Abstract
Dysregulation of cell cycle genes such as Cyclin D1 and Chk1 contributes to the undifferentiated phenotype of neuroblastoma (NB). CASZ1 functions as a tumor suppressor in NB; here we sought to determine how loss of CASZ1 contributes to cell cycle dysregulation in NB. CASZ1 restoration in NB cells delays NB cell cycle progression. The earliest changes occur within 8 h of CASZ1 restoration in SY5Y cells with a 2.8-fold increase in the level of p21, an inhibitor of Cdk2/4. By 16 h, there is a 40% decrease in the steady-state levels of Cdk6. Restoration of CASZ1 decreases Cdk2-dependent cyclins A and E protein levels and Cdk4/6-dependent Cyclin D1 protein levels. The restoration of CASZ1 resulted in a decrease in pRb phosphorylation and a significant reduction of E2F transcriptional activity. Subsequent to the changes in the G1/S transition, induction of CASZ1 results in a decrease in Cyclin B levels and Cdc25c phosphatase levels, an upstream activator of the G2/M regulator CyclinB:Cdk1. In addition, induction of CASZ1 results in a decrease in the levels of phospho-Chk1, a key M-phase regulatory kinase. Similar results were found in a NB cell line with MYCN amplification. Taken together, this study indicates that restoration of CASZ1 activates pRb in G1 and inhibits the G2/M regulators Cyclin B1 and Chk1, leading to a lengthening of NB cell cycle progression and a subsequent decrease in cell proliferation.
Keywords: CASZ1, tumor suppressor, neuroblastoma, cell cycle, Rb, Cyclin, Cdk, Chk1
Introduction
Neuroblastoma (NB) originates in tissues of the developing sympathetic nervous system and accounts for 15% of all pediatric cancer deaths.1,2 The transcriptome of poor prognosis NB tumors is enriched in cell cycle genes. Overexpression of Cyclin D1 has been reported in NB and is correlated with poor prognosis.3 Increased expression of Cyclin D1 and enhanced Cyclin D-dependent kinase activity contributes to hyper-phosphorylation of the retinoblastoma tumor suppressor protein (pRb) and release of E2F, which is known to activate the transcription of genes that are required for G1–S phase cell cycle progression.3,4 Abnormalities in the p53/Mdm2/p14Arf pathway,5 constitutive activation of Cdc25, and, consequently, Cyclin B-Cdk1 activity also have been observed in NB.6 In addition, Chk1 is highly expressed and/or constitutively phosphorylated in high-risk NB tumors,7 with treatment with Chk1 inhibitors inducing cell death in vitro and delays in NB xenograft growth in vivo.7,8
Human CASZ1 is a recently discovered tumor suppressor gene that maps to chromosome 1p36, whose deletion is a chromosomal anomaly implicated in NB tumorigenesis.2,9-12 Whether it has tumor suppressor activity in other cancers is still under investigation, but a case of cervical cancer has been reported in which human papillomavirus DNA integrated into the CASZ1 gene locus and disrupted its expression.13 Low expression of CASZ1 via loss of heterozygosity or epigenetic suppression is associated with poor prognoses in NB patients.9-11,14 The restoration of CASZ1 in neuroblastoma cells suppresses cell proliferation and induces cell differentiation.9,10 However, whether the loss of CASZ1 contributes to dysregulation of the cell cycle in neuroblastoma is unclear.
Dysregulation of cell cycle machinery is a hallmark of cancer progression.15-18 When normal, non-malignant cells respond to a pro-proliferative signal, D-type cyclins (Cyclin D1, D2, or D3) bind to either Cdk4 or Cdk6, while cyclins A or E bind to Cdk2. Such cyclin-Cdk complexes activate the kinase activity of Cdks, which enables these proteins to phosphorylate pRb.15,19,20 In the early G1 phase of the cell cycle, pRb predominately exists in a hypo-phosphorylated state and negatively regulates the cell cycle by sequestering E2F transcription factors.15,21 However, phosphorylation of pRb by cyclin-Cdks stimulates the release of the protein from E2F and thereby activates E2F-dependent transcription of genes required to promote G1–S phase cell cycle progression.4,15 Moreover, this cell cycle progression is also governed by the Cdk inhibitors such as p21 and p27,15,22 whose protein stability is partially regulated by ubiquitin protein ligase Skp2.22 As for NB, increased expression of Cyclin D1 and enhanced Cyclin D-dependent kinase activity is a major driver of hyper-phosphorylation of pRb.3
To understand how restoration of CASZ1 affects NB cell cycle progression, we systematically investigated proteins regulating G1/S and G2/M cell cycle transitions especially those are known to be altered in NB such as the pRb pathway. We found restoration of CASZ1 leads to reactivation of pRb, decreases in E2F transcriptional activity, and prolongation of NB cell cycle, which ultimately leads to suppression of NB cell growth.
Results
CASZ1 suppresses the proliferation of neuroblastoma cells and prolongs the cell cycle
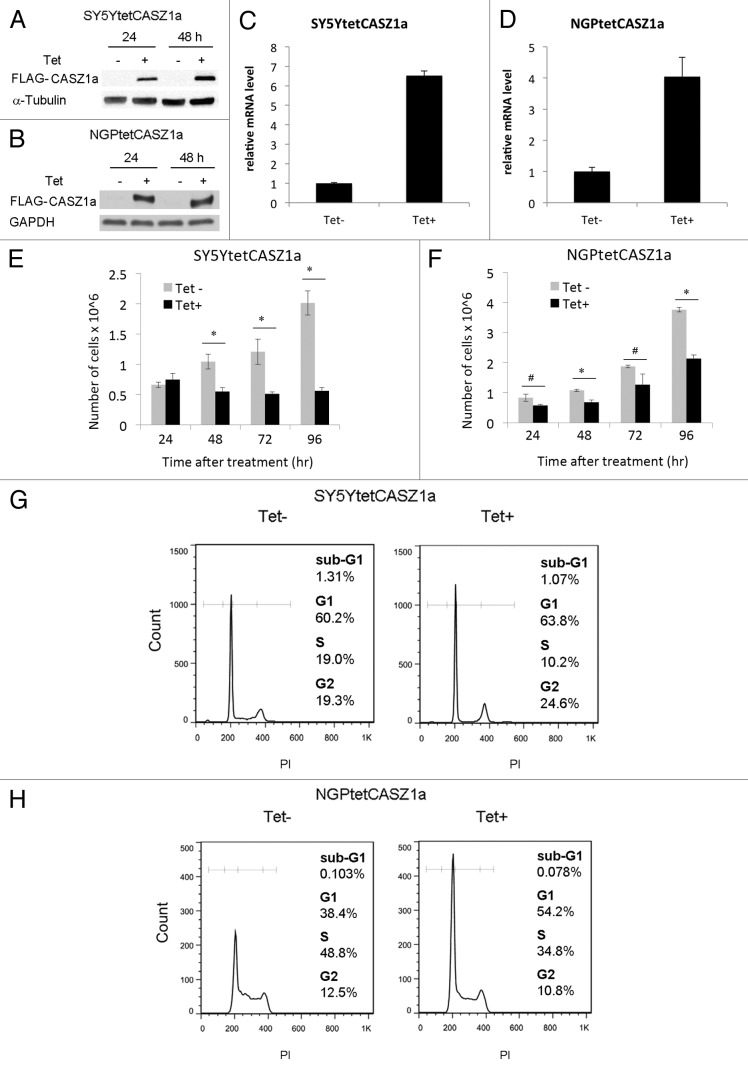
In SY5Y cells chromosome 1p36 is intact, while 1p36 is disrupted by translocation but not deleted in NGP cells. However, endogenous CASZ1 levels are relatively low in these cell lines because of epigenetic silencing by EZH2.14 To restore CASZ1 expression in these two cell lines, we generated SY5YtetCASZ1a and NGPtetCASZ1a cells, which are tetracycline (Tet)-inducible FLAG-tagged CASZ1a stable clones of the SY5Y cell line (MYCN single copy) and NGP cell line (MYCN amplified).9 CASZ1a (the full-length isoform of CASZ1) can be induced by treating the cells with 1 μg/mL Tet, and the induction was confirmed by immunoblotting with an antibody against FLAG (Fig. 1A and B). Real-time PCR results showed that CASZ1a mRNA levels increase 7-fold in SY5Y cells and 4-fold in NGP cells (Fig. 1C and D). The restoration of CASZ1a resulted in a 75% reduction in cell proliferation at 96 h in SY5Y cells and a 40% reduction in NGP cells (Fig. 1E and F). Tet treatment of empty vector-transfected SY5Y or NGP cells had no effect on cell proliferation at all time points analyzed (data not shown).
Figure 1. Restoration of CASZ1 suppresses NB cell proliferation and inhibits the cell cycle. Full-length FLAG-tagged CASZ1a in Tet-on vector was stably transfected into SY5Y (A) and NGP (B) neuroblastoma cell lines that express Tet repressor. Induction of CASZ1a expression by Tet (1 µg/mL) was visualized by immunoblotting whole-cell lysate with anti-FLAG antibody. The fold induction of CASZ1 mRNA at 24 h was detected by real-time PCR in SY5Y (C) and NGP (D). Restoration of CASZ1a in SY5Y (E) and NGP cells (F) inhibited cell proliferation (*P < 0.005; #P < 0.05). FACS analysis documenting the cell cycle distribution of SY5Ytet CASZ1a (G) or NGPtetCASZ1a (H) upon restoration of CASZ1a for 2 d.
We next performed FACS analysis to determine whether CASZ1a affects cell cycle progression. After 48 h of Tet treatment, the restoration of CASZ1a expression in SY5YtetCASZ1a cells was accompanied by increases in the G1 (60.2–63.5%) and G2/M (19.3–24.6%) phases, and a concomitant decrease in the S (19–10.2%) phase (Fig. 1G). In NGPtetCASZ1a cells, expression of CASZ1a induced an increase in the G1 (38.4–54.2%) phase, a decrease in the S (48.8–34.8%) phase, and a slight change in the G2/M (12.5% vs. 10.8%) phases (Fig. 1H). Consistent with these findings, a Click-iT EdU flow cytometric assay showed that the restoration of CASZ1a decreased DNA synthesis in both SY5Y cells and NGP cells (data not shown).
CASZ1 regulates the levels of proteins that promote G1–S phase cell cycle progression
To examine the effects of restored CASZ1a on the expression of cell cycle-regulatory proteins, SY5YtetCASZ1a and NGPtetCASZ1a cells were treated with or without 1 μg/mL Tet, and steady-state levels of key G1–S phase proteins were analyzed by western blot at 24 and 48 h following CASZ1a induction. We first examined cyclins and Cdks, as many are dysregulated in NB, and their activation contributes to the undifferentiated phenotype.3 The steady-state levels of Cdk2-dependent Cyclin A and Cyclin E decreased by more than 40% in SY5Y cells at 24 h following CASZ1a induction, and Cdk4/6-dependent Cyclin D1 protein levels were reduced by approximately 30% in SY5Y cells at 48 h (Fig. 2A). In addition, while the expression of Cdk2 and Cdk6 decreased almost 70% at 48 h, Cdk4 levels did not change (Fig. 2B). We also examined the status of several Cdk inhibitors. Within 24 h of CASZ1a expression, there was a transient 2-fold increase in the Cdk2/4 inhibitor p21 that declined at 48 h (Fig. 2C). There was no reproducible, detectable increases in p27, although Skp2 levels decreased by 3-fold at 24 h (Fig. 2C). Similar results were observed in NGP cells, as restoration of CASZ1a was accompanied by decreases in Cyclin D1, Cyclin A, Cdk6, and an increase in p21 (Fig. 2D).
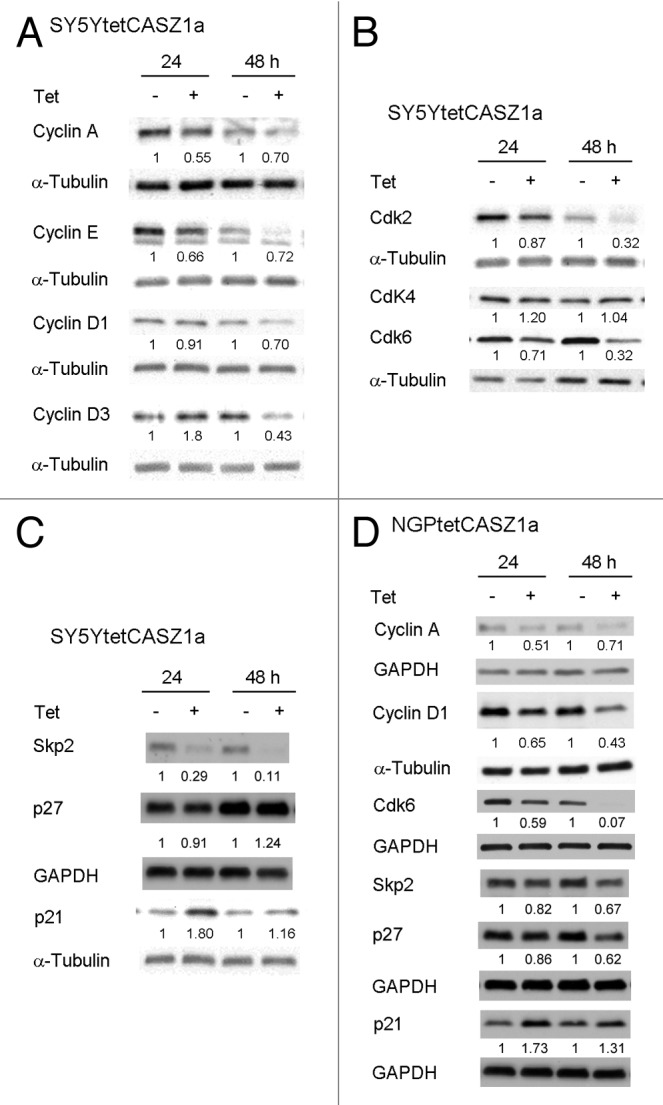
Figure 2. CASZ1 regulates the levels of proteins that regulate G1–S phase cell cycle progression. (A) The protein levels of cyclins in SY5Y cells upon CASZ1a induction were visualized by immunobloting whole-cell lysate with indicated antibodies. (B) The protein levels of Cdks in SY5Y cells upon CASZ1 induction were visualized by immunobloting whole-cell lysate with indicated antibodies. The relative densitometric units were normalized to control condition and the ratio shown under each condition. (C) The protein levels of Cdk inhibitors in SY5Y cells were visualized by immunobloting whole-cell lysate with indicated antibodies. (D) The protein levels of cell cycle regulators in NGP cells were visualized by immunobloting whole-cell lysate with indicated antibodies.
Because early cell cycle changes may reflect a more direct effect of CASZ1 induction, we analyzed the expression of several G1–S phase proteins at 8, 16, or 24 h following CASZ1a induction. Steady-state levels of Cdk6 decreased within 16 h after CASZ1a induction in both SY5Y cells and NGP cells (Fig. 3A and B). In SY5Y cells, p21 was upregulated by approximately 2.8-fold at 8 h, and this increase persisted until 24 h (Fig. 3A). In NGP cells, p21 increased by 20% at 8 h and had more than doubled by 24 h (70% increase) (Fig. 3B). P21 is known to be a transcriptional target of the p53 protein; however, there was a 27% increase in total p53 at 8 h but no other time point, suggesting that CASZ1a may regulate p21 through another pathway (Fig. 3A and B).
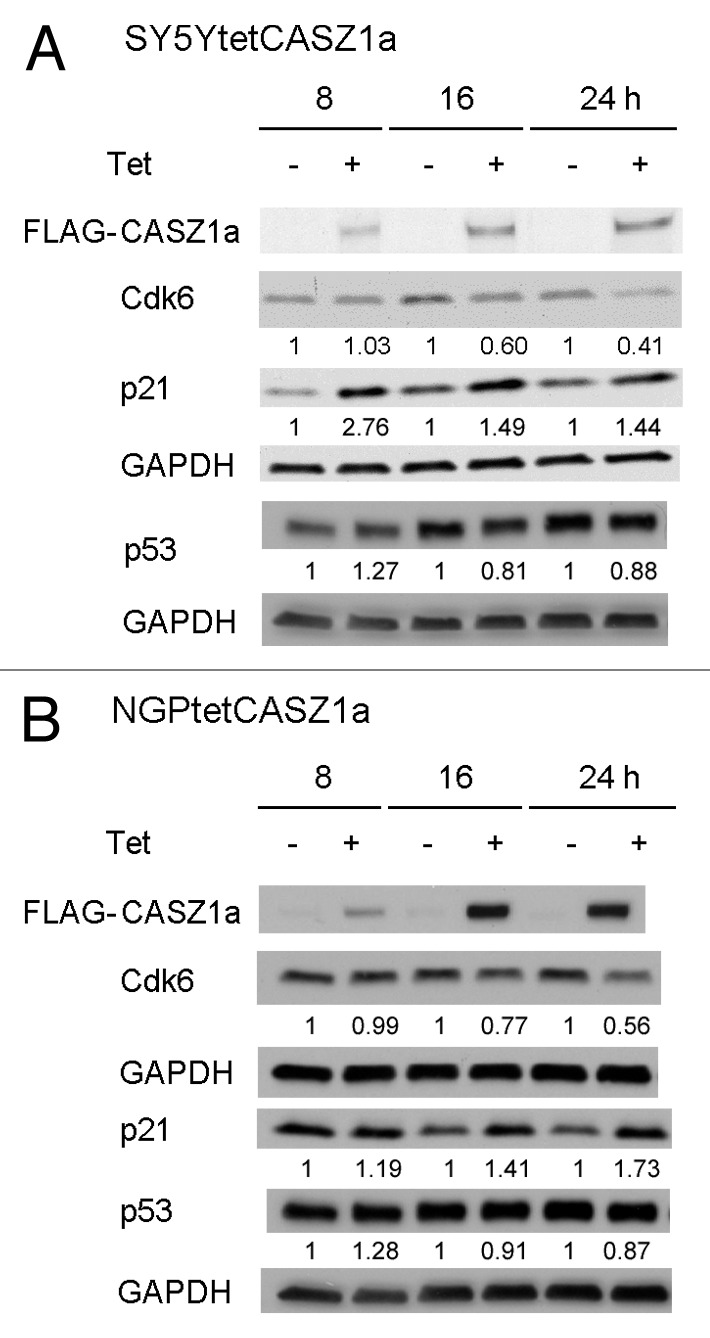
Figure 3. Cdk6 and p21 were regulated by CASZ1a shortly after CASZ1 restoration. The protein levels of cell cycle regulators in SY5Y cells (A) and NGP cells (B) were visualized by immunobloting whole-cell lysate with indicated antibody from 8–24 h after induction of CASZ1a. The relative densitometric units were normalized to timed control, and the ratio shown under each condition.
CASZ1 restores pRb activity
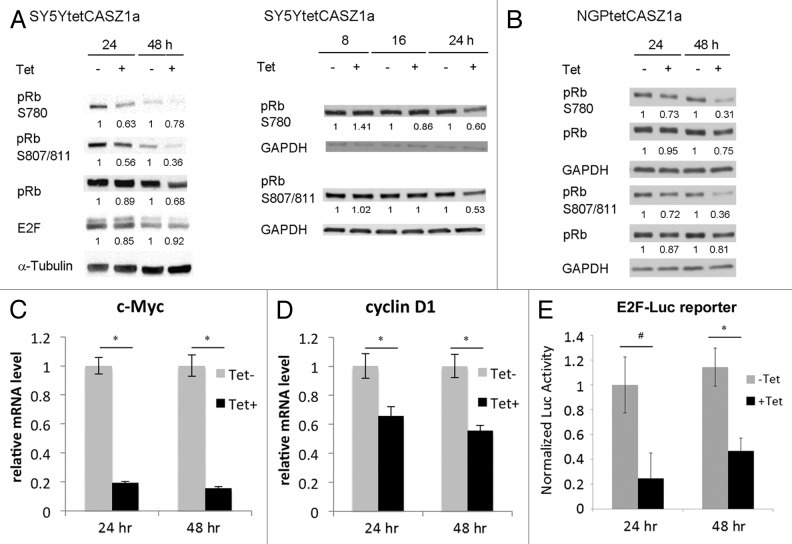
Both Cdk2 and Cdk6 are known to phosphorylate the pRb tumor suppressor protein. It is therefore likely that the CASZ1-mediated decrease in Cdk2 and Cdk6 as well as upregulation of the Cdk2/4 inhibitor p21 will result in a reduction in pRb phosphorylation. Indeed, at 24 h following CASZ1a induction, a 40% reduction in phosphorylation of pRb at both Ser807/811 and Ser780 sites (known to be phosphorylated by Cdks) was observed in SY5Y cells, and this decrease was sustained at 48 h (Fig. 4A, left panel). However, E2F-1 levels remained constant in the presence or absence of CASZ1a at the time points analyzed (Fig. 4A, left panel). We found that even though the P-pRBser780, 807/811 levels decrease in controls from 24 h to 48 h, the levels decreased even more upon CASZ1 induction. To determine the earliest decrease, we analyzed levels from 8–24 h. The earliest detected CASZ1 induced decrease in P-pRBser780, 807/811 levels occurred at 24 h, and during this time (8–24 h), P-pRBser780, 807/811 levels did not change in the control samples (Fig. 4A, right panel). A reduction in pRb phosphorylation upon CASZ1a induction was also observed in NGP cells at both 24 h and 48 h (Fig. 4B). In contrast to the dynamics in SY5Y cells, there were no significant changes in phosphorylated pRb levels in NGP control cells from 24 h to 48 h (Fig. 4B).
Figure 4. CASZ1a restores pRb activity. CASZ1a restoration leads to de-phosphorylation of pRb at Ser780 and Ser807/811 in SY5Y cells (A) and NGP cells (B) and was visualized by immunobloting whole-cell lysate with indicated antibody. The relative densitometric units were normalized to control condition and the ratio shown under each condition. (C) CASZ1 restoration significantly decreased E2F target gene c-Myc expression in SY5Y cells detected by real-time PCR (*P < 0.005). (D) CASZ1 restoration significantly decreased E2F target gene cyclin D1 expression in NGP cells detected by real-time PCR (*P < 0.01). (E) CASZ1 restoration inhibits E2F activity at both 24 h and 48 h time points as assessed using a E2F-luc reporter assay (*P < 0.005; #P < 0.02).
The decrease in pRb phosphorylation indicates there should be less free E2F to stimulate gene transcription after induction of CASZ1a. To test this, we performed real-time PCR to investigate E2F target gene expression after induction of CASZ1a. Cyclin D1 and c-Myc are two known E2F target genes.23 In SY5Y cells, the induction of CASZ1a significantly decreased E2F target gene c-Myc expression at 24 h and 48 h (Fig. 4C). In NGP cells, the induction of CASZ1a significantly decreased E2F target gene cyclin D1 expression at 24 h and 48 h (Fig. 4D). To further confirm that E2F transcriptional activity was inhibited by the induction of CASZ1a, we performed a luciferase assay with a reporter construct containing E2F binding sites that fused to a luciferase reporter (E2F-luc). SY5YtetCASZ1a cells were transiently transfected with a pE2F-luciferase plasmid by electroporation. Following transfection, the cells were treated with 1 ug/mL Tet, and the activity of E2F-luc was analyzed 24 h and 48 h later using the Promega luciferase reporter assay. In Figure 4E, the data were analyzed by normalizing the data from each sample to Tet-negative 24 h sample. The results showed that CASZ1a reduces E2F activity by ~80% at 24 h and 60% at 48 h (Fig. 4E). We found that there was no significant change of E2F transcriptional activity in the Tet-negative cells at 48 h when compared with the 24 h time point (Fig. 4E). These data indicate that the increase in CASZ1a levels results in restoration of pRb activity, thus reinstating regulation of the G1–S phase transition.
CASZ1 regulates the levels of proteins that promote G2–M phase cell cycle progression
In SY5Y cells, induction of CASZ1a also led to an increase in cells in the G2/M phases of the cell cycle suggesting that CASZ1a may regulate G2/M phase proteins. The steady-state levels of proteins that control the G2–M phase transition were analyzed by western blot following CASZ1a restoration in SY5Y cells. There was a 10% decline in Cdc25C levels at 24 h after CASZ1 induction; at 48 h there was a 35% decline (Fig. 5A). Inhibition of Cdc25C was associated with an early decrease in steady-state levels of Cyclin B1 in SY5YtetCASZ1a cells, with the first detectable decrease in Cyclin B1 levels occurring at 24 h (30% decrease) and reaching a 75% decrease by 48 h (Fig. 5A). Phospho-Chk1 (p-Chk1) overexpression occurs in high-risk NB tumors.7 Induction of CASZ1a caused a 50% decrease in p-Chk1 levels that was first detected at 24 h and was sustained at 48 h (Fig. 5A). Similar results were seen following CASZ1a restoration in NGP cells (Fig. 5B). These results indicate that restoration of CASZ1a in NB cells may also regulate G2–M phase proteins; however, these changes may be subsequent to changes in G1–S phase proteins.
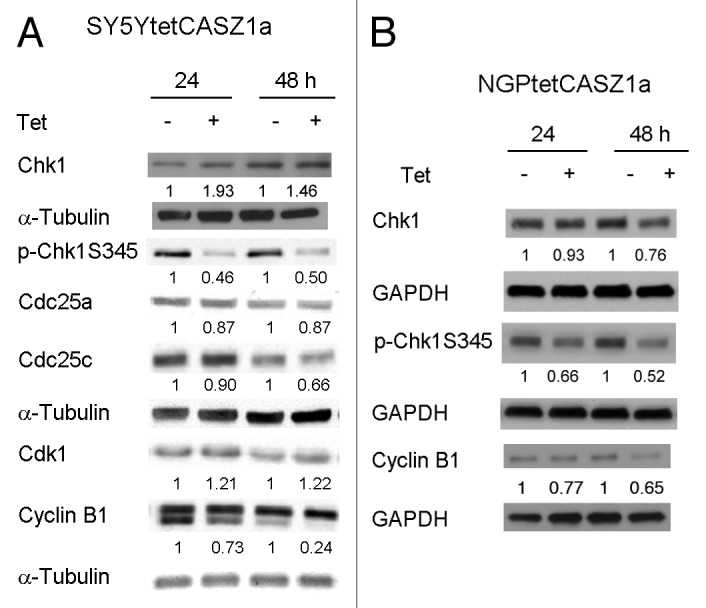
Figure 5. CASZ1 regulates the levels of proteins that promote G2–M phase cell cycle progression. The protein levels of G2–M cell cycle regulators after induction of CASZ1a in SY5Y cells (A) and NGP cells (B) were visualized by immunobloting whole-cell lysates with indicated antibody. The relative densitometric units were normalized to control condition and the ratio shown under each condition.
CASZ1 regulates some cell cycle regulators at mRNA level
CASZ1 is a zinc finger transcription factor and may regulate gene expression at a transcriptional level. Our pilot microarray data showed that Cdk6, Skp2, and p21 were among cell cycle regulators regulated by CASZ1a at the mRNA level (unpublished data). To confirm the microarray data, quantitative real-time PCR was performed after restoration of CASZ1a in SY5Y cells. The restoration of CASZ1 mRNA levels is shown in Figure 6A. The level of Cdk6 mRNA decreased from 8 h to 24 h by 30–40% following CASZ1a induction (Fig. 6B). The level of p21 mRNA increased at 8 h by 50% and at 24 h by 2-fold after CASZ1a induction (Fig. 6C). Skp2 mRNA levels decreased from 8 h to 16 h by 40% (Fig. 6D). These results are consistent with transcriptional activation of p21 and transcriptional repression of Cdk6 and Skp2 by CASZ1a and provide a mechanism for the observed increase in p21 protein and decrease in Cdk6 following CASZ1 induction.
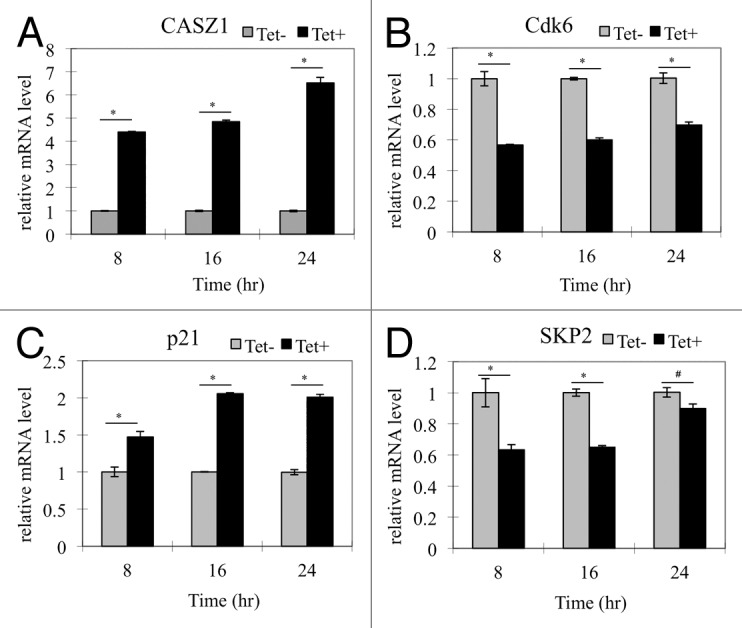
Figure 6. CASZ1 modulates transcription of select cell cycle regulators. The mRNA level changes of (A) CASZ1, (B) Cdk6, (C) p21, and (D) Skp2 detected by real-time-PCR in SY5YtetCASZ1a cells treated with or without Tet for 8, 16, and 24 h (*P < 0.005; #P < 0.05).
From this study, we propose a model in which CASZ1 increases Cdks inhibitor p21; decreases cyclins such as Cyclin D1 and Cyclin D3; decreases Cdk2 and Cdk6, which ultimately lead to the de-phosphorylation of pRb, suppression of E2F transcription activity, and inhibition of G1–S progression (Fig. 7). The dephosphorylation of Chk1 by CASZ1a overexpression suggests CASZ1a also impacts G2–M progression. Consistent with these observations, restoration of CASZ1a levels in NB cells increased cell-doubling times.
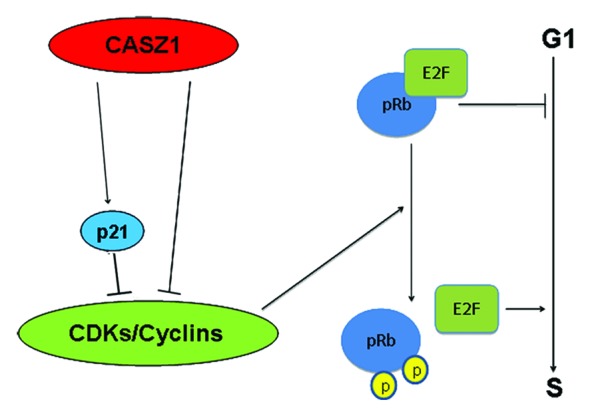
Figure 7. Model of CASZ1 function in cell cycle regulation. Restoration of CASZ1 in NB cells results in upregulation of p21 a Cdk2/4 inhibitor and downregulation of cyclins such as Cyclin D1 and Cdk2/6. Thus CASZ1 inhibits Cdk enzyme activity, resulting in hypo-phosphorylation of pRb that binds E2F, thus decreasing E2F-mediated transcription and restoring the G1–S checkpoint and inhibition of cell cycle progression.
Discussion
Recent evidence indicates that CASZ1a is a suppressor of neuroblastoma tumor growth.9 Due to the well-established involvement of tumor suppressor genes in regulation of cell cycle machinery,24-26 we examined how reconstitution of CASZ1a affected the level of expression of cell cycle-regulatory proteins in NB cells. We found that induction of CASZ1a reprogrammed the overall cell cycle to an quiescent state. Results show that CASZ1a expression inhibited cell cycle progression mainly by restoring pRb activity and inhibiting Chk1 phosphorylation. This suggests that during NB tumorigenesis, the loss of CASZ1a through LOH and/or epigenetic silencing contributes to the deregulation of cell cycle found in NB.
In NB, relatively high expression of Cyclin D1, Cdk4, and Cdk6 correlates with unfavorable prognosis and an undifferentiated histologic classification.3 Their elevated levels lead to hyperphosphorylation of pRb and constitutive E2F-dependent gene transcription, which stimulates proliferation and accelerates the G1–S phase transition in NB cells.3 In our study, CASZ1a increases the Cdk2/4 inhibitor p21 and decreases the steady-state levels of Cyclin D1 and Cdk6, which leads to the restoration of pRb tumor suppressor activity as evidenced by the de-phosphorylation of pRb. Attenuation of the deregulated G1–S phase transition via rescue of pRb activity represents an important mechanism by which CASZ1 suppresses neuroblastoma growth in vitro. Immunoblotting at earlier time points for proteins revealed a possible mechanism of how CASZ1 restores the activity of G1–S phase checkpoint proteins. Our data supports a model in which the induction of CASZ1 leads to a transcriptional increase in the levels of the Cdk2/4 inhibitor p21. CASZ1 restoration decreases the steady-state levels of Cdk6 within 16 h of induction with subsequent decreases in the steady-state levels of Cyclin D1 occurring at 24 h. In the absence of CASZ1, the steady-state levels of Cdk2,4,6/Cyclin-dependent kinases phosphorylate pRb and release E2F from the pRb-E2F complex; E2F is then free to drive gene expression required for G1–S progression. The CASZ1-mediated increases in p21 and decreases in Cdk6 and Cyclin D1 attenuate the activity of the G1 cyclin-dependent kinase, resulting in decreased phosphorylated pRb, causing Rb activation (Fig. 7). The finding that induction of CASZ1 leads to decreases in the activity of an E2F-luciferase reporter (Fig. 4E) and E2F target genes (Fig. 4C and D) is consistent with the known function of activated pRb to bind E2F and inhibit its activity.
Cyclin protein levels rise and fall during the cell cycle, and in this way they periodically activate Cdks.27 By including controls at every time point, we have been able to assess the impact of CASZ1 expression as a function of the dynamic expression of these cell cycle proteins in the NB tumor cells. Our results indicate that there are essentially two types of effects on cell cycle gene expression induced by CASZ1: (1) those in which CASZ1 induction enhances the normal cell cycle changes, such as the decreases of Cyclin A or E (Fig. 2A) and (2) those in which CASZ1 induction causes a change when the levels of these proteins are unchanged in controls, such as Skp2 and p21 (Fig. 2C and D). Our data indicate that CASZ1 is an important regulator of cell cycle in NB cells. Although CASZ1 does induce an increase in cells in the G1 phase, it is not to the same extent as the increases in the G1 phase induced by retinoid treatment.28 Thus, it is probable that other factors are needed to work in concert with CASZ1 to exert growth arrest in NB.
Regulation of cell cycle machinery by CASZ1 was not confined to regulators of the G1–S phase transition; CASZ1 was also found to decrease the levels of multiple proteins that promote G2–M phase progression, including the Cdc25C phosphatase, Cyclin B1, and p-Chk1. Thus, these data indicate that CASZ1 modulates the activities of key cell cycle-regulatory proteins in a way that attenuates the deregulation of both the G1–S and G2–M phase transitions that are found in NB cells. Interestingly, while some of the major cell cycle proteins active in the G1–S phase transitions were significantly reduced in NB cells within 24 h of induction of CASZ1 expression, the decrease in the expression of many G2–M phase transition proteins occurred at 48 h. This delay suggests that CASZ1 does not directly regulate G2/M phase proteins. Instead, it is likely that the observed effects in the G2–M phase transition are a consequence of restored G1–S phase regulation. For example, the observed decrease in steady-state levels of Cyclin B1 may be due to downregulation of the Cyclin via CASZ1-mediated decreases in G1–S phase proteins. We did see an earlier response of G2–M phase protein Chk1 at 24 h after CASZ1 restoration; p-Chk1 showed a 50% decrease at 24 h of induction of CASZ1 expression (Fig. 5) but not prior to this time (data not shown). Since Chk1 acts downstream of ATR kinase and plays an important role in DNA damage checkpoint control,29 it is possible that CASZ1 may interfere with the ATR pathway and lead to the dephosphorylation of Chk1.
The ability of CASZ1 to inhibit NB cellular proliferation via reprogramming of cell cycle machinery was found to be independent of MYCN status. Clinically, patients whose tumors have amplification of MYCN have a worse prognosis and decreased overall survival.2 The fact that pRb levels and the levels of G1–S phase Cdks decreased in a similar manner in NGP cells (MYCN amplified) as in SY5Y cells (MYCN single copy) after CASZ1 restoration indicates that the effects of CASZ1 on the cell cycle are downstream of MYCN’s effects on the cell cycle. Because primary neuroblastoma tumors are very heterogeneous with respect to MYCN status, the observation that CASZ1 function is independent of MYCN is clinically significant—therapeutic strategies designed to increase CASZ1 expression in neuroblastoma patients should be effective regardless of MYCN status.
The identification of CASZ1 involvement in regulation of cell cycle in tumor cells suggests a mechanism by which CASZ1 may control cellular processes during normal development. It has been shown that the Drosophila CASZ1 homolog (dcas) regulates neuronal differentiation,30,31 and CASZ1 is required for heart development and onset of cardiomyocyte differentiation in Xenopus.32 In NB cells, the restoration of CASZ1 induces neurite extension.9 Neural differentiation is usually coupled with a lengthening of the cell cycle during development.33,34 That the changes induced by CASZ1 cause a lengthening in the cell doubling time may be an aspect of the mechanism by which CASZ1 regulates neuronal differentiation.
Inactivation of pRb and activation of CHK1 are two anomalies reported in NB.3,7 The restoration of CASZ1 levels in NB cells activates pRb and inhibits Chk1 phosphorylation. Thus it is possible that loss of CASZ1 during NB tumorigenesis contributes to the deregulation of pRb pathway and Chk1 phosphorylation and accelerates NB tumor progression. These results imply that restoration of CASZ1 may be able to improve the survival of neuroblastoma patients by restoring regulation of the cell cycle transition.
Materials and Methods
Cell culture
The SH-SY5Y (SY5Y) and NGP human neuroblastoma cell lines used in this study were obtained from the cell line bank of the Pediatric Oncology Branch of the National Cancer Institute and were determined to be genetically pure using a STS genotype assay (SJ Chanock, Division of Cancer Genetics and Epidemiology, National Cancer Institute). Neither cell line harbors mutations in any of the cell cycle-regulatory genes analyzed. SY5Y has a single copy of MYCN, while NGP has a 1p translocation and the MYCN locus is amplified. The cell lines were cultured in RPMI-1640 medium (GIBCO) supplemented with 10% fetal bovine serum (Gemini Bio-Products), 2 mmol/L L-glutamine, 100 units/mL penicillin, and 100 μg/mL streptomycin (Invitrogen) at 37 °C in 5% CO2.
Stable clones
Stable clones expressing CASZ1a are designated as SY5YtetCASZ1a and NGPtetCASZ1a, while stable clones transfected with the empty vector are labeled SY5Ytetemv and NGPtetemv as described previously.9,10 These transfectants were cultured in RPMI 1640 complete medium containing 5 μg/mL blasticidin and 500 μg/mL Geneticin. CASZ1a expression was induced by treatment with 1 μg/mL Tetracycline (Tet). For the cell treatment, SY5YtetCASZ1a and NGPtetCASZ1a cells were seeded into 10 cm plates at a density of 4 × 106 cells per plate and cultured in RPMI-1640 containing 10% FBS. After 24 h of culture, cells were treated with 1 μg/mL Tet (+ Tet) or with RPMI-1640 (– Tet, negative control). For cell proliferation assay, cell number was counted at different time point using hemocytometer.
Protein extraction
SY5YtetCASZ1a and NGPtetCASZ1a cells treated with Tet or RPMI-1640 were washed and collected in ice-cold PBS at 8, 16, 24, and 48 h post treatment. Suspensions were centrifuged at 1400 rpm for 5 min at 4 °C, and total protein lysates were prepared by resuspending cells in mammalian protein extraction reagent lysis buffer (Thermo Scientific) or RIPA buffer containing halt protease/phosphatase inhibitor cocktail (Thermo Scientific) and EDTA. After extraction, suspensions were centrifuged for 10 min at 1400 rpm at 4 °C, and the protein supernatant was collected and quantified using a Bradford protein assay (Sigma-Aldrich).
Immunoblotting
Protein (20 μg) in 4× NuPAGE LDS loading buffer (Invitrogen) containing 10% β-mercaptoethanol (Sigma-Aldrich) were electrophoresed in a 4–15% or 4–20% polyacrylamide gel (Bio-Rad) and transferred electrophoretically to 0.2 μm nitrocellulose membranes (Schleicher and Schuell). Membranes were incubated overnight at 4 °C in 5% nonfat dry milk in Tris-buffered saline with Tween 20 (20 mM Tris-HCL [pH 7.4], 150 mM NaCl, and 0.5% Tween 20) and subsequently incubated overnight at 4 °C in the following primary antibodies (diluted according to manufacturer’s protocol): Cyclin A BF683, Cyclin D1/2 5D4, E2F-1 KH20, and KH95, Cdk2 AN4.3, Cdc25C TC-15, Cyclin B1, Cyclin E HE12, Skp2, Cdk1/cdc2 PSTAIR, p27 (Santa Cruz Biotechnology), Cyclin D1 DCS6, Cdk6 DCS83, Cyclin D3 DCS22, p21 Waf/Cip DCS60, Cdk4 DCS156, Rb 4H1, p-Rb S780, p-Rb S807/811, Chk1, p-Chk1 S345 (Cell Signaling), p53 OP43 DO-1 (Calbiochem). The membranes were then washed with Tris-buffered saline–Tween 20 and incubated with either goat anti-rabbit or anti-mouse antibodies (1:2000, Santa Cruz Biotechnology) for 1 h at room temperature. Bound antibodies were detected using the enhanced chemiluminescence immunoblotting detection reagent (Amersham Biosciences Inc) and exposed to X-ray film. Appropriately exposed autoradiographs were analyzed by densitometry using ImageJ software and the relative densitometric units were normalized to timed control and the ratio shown under each time point and condition.
Fluorescence-activated cell sorting analysis
NB cells were washed and collected in ice-cold phosphate-buffered saline (PBS) and fixed with ethanol by centrifuging for 5 min at 1400 rpm at 4 °C and resuspending the resulting pellet in a solution containing 0.5 mL PBS and 4.5 mL of 70% EtOH. Fixed cells were stored at –20 °C for several weeks and then pelleted by centrifuging for 5 min at 1400 rpm. Cells were resuspended in PBS and pelleted again for 5 min at 1400 rpm. Residual PBS was decanted, and cells were incubated in a solution of 50 μg/mL propidium iodide/0.1% Triton X-100 (Sigma-Aldrich) containing 100 μg/mL RNase A (Roche) for 20 min in the dark at room temperature. Stained cells were then analyzed for DNA content by fluorescence-activated cell sorting using a FACScan flow cytometer (Becton Dickinson), and percentages of cells in the G1, S, or G2/M phases of the cell cycle were quantified with FlowJo software. Click-iT-EdU flow cytometry assay was performed following the manufacturer’s protocol (Life Technologies). In brief, SY5YtetCASZ1a cells or NGPtetCASZ1 cells were treated with or without 1 µg/ml Tet for 72 h followed by incubation with 10 µM EdU for 4 h, after which the cells were harvested and fixed for the flow cytometry assay.
Transient transfections and luciferase assay
In luciferase experiments, plasmids were transiently transfected into SY5YtetCASZ1a cells using the amaxa nucleofector solution V (Lonza) according to the manufacturer’s protocol. The E2F-luciferase constructs (E2F-Luc), which contains E2F binding element in the promoter region was from Clontech. A CMV-driven β-galactosidase construct was co-transfected with E2F-Luc in order to provide an internal control for transfection efficiency. Three hours after transfection, the SY5YtetCASZ1a cells were treated with or without 1 µg/ml Tet. Luciferase activity was quantified after 24 or 48 h using the Luciferase Reporter Assay System (Promega), and β-galactosidase activity was measured in conjunction with the Luminescent Beta-galactosidase Detection Kit II (Clontech) to normalize the luciferase signals. All of the above experiments have been performed at least twice.
Real-time quantitative PCR
The primer sets used for the real-time PCR will be provided upon request. Quantitative real-time PCR was performed on ABI Prism 7900 (PE Applied Biosystems) using Power SYBR Green PCR master mix (AB applied biosystems) as described previously and β-Actin was used as normalization control.9 PCR reactions were performed according to the manufacture’s protocol. All the PCR experiments have been performed at least twice.
Statistical analyses
Statistical analyses of the data were performed using a t test with P < 0.05 considered significant. Values in the graphs are expressed as means ± SD. The statistical tests were two-sided.
Acknowledgments
This work was supported by the Intramural Research Program of the NIH, National Cancer Institute, and Center for Cancer Research.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Footnotes
Previously published online: www.landesbioscience.com/journals/cc/article/25265
Reference
- 1.Maris JM. Recent advances in neuroblastoma. N Engl J Med. 2010;362:2202–11. doi: 10.1056/NEJMra0804577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brodeur GM. Neuroblastoma: biological insights into a clinical enigma. Nat Rev Cancer. 2003;3:203–16. doi: 10.1038/nrc1014. [DOI] [PubMed] [Google Scholar]
- 3.Molenaar JJ, Ebus ME, Koster J, van Sluis P, van Noesel CJ, Versteeg R, et al. Cyclin D1 and CDK4 activity contribute to the undifferentiated phenotype in neuroblastoma. Cancer Res. 2008;68:2599–609. doi: 10.1158/0008-5472.CAN-07-5032. [DOI] [PubMed] [Google Scholar]
- 4.Müller H, Bracken AP, Vernell R, Moroni MC, Christians F, Grassilli E, et al. E2Fs regulate the expression of genes involved in differentiation, development, proliferation, and apoptosis. Genes Dev. 2001;15:267–85. doi: 10.1101/gad.864201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Carr-Wilkinson J, O’Toole K, Wood KM, Challen CC, Baker AG, Board JR, et al. High Frequency of p53/MDM2/p14ARF Pathway Abnormalities in Relapsed Neuroblastoma. Clin Cancer Res. 2010;16:1108–18. doi: 10.1158/1078-0432.CCR-09-1865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Timofeev O, Cizmecioglu O, Settele F, Kempf T, Hoffmann I. Cdc25 phosphatases are required for timely assembly of CDK1-cyclin B at the G2/M transition. J Biol Chem. 2010;285:16978–90. doi: 10.1074/jbc.M109.096552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cole KA, Huggins J, Laquaglia M, Hulderman CE, Russell MR, Bosse K, et al. RNAi screen of the protein kinome identifies checkpoint kinase 1 (CHK1) as a therapeutic target in neuroblastoma. Proc Natl Acad Sci USA. 2011;108:3336–41. doi: 10.1073/pnas.1012351108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Xu H, Cheung IY, Wei XX, Tran H, Gao X, Cheung NK. Checkpoint kinase inhibitor synergizes with DNA-damaging agents in G1 checkpoint-defective neuroblastoma. Int J Cancer. 2011;129:1953–62. doi: 10.1002/ijc.25842. [DOI] [PubMed] [Google Scholar]
- 9.Liu Z, Yang X, Li Z, McMahon C, Sizer C, Barenboim-Stapleton L, et al. CASZ1, a candidate tumor-suppressor gene, suppresses neuroblastoma tumor growth through reprogramming gene expression. Cell Death Differ. 2011;18:1174–83. doi: 10.1038/cdd.2010.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liu Z, Naranjo A, Thiele CJ. CASZ1b, the short isoform of CASZ1 gene, coexpresses with CASZ1a during neurogenesis and suppresses neuroblastoma cell growth. PLoS One. 2011;6:e18557. doi: 10.1371/journal.pone.0018557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liu Z, Yang X, Tan F, Cullion K, Thiele CJ. Molecular cloning and characterization of human Castor, a novel human gene upregulated during cell differentiation. Biochem Biophys Res Commun. 2006;344:834–44. doi: 10.1016/j.bbrc.2006.03.207. [DOI] [PubMed] [Google Scholar]
- 12.Henrich KO, Schwab M, Westermann F. 1p36 tumor suppression--a matter of dosage? Cancer Res. 2012;72:6079–88. doi: 10.1158/0008-5472.CAN-12-2230. [DOI] [PubMed] [Google Scholar]
- 13.Schmitz M, Driesch C, Beer-Grondke K, Jansen L, Runnebaum IB, Dürst M. Loss of gene function as a consequence of human papillomavirus DNA integration. Int J Cancer. 2012;131:E593–602. doi: 10.1002/ijc.27433. [DOI] [PubMed] [Google Scholar]
- 14.Wang C, Liu Z, Woo CW, Li Z, Wang L, Wei JS, et al. EZH2 Mediates epigenetic silencing of neuroblastoma suppressor genes CASZ1, CLU, RUNX3, and NGFR. Cancer Res. 2012;72:315–24. doi: 10.1158/0008-5472.CAN-11-0961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lundberg AS, Weinberg RA. Control of the cell cycle and apoptosis. Eur J Cancer. 1999;35:531–9. doi: 10.1016/S0959-8049(99)00292-0. [DOI] [PubMed] [Google Scholar]
- 16.Schwartz GK, Shah MA. Targeting the cell cycle: a new approach to cancer therapy. J Clin Oncol. 2005;23:9408–21. doi: 10.1200/JCO.2005.01.5594. [DOI] [PubMed] [Google Scholar]
- 17.Senderowicz AM. Targeting cell cycle and apoptosis for the treatment of human malignancies. Curr Opin Cell Biol. 2004;16:670–8. doi: 10.1016/j.ceb.2004.09.014. [DOI] [PubMed] [Google Scholar]
- 18.Hartwell LH, Kastan MB. Cell cycle control and cancer. Science. 1994;266:1821–8. doi: 10.1126/science.7997877. [DOI] [PubMed] [Google Scholar]
- 19.Blagosklonny MV, Pardee AB. The restriction point of the cell cycle. Cell Cycle. 2002;1:103–10. doi: 10.4161/cc.1.2.108. [DOI] [PubMed] [Google Scholar]
- 20.Paternot S, Bockstaele L, Bisteau X, Kooken H, Coulonval K, Roger PP. Rb inactivation in cell cycle and cancer: the puzzle of highly regulated activating phosphorylation of CDK4 versus constitutively active CDK-activating kinase. Cell Cycle. 2010;9:689–99. doi: 10.4161/cc.9.4.10611. [DOI] [PubMed] [Google Scholar]
- 21.Chellappan SP, Hiebert S, Mudryj M, Horowitz JM, Nevins JR. The E2F transcription factor is a cellular target for the RB protein. Cell. 1991;65:1053–61. doi: 10.1016/0092-8674(91)90557-F. [DOI] [PubMed] [Google Scholar]
- 22.Lu Z, Hunter T. Ubiquitylation and proteasomal degradation of the p21(Cip1), p27(Kip1) and p57(Kip2) CDK inhibitors. Cell Cycle. 2010;9:2342–52. doi: 10.4161/cc.9.12.11988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bracken AP, Ciro M, Cocito A, Helin K. E2F target genes: unraveling the biology. Trends Biochem Sci. 2004;29:409–17. doi: 10.1016/j.tibs.2004.06.006. [DOI] [PubMed] [Google Scholar]
- 24.Baldi A, De Luca A, Esposito V, Campioni M, Spugnini EP, Citro G. Tumor suppressors and cell cycle proteins in lung cancer. Patholog Res Int. 2011;2011:605042. doi: 10.4061/2011/605042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ho A, Dowdy SF. Regulation of G(1) cell cycle progression by oncogenes and tumor suppressor genes. Curr Opin Genet Dev. 2002;12:47–52. doi: 10.1016/S0959-437X(01)00263-5. [DOI] [PubMed] [Google Scholar]
- 26.Giordano A, Rustum YM, Wenner CE. Cell cycle: molecular targets for diagnosis and therapy: tumor suppressor genes and cell cycle progression in cancer. J Cell Biochem. 1998;70:1–7. doi: 10.1002/(SICI)1097-4644(19980701)70:1<1::AID-JCB1>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- 27.Vermeulen K, Van Bockstaele DR, Berneman ZN. The cell cycle: a review of regulation, deregulation and therapeutic targets in cancer. Cell Prolif. 2003;36:131–49. doi: 10.1046/j.1365-2184.2003.00266.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gaetano C, Matsumoto K, Thiele CJ. In vitro activation of distinct molecular and cellular phenotypes after induction of differentiation in a human neuroblastoma cell line. Cancer Res. 1992;52:4402–7. [PubMed] [Google Scholar]
- 29.Liu Q, Guntuku S, Cui XS, Matsuoka S, Cortez D, Tamai K, et al. Chk1 is an essential kinase that is regulated by Atr and required for the G(2)/M DNA damage checkpoint. Genes Dev. 2000;14:1448–59. [PMC free article] [PubMed] [Google Scholar]
- 30.Cui X, Doe CQ. ming is expressed in neuroblast sublineages and regulates gene expression in the Drosophila central nervous system. Development. 1992;116:943–52. doi: 10.1242/dev.116.4.943. [DOI] [PubMed] [Google Scholar]
- 31.Mellerick DM, Kassis JA, Zhang SD, Odenwald WF. castor encodes a novel zinc finger protein required for the development of a subset of CNS neurons in Drosophila. Neuron. 1992;9:789–803. doi: 10.1016/0896-6273(92)90234-5. [DOI] [PubMed] [Google Scholar]
- 32.Christine KS, Conlon FL. Vertebrate CASTOR is required for differentiation of cardiac precursor cells at the ventral midline. Dev Cell. 2008;14:616–23. doi: 10.1016/j.devcel.2008.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.McClellan KA, Slack RS. Novel functions for cell cycle genes in nervous system development. Cell Cycle. 2006;5:1506–13. doi: 10.4161/cc.5.14.2980. [DOI] [PubMed] [Google Scholar]
- 34.Fasano CA, Dimos JT, Ivanova NB, Lowry N, Lemischka IR, Temple S. shRNA knockdown of Bmi-1 reveals a critical role for p21-Rb pathway in NSC self-renewal during development. Cell Stem Cell. 2007;1:87–99. doi: 10.1016/j.stem.2007.04.001. [DOI] [PubMed] [Google Scholar]