The regulated loading of the replicative helicase MCM2–7 onto DNA is central to DNA replication. Errors during this process lead to genetic disorders, like Meier-Gorlin syndrome, or to genomic instability and cancer. Analyzing DNA replication will allow us to understand how helicase loading is misregulated in disease and will promote the development of diagnostic tools and therapeutics that target DNA replication proteins.
The MCM2–7 helicase is a hetero-hexamer, and its subunits form a ring-shaped structure. Helicase loading onto DNA, also termed pre-replicative complex (pre-RC) formation, is facilitated by the six-subunit origin recognition complex (ORC), cell-division-cycle 6 (Cdc6), and Cdc10 protein-dependent transcript 1 (Cdt1). These proteins can open the MCM2–7 ring and load two MCM2–7 hexamers into a head-to-head double-hexamer around double-stranded DNA.1,2 Helicase loading is an energy-consuming process and requires ATP hydrolysis by Cdc6. During late M-phase, Cdc6 binds to ORC, and then the ORC/Cdc6 complex is competent for pre-RC formation. Equally, MCM2–7 forms a tight complex with Cdt1, which is required in budding yeast for nuclear import of the MCM2–7 helicase. Furthermore, a specific Cdt1-MCM2–7 interaction involving the Cdt1- and Mcm6 C-terminal domains has been characterized in budding yeast and higher eukaryotes, and this interaction could be important for pre-RC formation.3
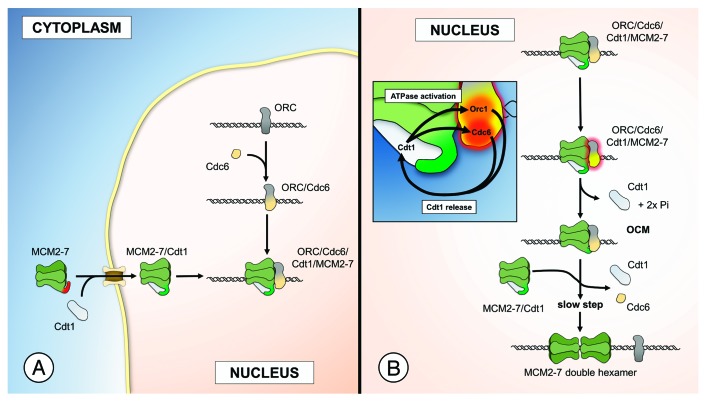
The question of how Cdt1 recruits MCM2–7 toward ORC/Cdc6 has recently been under intense scrutiny. Takara et al. showed that the Cdt1 C terminus is sufficient to promote MCM2–7 recruitment to ORC/Cdc6.4 Using a reconstituted system employing purified proteins, we dissected the process in more detail.5 In a series of experiments, we showed that the Mcm6 C terminus contains an auto-inhibitory domain, which blocks the recruitment of MCM2–7 toward ORC/Cdc6 (Fig. 1A). Interestingly, Cdt1 binding to MCM2–7 alleviates the inhibition by Mcm6 and promotes ORC/Cdc6/Cdt1/MCM2–7 complex formation. Consistently with that model, we found that MCM2–7-ΔC6, which is missing the conserved Mcm6 C terminus, can bind to ORC/Cdc6 in a Cdt1-independent manner. Supporting this concept, Frigola et al. reported that an MCM complex that is missing the Mcm6 subunit can bind to ORC/Cdc6 in a Cdt1-independent manner.6
Figure 1. (A) Cdt1 regulated nuclear import and ORC/Cdc6/Cdt1/MCM2–7 complex formation. (B) Orc1 and Cdc6 mediated ATP hydrolysis promotes OCM formation and MCM2–7 double-hexamer formation.
After ORC/Cdc6- and Cdt1-dependent recruitment of MCM2–7 toward the replication origin an MCM2–7 double hexamer needs to be assembled around DNA in an ATP hydrolysis-dependent fashion; however, this process is only partially understood. ATPγS, which is an ATP analog that can be only very slowly hydrolyzed, does block Cdc6 ATP hydrolysis and Cdt1 release during pre-RC formation. Thus, ATPγS offers the possibility to arrest complex formation prior to stable MCM2–7 double hexamer assembly. We studied such an arrested complex, and our work revealed that the ATPγS-arrested complex contained only a single MCM2–7 hexamer7 and allowed us to suggest a novel model of MCM2–7 loading. In this model, two MCM2–7 hexamers are recruited in a step-wise manner. We predicted that the recruitment of the second MCM2–7 hexamer would require ATP hydrolysis. In a consecutive study, we addressed the role of ATP hydrolysis during complex assembly, employing time-resolved pre-RC formation assays.5 We found that MCM2–7 recruitment and ATP hydrolysis occurred within seconds, while MCM2–7 double hexamer formation was a slow process that took several minutes. This work suggested that ATP hydrolysis would generate an ATP hydrolysis-dependent pre-RC intermediate, which could mature slowly into an MCM2–7 double hexamer. Analysis of this intermediate revealed that Cdc6 ATP hydrolysis promoted the formation of a novel ORC/Cdc6/MCM2–7 (OCM) complex, which had a 1:1:1 stoichiometry. Importantly, we showed that the OCM complex was competent for MCM2–7 double-hexamer formation. In an unexpected twist, we discovered that not only Cdc6, but also Orc1, participates in OCM formation and pre-RC assembly as well. Thus, two different ATPases act in a redundant manner to promote Cdt1 release (Fig. 1B). This suggests that Cdt1 release is of particular importance for successful pre-RC formation and could also indicate a potential way to block pre-RC assembly.
As ATP hydrolysis during pre-RC formation promotes Cdt1 release, we hypothesized that a productive Cdt1-Mcm6 interaction could be required to induce Orc1 and Cdc6 ATPase activity. In fact, we observed that an ORC/Cdc6/Cdt1/MCM2–7-ΔC6 complex blocked ATP hydrolysis, and that Cdt1 addition to ORC-Cdc6 was not sufficient to induce ATP hydrolysis. Furthermore, we found that MCM2–7-ΔC6 also blocked Cdt1 release during pre-RC formation. Thus, the Mcm6-C terminus and Cdt1 have, besides their role in nuclear import and auto-inhibition, another important function in promoting ATP hydrolysis and in facilitating Cdt1 release. In summary, our 2 recent publications describe a series of reaction intermediates and mechanisms that together detail a novel pathway for MCM2–7 double-hexamer formation. Although not every-step is known, these studies have generated a significant framework that will allow us to discover new and exciting mechanisms of helicase loading.
Footnotes
Previously published online: www.landesbioscience.com/journals/cc/article/25336
References
- 1.Evrin C, Clarke P, Zech J, Lurz R, Sun J, Uhle S, et al. A double-hexameric MCM2-7 complex is loaded onto origin DNA during licensing of eukaryotic DNA replication. Proc Natl Acad Sci USA. 2009;106:20240–5. doi: 10.1073/pnas.0911500106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Remus D, Beuron F, Tolun G, Griffith JD, Morris EP, Diffley JF. Concerted loading of Mcm2-7 double hexamers around DNA during DNA replication origin licensing. Cell. 2009;139:719–30. doi: 10.1016/j.cell.2009.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Masai H, Matsumoto S, You Z, Yoshizawa-Sugata N, Oda M. Eukaryotic chromosome DNA replication: where, when, and how? Annu Rev Biochem. 2010;79:89–130. doi: 10.1146/annurev.biochem.052308.103205. [DOI] [PubMed] [Google Scholar]
- 4.Takara TJ, Bell SP. Multiple Cdt1 molecules act at each origin to load replication-competent Mcm2-7 helicases. EMBO J. 2011;30:4885–96. doi: 10.1038/emboj.2011.394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fernández-Cid A, Riera A, Tognetti S, Herrera MC, Samel S, Evrin C, et al. An ORC/Cdc6/MCM2-7 Complex Is Formed in a Multistep Reaction to Serve as a Platform for MCM Double-Hexamer Assembly. Mol Cell. 2013;50:577–88. doi: 10.1016/j.molcel.2013.03.026. [DOI] [PubMed] [Google Scholar]
- 6.Frigola J, Remus D, Mehanna A, Diffley JF. ATPase-dependent quality control of DNA replication origin licensing. Nature. 2013;495:339–43. doi: 10.1038/nature11920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Evrin C, Fernández-Cid A, Zech J, Herrera MC, Riera A, Clarke P, et al. In the absence of ATPase activity, pre-RC formation is blocked prior to MCM2-7 hexamer dimerization. Nucleic Acids Res. 2013;41:3162–72. doi: 10.1093/nar/gkt043. [DOI] [PMC free article] [PubMed] [Google Scholar]