doi: 10.1073/pnas.0602460103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Divito SJ, Hendricks RL. Activated Inflammatory Infiltrate in HSV-1–Infected Corneas without Herpes Stromal Keratitis. Invest Ophthalmol Vis Sci. 2008;49:1488–1495. doi: 10.1167/iovs.07-1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebong S, Call D, Nemzek J, Bolgos G, Newcomb D, Remick D. Immunopathologic Alterations in Murine Models of Sepsis of Increasing Severity. Infect. Immun. 1999;67:6603–6610. doi: 10.1128/iai.67.12.6603-6610.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farber JM. Mig and IP-10: CXC chemokines that target lymphocytes. J. Leukoc Biol. 1997;61:246–257. [PubMed] [Google Scholar]
- Fenton RR, Molesworth-Kenyon S, Oakes JE, Lausch RN. Linkage of IL-6 with neutrophil chemoattractant expression in virus-induced ocular inflammation. Invest. Ophthalmol. Vis. Sci. 2002;43:737–743. [PubMed] [Google Scholar]
- Fielding CA, McLoughlin RM, McLeod L, Colmont CS, Najdovska M, Grail D, Ernst M, Jones SA, Topley N, Jenkins BJ. IL-6 regulates neutrophil trafficking during acute inflammation via STAT3. J. Immunol. 2008;181:2189–2195. doi: 10.4049/jimmunol.181.3.2189. [DOI] [PubMed] [Google Scholar]
- Fu XY, Schindler C, Improta T, Aebersold R, Darnell JE., Jr The proteins of ISGF-3, the interferon alpha-induced transcriptional activator, define a gene family involved in signal transduction. Proc. Natl. Acad. Sci U.S.A. 1992;89:7840–7843. doi: 10.1073/pnas.89.16.7840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo Y, Audry M, Ciancanelli M, Alsina L, Azevedo J, Herman M, Anguiano E, Sancho-Shimizu V, Lorenzo L, Pauwels E, Philippe PB, Pérez de Diego R, Cardon A, Vogt G, Picard C, Andrianirina ZZ, Rozenberg F, Lebon P, Plancoulaine S, Tardieu M, Valérie Doireau Jouanguy E, Chaussabel D, Geissmann Frederic, Abel L, Casanova J-L, Zhang S-Y. Herpes simplex virus encephalitis in a patient with complete TLR3 deficiency: TLR3 is otherwise redundant in protective immunity. J. Exp. Med. 2011;208:2083–2098. doi: 10.1084/jem.20101568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He B, Gross Martin, Roizman Bernard. The γ134.5 protein of herpes simplex virus 1 complexes with protein phosphatase 1 αto dephosphorylate the α subunit of the eukaryotic translation initiation factor 2 and preclude the shutoff of protein synthesis by double-stranded RNA-activated protein kinase. Proc Natl Acad Sci U S A. 1997;94:843–848. doi: 10.1073/pnas.94.3.843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiscott J, Pitha P, Genin P, Nguyen H, Heylbroeck C, Mamane Y, Algarte M, Lin R. Triggering the interferon response: the role of IRF-3 transcription factor. J. Interferon Cytokine Res. 1999;19:1–13. doi: 10.1089/107999099314360. [DOI] [PubMed] [Google Scholar]
- Honda K, Yanai H, Negishi H, Asagiri M, Sato Mitsuharu, Mizutani T, Shimada N, Ohba Y, Takaoka A, Yoshida N, Taniguchi Tadatsugu. IRF-7 is the master regulator of type-I interferon-dependent immune responses. Nature. 2005;434:772–777. doi: 10.1038/nature03464. [DOI] [PubMed] [Google Scholar]
- Hotchkiss RS, Coopersmith CM, McDunn JE, Ferguson TA. The sepsis seesaw: tilting toward immunosuppression. Nature Medicine. 2009;15:496–497. doi: 10.1038/nm0509-496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsiao C-H, Yeung L, Yeh L-K, Kao L-Y, Tan H-Y, Wang N-K, Lin K-K, Ma DHK. Pediatric herpes simplex virus keratitis. Cornea. 2009;28:249–253. doi: 10.1097/ICO.0b013e3181839aee. [DOI] [PubMed] [Google Scholar]
- Hua LL, Kim M-O, Brosnan CF, Lee SC. Modulation of astrocyte inducible nitric oxide synthase and cytokine expression by interferon β is associated with induction and inhibition of interferon y-activated sequence binding activity. Journal of Neurochemistry. 2002;83:1120–1128. doi: 10.1046/j.1471-4159.2002.01226.x. [DOI] [PubMed] [Google Scholar]
- Inoue Y. Immunological aspects of herpetic stromal keratitis. Semin Ophthalmol. 2008;23:221–227. doi: 10.1080/08820530802111390. [DOI] [PubMed] [Google Scholar]
- Ishikawa H, Barber GN. STING is an endoplasmic reticulum adaptor that facilitates innate immune signalling. Nature. 2008;455:674–678. doi: 10.1038/nature07317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishikawa H, Ma Z, Barber GN. STING regulates intracellular DNA-mediated, type I interferon-dependent innate immunity. Nature. 2009;461:788–792. doi: 10.1038/nature08476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson KE, Song B, Knipe David M. Role for herpes simplex virus 1 ICP27 in the inhibition of type I interferon signaling. Virology. 2008;374:487–494. doi: 10.1016/j.virol.2008.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juang YT, Lowther W, Kellum M, Au WC, Lin R, Hiscott J, Pitha PM. Primary activation of interferon A and interferon B gene transcription by interferon regulatory factor 3. Proc. Natl. Acad. Sci. U.S.A. 1998;95:9837–9842. doi: 10.1073/pnas.95.17.9837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaempfer R. Interferon- mRNA attenuates its own translation by activating PKR: A molecular basis for the therapeutic effect of interferon- in multiple sclerosis. Cell Research. 2006;16:148–153. doi: 10.1038/sj.cr.7310020. [DOI] [PubMed] [Google Scholar]
- Kang D, Gopalkrishnan RV, Wu Q, Jankowsky E, Pyle AM, Fisher PB. mda-5: An interferon-inducible putative RNA helicase with double-stranded RNA-dependent ATPase activity and melanoma growth-suppressive properties. Proc Natl Acad Sci U S A. 2002;99:637–642. doi: 10.1073/pnas.022637199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keadle TL, Usui N, Laycock KA, Miller Judith Kelvin, Pepose Jay S, Stuart PM. IL-1 and TNF-α Are Important Factors in the Pathogenesis of Murine Recurrent Herpetic Stromal Keratitis. IOVS. 2000;41:96–102. [PubMed] [Google Scholar]
- Lafaille FG, Pessach IM, Zhang S-Y, Ciancanelli MJ, Herman M, Abhyankar A, Ying S-W, Keros S, Goldstein PA, Mostoslavsky G, Ordovas-Montanes J, Jouanguy E, Plancoulaine S, Tu E, Elkabetz Y, Al-Muhsen S, Tardieu M, Schlaeger TM, Daley GQ, Abel L, Casanova J-L, Studer L, Notarangelo LD. Impaired intrinsic immunity to HSV-1 in human iPSC-derived TLR3-deficient CNS cells. Nature. 2012;491:769–773. doi: 10.1038/nature11583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawlor KE, Campbell IK, Metcalf D, O’Donnell K, van Nieuwenhuijze A, Roberts AW, Wicks IP. Critical role for granulocyte colony-stimulating factor in inflammatory arthritis. Proc Natl Acad Sci U S A. 2004;101:11398–11403. doi: 10.1073/pnas.0404328101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leib DA, Coen DM, Bogard CL, Hicks KA, Yager DR, Knipe DM, Tyler KL, Schaffer PA. Immediate-early regulatory gene mutants define different stages in the establishment and reactivation of herpes simplex virus latency. J Virol. 1989;63:759–768. doi: 10.1128/jvi.63.2.759-768.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemaitre B, Nicolas E, Michaut L, Reichhart JM, Hoffmann JA. The dorsoventral regulatory gene cassette spätzle/Toll/cactus controls the potent antifungal response in Drosophila adults. Cell. 1996;86:973–983. doi: 10.1016/s0092-8674(00)80172-5. [DOI] [PubMed] [Google Scholar]
- Levy DE, García-Sastre A. The virus battles: IFN induction of the antiviral state and mechanisms of viral evasion. Cytokine Growth Factor Rev. 2001;12:143–156. doi: 10.1016/s1359-6101(00)00027-7. [DOI] [PubMed] [Google Scholar]
- Lin R, Heylbroeck C, Pitha PM, Hiscott J. Virus-dependent phosphorylation of the IRF-3 transcription factor regulates nuclear translocation, transactivation potential, and proteasome-mediated degradation. Mol. Cell. Biol. 1998;18:2986–2996. doi: 10.1128/mcb.18.5.2986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin Rongtuan, Noyce RS, Collins SE, Everett RD, Mossman KL. The Herpes Simplex Virus ICP0 RING Finger Domain Inhibits IRF3- and IRF7-Mediated Activation of Interferon-Stimulated Genes. J Virol. 2004;78:1675–1684. doi: 10.1128/JVI.78.4.1675-1684.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luker GD, Prior JL, Song J, Pica CM, Leib David A. Bioluminescence imaging reveals systemic dissemination of herpes simplex virus type 1 in the absence of interferon receptors. J. Virol. 2003;77:11082–11093. doi: 10.1128/JVI.77.20.11082-11093.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundberg P, Ramakrishna C, Brown J, Tyszka JM, Hamamura M, Hinton DR, Kovats S, Nalcioglu O, Weinberg K, Openshaw H, Cantin EM. The Immune Response to Herpes Simplex Virus Type 1 Infection in Susceptible Mice Is a Major Cause of Central Nervous System Pathology Resulting in Fatal Encephalitis. J Virol. 2008;82:7078–7088. doi: 10.1128/JVI.00619-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchant A, Devière J, Byl B, De Groote D, Vincent JL, Goldman M. Interleukin-10 production during septicaemia. Lancet. 1994;343:707–708. doi: 10.1016/s0140-6736(94)91584-9. [DOI] [PubMed] [Google Scholar]
- Marichal T, Bedoret D, Mesnil C, Pichavant M, Goriely S, Trottein F, Cataldo D, Goldman Michel, Lekeux P, Bureau F, Desmet CJ. Interferon response factor 3 is essential for house dust mite-induced airway allergy. Journal of Allergy and Clinical Immunology. 2010;126:836–844. e13. doi: 10.1016/j.jaci.2010.06.009. [DOI] [PubMed] [Google Scholar]
- Marié I, Durbin JE, Levy DE. Differential viral induction of distinct interferon-alpha genes by positive feedback through interferon regulatory factor-7. EMBO J. 1998;17:6660–6669. doi: 10.1093/emboj/17.22.6660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathieu M-E, Saucourt C, Mournetas V, Gauthereau X, Thézé N, Praloran V, Thiébaud P, Bœuf H. LIF-Dependent Signaling: New Pieces in the Lego. Stem Cell Rev. 2012;8:1–15. doi: 10.1007/s12015-011-9261-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGrath N, Anderson NE, Croxson MC, Powell KF. Herpes simplex encephalitis treated with acyclovir: diagnosis and long term outcome. J. Neurol. Neurosurg. Psychiatr. 1997;63:321–326. doi: 10.1136/jnnp.63.3.321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melroe GT, DeLuca NA, Knipe David M. Herpes Simplex Virus 1 Has Multiple Mechanisms for Blocking Virus-Induced Interferon Production. J. Virol. 2004;78:8411–8420. doi: 10.1128/JVI.78.16.8411-8420.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menachery VD, Leib David A. Control of Herpes Simplex Virus Replication Is Mediated through an Interferon Regulatory Factor 3-Dependent Pathway. J. Virol. 2009;83:12399–12406. doi: 10.1128/JVI.00888-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menachery VD, Pasieka TJ, Leib David A. Interferon Regulatory Factor 3-Dependent Pathways Are Critical for Control of Herpes Simplex Virus Type 1 Central Nervous System Infection. J. Virol. 2010;84:9685–9694. doi: 10.1128/JVI.00706-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minuk GY, Nicolle LE. Genital herpes and hepatitis in healthy young adults. J. Med. Virol. 1986;19:269–275. doi: 10.1002/jmv.1890190309. [DOI] [PubMed] [Google Scholar]
- Mogensen KE, Lewerenz M, Reboul J, Lutfalla G, Uzé G. The type I interferon receptor: structure, function, and evolution of a family business. J. Interferon Cytokine Res. 1999;19:1069–1098. doi: 10.1089/107999099313019. [DOI] [PubMed] [Google Scholar]
- Mulvey M, Camarena V, Mohr I. Full Resistance of Herpes Simplex Virus Type 1-Infected Primary Human Cells to Alpha Interferon Requires both the Us11 and γ134.5 Gene Products. J Virol. 2004;78:10193–10196. doi: 10.1128/JVI.78.18.10193-10196.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norvell JP, Blei AT, Jovanovic BD, Levitsky J. Herpes simplex virus hepatitis: an analysis of the published literature and institutional cases. Liver Transpl. 2007;13:1428–1434. doi: 10.1002/lt.21250. [DOI] [PubMed] [Google Scholar]
- Orvedahl A, Alexander D, Tallóczy Z, Sun Q, Wei Y, Zhang W, Burns D, Leib David A, Levine B. HSV-1 ICP34.5 Confers Neurovirulence by Targeting the Beclin 1 Autophagy Protein. Cell Host & Microbe. 2007;1:23–35. doi: 10.1016/j.chom.2006.12.001. [DOI] [PubMed] [Google Scholar]
- Ousman SS, Wang J, Campbell IL. Differential Regulation of Interferon Regulatory Factor (IRF)-7 and IRF-9 Gene Expression in the Central Nervous System during Viral Infection. J Virol. 2005;79:7514–7527. doi: 10.1128/JVI.79.12.7514-7527.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasieka TJ, Cilloniz C, Lu B, Teal TH, Proll SC, Katze MG, Leib David A. Host Responses to Wild-Type and Attenuated Herpes Simplex Virus Infection in the Absence of Stat1. J. Virol. 2009;83:2075–2087. doi: 10.1128/JVI.02007-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasieka TJ, Collins L, O’Connor MA, Chen Y, Parker ZM, Berwin BL, Piwnica-Worms DR, Leib David A. Bioluminescent Imaging Reveals Divergent Viral Pathogenesis in Two Strains of Stat1-Deficient Mice, and in αβγInterferon Receptor-Deficient Mice. PLoS One. 2011:6. doi: 10.1371/journal.pone.0024018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasieka TJ, Lu B, Leib David A. Enhanced Pathogenesis of an Attenuated Herpes Simplex Virus for Mice Lacking Stat1. J. Virol. 2008;82:6052–6055. doi: 10.1128/JVI.00297-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasieka TJ, Menachery VD, Rosato PC, Leib David A. Corneal replication is an interferon response-independent bottleneck for virulence of herpes simplex virus 1 in the absence of virion host shutoff. J. Virol. 2012;86:7692–7695. doi: 10.1128/JVI.00761-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfaffl MW. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids. 2001;29:e45. doi: 10.1093/nar/29.9.e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rader KA, Ackland-Berglund CE, Miller JK, Pepose JS, Leib DA. In vivo characterization of site-directed mutations in the promoter of the herpes simplex virus type 1 latency-associated transcripts. J. Gen. Virol. 1993;74(Pt 9):1859–1869. doi: 10.1099/0022-1317-74-9-1859. [DOI] [PubMed] [Google Scholar]
- Reich NC. STAT dynamics. Cytokine Growth Factor Rev. 2007;18:511–518. doi: 10.1016/j.cytogfr.2007.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riediger C, Sauer P, Matevossian E, Müller MW, Büchler P, Friess H. Herpes simplex virus sepsis and acute liver failure. Clin Transplant. 2009;23(23 Suppl):37–41. doi: 10.1111/j.1399-0012.2009.01108.x. [DOI] [PubMed] [Google Scholar]
- Sànchez R, Mohr I. Inhibition of cellular 2’-5’ oligoadenylate synthetase by the herpes simplex virus type 1 Us11 protein. J. Virol. 2007;81:3455–3464. doi: 10.1128/JVI.02520-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sancho-Shimizu V, Pérez de Diego R, Lorenzo L, Halwani R, Alangari A, Israelsson E, Fabrega S, Cardon A, Maluenda J, Tatematsu M, Mahvelati F, Herman M, Ciancanelli M, Guo Y, AlSum Z, Alkhamis N, Al-Makadma AS, Ghadiri A, Boucherit S, Plancoulaine S, Picard C, Rozenberg F, Tardieu M, Lebon P, Jouanguy E, Rezaei N, Seya T, Matsumoto M, Chaussabel D, Puel A, Zhang S-Y, Abel L, Al-Muhsen S, Casanova J-L. Herpes simplex encephalitis in children with autosomal recessive and dominant TRIF deficiency. J. Clin. Invest. 2011;121:4889–4902. doi: 10.1172/JCI59259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sancho-Shimizu V, Zhang S-Y, Abel L, Tardieu M, Rozenberg F, Jouanguy E, Casanova J-L. Genetic susceptibility to herpes simplex virus 1 encephalitis in mice and humans. Curr Opin Allergy Clin Immunol. 2007;7:495–505. doi: 10.1097/ACI.0b013e3282f151d2. [DOI] [PubMed] [Google Scholar]
- Sato M, Tanaka N, Hata N, Oda E, Taniguchi T. Involvement of the IRF family transcription factor IRF-3 in virus-induced activation of the IFN-beta gene. FEBS Lett. 1998;425:112–116. doi: 10.1016/s0014-5793(98)00210-5. [DOI] [PubMed] [Google Scholar]
- Sato Mitsuharu, Suemori H, Hata Naoki, Asagiri M, Ogasawara K, Nakao K, Nakaya T, Katsuki M, Noguchi S, Tanaka Nobuyuki, Taniguchi Tadatsugu. Distinct and Essential Roles of Transcription Factors IRF-3 and IRF-7 in Response to Viruses for IFN-α/β Gene Induction. Immunity. 2000;13:539–548. doi: 10.1016/s1074-7613(00)00053-4. [DOI] [PubMed] [Google Scholar]
- Schilte C, Buckwalter MR, Laird ME, Diamond MS, Schwartz O, Albert ML. Cutting Edge: Independent Roles for IRF-3 and IRF-7 in Hematopoietic and Nonhematopoietic Cells during Host Response to Chikungunya Infection. J Immunol. 2012;188:2967–2971. doi: 10.4049/jimmunol.1103185. [DOI] [PubMed] [Google Scholar]
- Sharma Sonia, tenOever BR, Grandvaux N, Zhou G-P, Lin Rongtuan, Hiscott John. Triggering the interferon antiviral response through an IKK-related pathway. Science. 2003;300:1148–1151. doi: 10.1126/science.1081315. [DOI] [PubMed] [Google Scholar]
- Sköldenberg B. Herpes simplex encephalitis. Scand J Infect Dis. 1996;(Suppl 100):8–13. [PubMed] [Google Scholar]
- Spruance SL. The natural history of recurrent oral-facial herpes simplex virus infection. Semin Dermatol. 1992;11:200–206. [PubMed] [Google Scholar]
- Sriskandan S, Altmann D. The immunology of sepsis. The Journal of Pathology. 2008;214:211–223. doi: 10.1002/path.2274. [DOI] [PubMed] [Google Scholar]
- Suh H-S, Zhao M-L, Choi N, Belbin TJ, Brosnan CF, Lee SC. TLR3 and TLR4 are innate antiviral immune receptors in human microglia: role of IRF3 in modulating antiviral and inflammatory response in the CNS. Virology. 2009;392:246–259. doi: 10.1016/j.virol.2009.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Summers BC, Margolis TP, Leib David A. Herpes Simplex Virus Type 1 Corneal Infection Results in Periocular Disease by Zosteriform Spread. J. Virol. 2001;75:5069–5075. doi: 10.1128/JVI.75.11.5069-5075.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takaoka A, Wang Z, Choi MK, Yanai H, Negishi H, Ban T, Lu Y, Miyagishi M, Kodama T, Honda K, Ohba Y, Taniguchi Tadatsugu. DAI (DLM-1/ZBP1) is a cytosolic DNA sensor and an activator of innate immune response. Nature. 2007;448:501–505. doi: 10.1038/nature06013. [DOI] [PubMed] [Google Scholar]
- Tarassishin L, Loudig O, Bauman A, Shafit-Zagardo B, Suh H-S, Lee SC. Interferon regulatory factor 3 inhibits astrocyte inflammatory gene expression through suppression of the proinflammatory miR-155 and miR-155*. Glia. 2011a;59:1911–1922. doi: 10.1002/glia.21233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarassishin L, Suh H-S, Lee SC. Interferon regulatory factor 3 plays an anti-inflammatory role in microglia by activating the PI3K/Akt pathway. J. Neuroinflammation. 2011b;8:187. doi: 10.1186/1742-2094-8-187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thackray LB, Duan E, Lazear HM, Kambal A, Schreiber RD, Diamond MS, Virgin HW. Critical Role for Interferon Regulatory Factor 3 (IRF-3) and IRF-7 in Type I Interferon-Mediated Control of Murine Norovirus Replication. J. Virol. 2012;86:13515–13523. doi: 10.1128/JVI.01824-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyler Kenneth L. Herpes simplex virus infections of the central nervous system: encephalitis and meningitis, including Mollaret’s. Herpes. 2004;11(Suppl 2):57A–64A. [PubMed] [Google Scholar]
- Unterholzner L, Keating SE, Baran M, Horan KA, Jensen SB, Sharma Shruti, Sirois CM, Jin T, Latz E, Xiao TS, Fitzgerald Katherine A, Paludan Soren R, Bowie AG. IFI16 is an innate immune sensor for intracellular DNA. Nat Immunol. 2010;11:997–1004. doi: 10.1038/ni.1932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villers D, Dao T, Nguyen JM, Bironneau E, Godard A, Moreau M, De Groote D, Nicolas F, Soulillou JP, Anegon I. Increased plasma levels of human interleukin for DA1.a cells/leukemia inhibitory factor in sepsis correlate with shock and poor prognosis. J. Infect. Dis. 1995;171:232–236. doi: 10.1093/infdis/171.1.232. [DOI] [PubMed] [Google Scholar]
- Whitley RJ, Kimberlin DW, Roizman B. Herpes simplex viruses. Clin. Infect. Dis. 1998;26:541–553. doi: 10.1086/514600. quiz 554–555. [DOI] [PubMed] [Google Scholar]
- Xing J, Wang S, Lin Rongtuan, Mossman KL, Zheng C. Herpes simplex virus 1 tegument protein US11 downmodulates the RLR signaling pathway via direct interaction with RIG-I and MDA-5. J. Virol. 2012;86:3528–3540. doi: 10.1128/JVI.06713-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu S, Höglund M, Venge P. The effect of granulocyte colony-stimulating factor (G-CSF) on the degranulation of secondary granule proteins from human neutrophils in vivo may be indirect. Br. J. Haematol. 1996;93:558–568. doi: 10.1046/j.1365-2141.1996.d01-1697.x. [DOI] [PubMed] [Google Scholar]
- Yoneyama M, Kikuchi M, Natsukawa T, Shinobu N, Imaizumi T, Miyagishi M, Taira K, Akira S, Fujita T. The RNA helicase RIG-I has an essential function in double-stranded RNA-induced innate antiviral responses. Nature Immunology. 2004;5:730–737. doi: 10.1038/ni1087. [DOI] [PubMed] [Google Scholar]
- Yong KL. Granulocyte colony-stimulating factor (G-CSF) increases neutrophil migration across vascular endothelium independent of an effect on adhesion: comparison with granulocyte-macrophage colony-stimulating factor (GM-CSF) Br. J. Haematol. 1996;94:40–47. doi: 10.1046/j.1365-2141.1996.d01-1752.x. [DOI] [PubMed] [Google Scholar]
- Zhang S-Y, Jouanguy E, Ugolini S, Smahi A, Elain G, Romero P, Segal D, Sancho-Shimizu V, Lorenzo L, Puel A, Picard C, Chapgier A, Plancoulaine S, Titeux M, Cognet C, von Bernuth H, Ku C-L, Casrouge A, Zhang X-X, Barreiro L, Leonard J, Hamilton C, Lebon P, Héron B, Vallée L, Quintana-Murci L, Hovnanian A, Rozenberg F, Vivier E, Geissmann Frédéric, Tardieu M, Abel L, Casanova J-L. TLR3 deficiency in patients with herpes simplex encephalitis. Science. 2007;317:1522–1527. doi: 10.1126/science.1139522. [DOI] [PubMed] [Google Scholar]
- Zimmerer JM, Lesinski GB, Radmacher MD, Ruppert A, Carson WE., 3rd STAT1-dependent and STAT1-independent gene expression in murine immune cells following stimulation with interferon-alpha. Cancer Immunol. Immunother. 2007;56:1845–1852. doi: 10.1007/s00262-007-0329-9. [DOI] [PMC free article] [PubMed] [Google Scholar]