Abstract
Caveolar domains act as platforms for the organization of molecular complexes involved in signal transduction. Caveolin proteins, the principal structural components of caveolae, have been involved in many cellular processes. Caveolin-1 (Cav-1) and caveolin-2 (Cav-2) are highly expressed in the lung. Cav-1-deficient mice (Cav-1−/−) and Cav-2-deficient mice (Cav-2−/−) exhibit severe lung dysfunction attributed to a lack of Cav-2 expression. Recently, Cav-1 has been shown to regulate lung fibrosis in different models. Here, we show that Cav-2 is also involved in modulation of the fibrotic response, but through distinct mechanisms. Treatment of wild-type mice with the pulmonary fibrosis-inducer bleomycin reduced the expression of Cav-2 and its phosphorylation at tyrosine 19. Importantly, Cav-2−/− mice, but not Cav-1−/− mice, were more sensitive to bleomycin-induced lung injury in comparison to wild-type mice. Bleomycin-induced lung injury was characterized by alveolar thickening, increase in cell density, and extracellular matrix deposition. The lung injury observed in bleomycin-treated Cav-2−/− mice was not associated with alterations in the TGF-β signaling pathway and/or in the ability to produce collagen. However, apoptosis and proliferation were more prominent in lungs of bleomycin-treated Cav-2−/− mice. Since Cav-1−/− mice also lack Cav-2 expression and show a different outcome after bleomycin treatment, we conclude that Cav-1 and Cav-2 have distinct roles in bleomycin induced-lung fibrosis, and that the balance of both proteins determines the development of the fibrotic process.
Keywords: caveolin, caveolae, fibrosis, bleomycin, lung
Introduction
Caveolins are structural proteins essential for the formation of caveolae. The caveolin family comprises three members: caveolin-1 (Cav-1), caveolin-2 (Cav-2), and caveolin-3 (Cav-3). Cav-1 and Cav-2 proteins show ubiquitous expression, while Cav-3 protein expression is restricted to muscle cells.1,2 The human Cav-1 and Cav-3 proteins share 65% identity and 85% similarity, and both have a conserved caveolin scaffolding domain (CSD) that confers the ability to interact with and inactivate other signaling proteins. The human Cav-2 protein is the most divergent member, sharing only 38% identity and 58% similarity with the human Cav-1 protein.3 Also, its CSD does not appear to have any regulatory function on signaling proteins, but may rather localize Cav-2 to the Golgi apparatus.4
Caveolin proteins are highly expressed in the lungs. Mice carrying disrupted Cav-1 and/or Cav-2 genes show severe pulmonary defects. The lung parenchyma of these mice is disorganized due to thickening of the alveolar septa, proliferation of endothelial cells, and fibrosis.5,6 These alterations were attributed to a lack of Cav-2 expression, since Cav-1−/− mice show a very low expression of Cav-2, which is retained in the Golgi, while Cav-2−/− mice express Cav-1 in the right localization (approximately 50% in comparison to wild-type mice).
It has consistently been shown that Cav-1 plays an important role in fibrosis, especially in skin and lung-related models. For instance, Tourkina et al. showed that Cav-1 silencing in normal lung fibroblasts resulted in increased collagen expression and activation of the MEK/ERK signaling pathway. Interestingly, lung fibroblasts isolated from scleroderma patients with pulmonary fibrosis show reduced Cav-1 expression and increased MEK/ERK activities as compared with normal lung fibroblasts.7 Cav-1 expression is also reduced in lung fibroblasts derived from idiopathic pulmonary fibrosis, and Cav-1 gene transfer ameliorates bleomycin-induced fibrosis in mice through inhibition of the TGF-β signaling pathway.8 Tourkina et al. further showed that treatment of mice with a peptide corresponding to the Cav-1 scaffolding domain diminishes bleomycin-induced lung injury.9 Our group showed corroborating results using Cav-1−/− mice and the CSD peptide, confirming the anti-fibrotic properties attributed to Cav-1.10 Interestingly, while bleomycin induces Cav-1 and Cav-2 expressions in the human epithelial lung cancer cell line A549,11 it conversely decreases Cav-1 and Cav-2 expressions in the rat type I-like alveolar epithelial cell line R3/1.12 Nonetheless, although Cav-2 is highly expressed in the lungs, investigation of its role in lung fibrosis is still neglected.
Here, we examined the impact of a loss of Cav-2 expression in a pulmonary fibrotic mouse model. We observed that lungs of wild-type mice treated with bleomycin display decreased expression of the Cav-2 β isoform as well as reduced phosphorylation of Cav-2 β at the tyrosine 19 residue. Cav-2−/− mice are also more sensitive to bleomycin-induced lung injury as compared with wild-type mice. Interestingly, despite their profibrotic phenotype in basal condition, lungs of Cav-1−/− mice show similar lung injury as wild-type mice following bleomycin treatment. The damage induced by bleomycin in Cav-2−/− mice is not due to an altered capacity to induce fibrosis, as bleomycin-treated wild-type, Cav-1−/−, and Cav-2−/− mice show comparable increases in hydroxyproline content. Furthermore, bleomycin-treated wild-type and Cav-2−/− mice show similar TGF-β levels, TGF-β receptor expression as well as similar activation of regulatory proteins of the TGF-β signaling pathway. By contrast, the higher susceptibility of Cav-2−/− mouse lungs to bleomycin is correlated with increased apoptosis and proliferation. These data indicate that Cav-1 and Cav-2 use distinct mechanisms to repair tissues after a fibrotic insult, and that the balance of both proteins determines the outcome of the fibrotic process.
Results
Bleomycin treatment decreases the expression and tyrosine phosphorylation of the Cav-2 β isoform
The expression of Cav-2 was determined by immunoblot analyses using the right cranial lobe of the lung of WT mice 14 d after bleomycin instillation. The expression of total and phosphorylated isoforms of Cav-2 was normalized to the expression of β-actin in each sample. As shown in Figure 1, expression of the β isoform of Cav-2 was highly diminished in bleomycin-treated mice (46% reduction compared with saline-treated mice, P = 0.0003), while expression of the α isoform was not altered (P = ns). Importantly, phosphorylation of Cav-2 β at tyrosine 19 was also greatly reduced, even considering that the total Cav-2 β levels were decreased after bleomycin treatment (Fig. 1, P < 0.0001). Indeed, while total levels of Cav-2 β were reduced by 46% in lungs of bleomycin-treated mice, the levels of PY19-Cav-2 β were reduced by 82%. In contrast, the reductions observed in total Cav-2 β levels and the phosphorylation level of Cav-2 β at serine 23 were similar, indicating that the capacity to phosphorylate Cav-2 at serine 23 is not altered after bleomycin treatment. Moreover, the α isoform of Cav-2 and its phosphorylated forms, either at tyrosine 19 or serine 23, were not altered after bleomycin instillation. Therefore the expression and activation of a specific isoform of Cav-2, i.e., the β isoform, are highly altered in the injured pulmonary tissue.
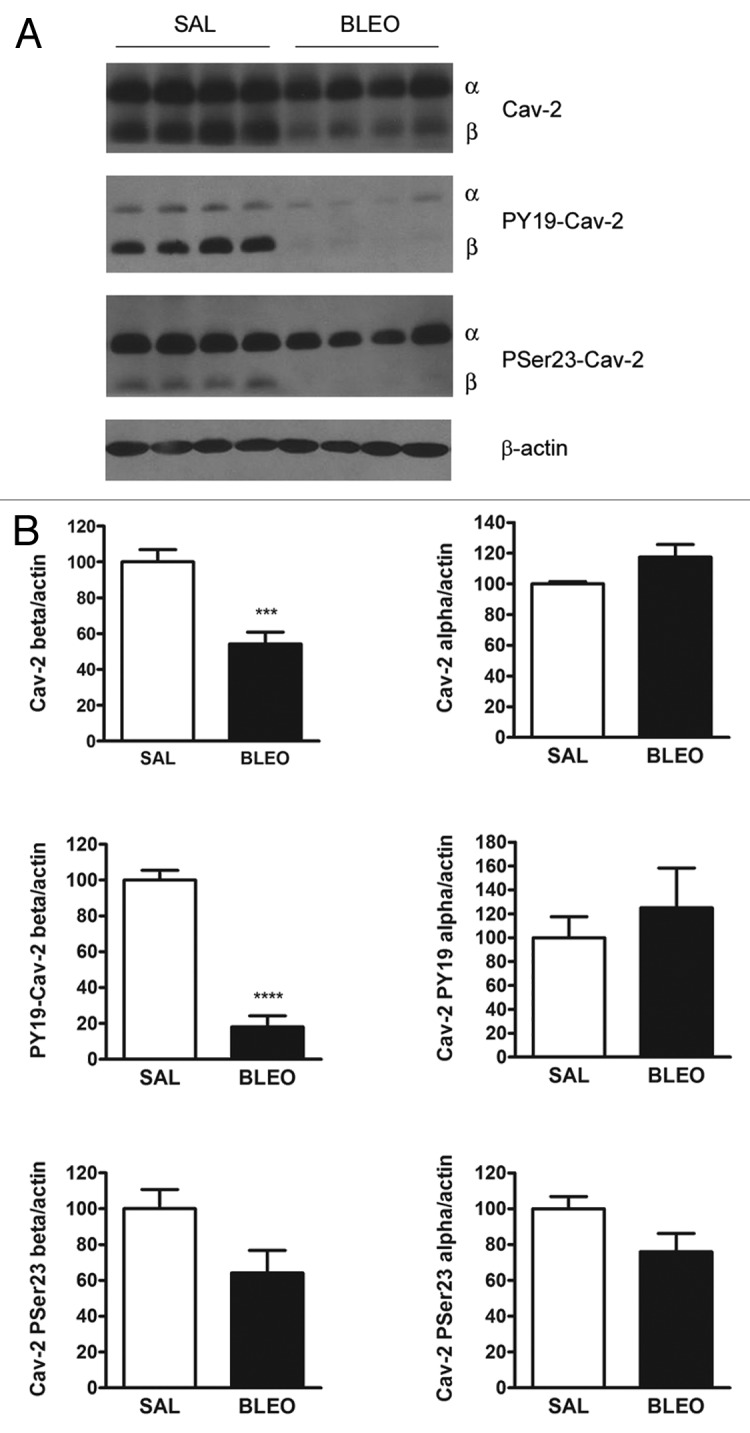
Figure 1. bleomycin treatment decreases the expression and phosphorylation of the Cav-2 beta isoform. (A) Immunoblot analyses of the expression of Cav-2 and its phosphorylated isoforms in lung lysates of wild-type mice treated with saline or bleomycin. β-actin is shown as a control for equal protein loading. Four mice are shown for each group (n = 7–10 for each group). (B) Densitometric analyses of the expression of Cav-2 and its phosphorylated isoforms were performed with the ImageJ software. The expression of Cav-2 and its phosphorylated isoforms were then normalized to β-actin expression and expressed as a percentage of the WT-SAL group. Bleomycin treatment decreases the protein levels of total Cav-2 β (***P = 0.0003) and PY19-Cav-2 β (****P < 0.0001) (n = 7–10 for each group).
Cav-2−/− mice are more sensitive to bleomycin-induced lung injury
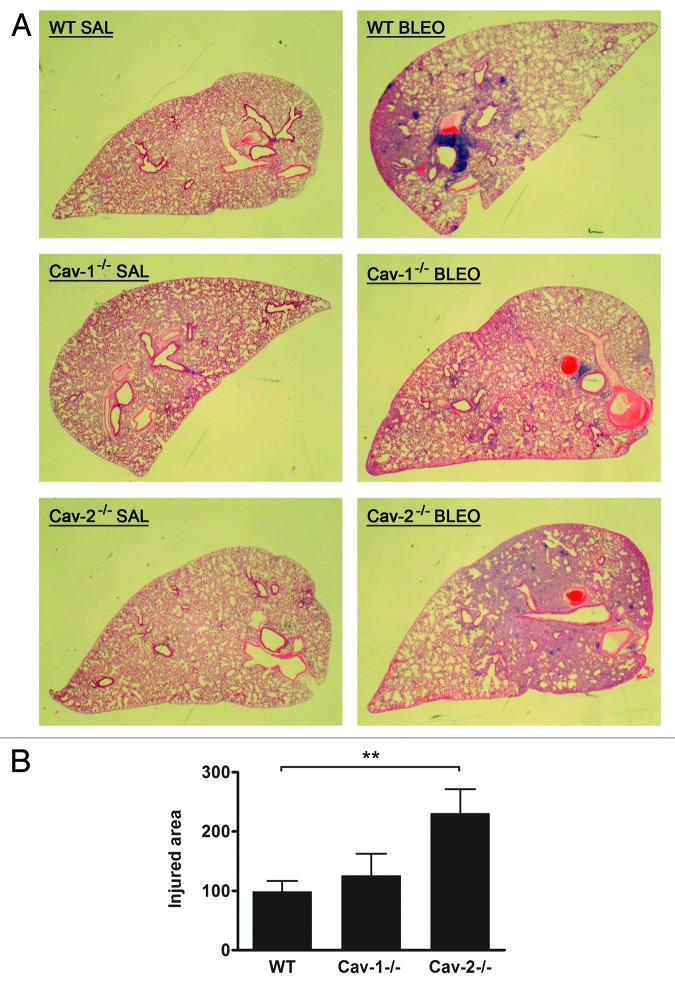
In order to investigate the effect of a lack of Cav-2 expression on bleomycin-induced lung injury, wild-type, Cav-1−/−, and Cav-2−/− mice were intratracheally instilled with bleomycin and sacrificed 14 d after for histological analysis of the lung. Lung injury was quantified by measuring the percentage of total area of the section composed of reorganized parenchyma and was expressed as a percentage of the WT group. Bleomycin-induced lung injury was characterized by a loss of pulmonary architecture caused by thickening of alveolar septa, accumulation of proliferating and inflammatory cells, and filling of alveolar spaces with cells and/or extracellular matrix. Interestingly, wild-type and Cav-1−/− mice treated with bleomycin developed similar lung injury (Fig. 2A and B, P = ns). In contrast, Cav-2−/− mice were more susceptible to bleomycin, showing larger areas of injured tissue in comparison to wild-type mice (Fig. 2A and B, P < 0.01). Therefore, bleomycin-induced lung damage is exacerbated in Cav-2−/− mice.
Figure 2.Cav-2−/− mice are more susceptible to bleomycin-induced lung injury. (A) Cav-2−/− mice show larger areas of injured parenchyma after bleomycin instillation. Wild-type, Cav-1−/−, and Cav-2−/− mice were intratracheally instilled with saline or bleomycin (1 U/kg) (n-12–22 for each group). Fourteen days later, the left lung was processed for histological analysis and stained with H&E. Bleomycin leads to reorganization of the lung parenchyma characterized by thickened alveolar septa and accumulation of proliferating and inflammatory cells. (B) Quantification of lung injury after bleomycin instillation. Lung injury was quantified by measuring the percentage of total area of the section composed of reorganized parenchyma. Measurement of the total and damaged areas was achieved with the ImageJ software. Data were expressed as a percentage of the WT group (**P < 0.01, n = 12–22 for each group).
Fibrosis is not altered in Cav-2−/− mice
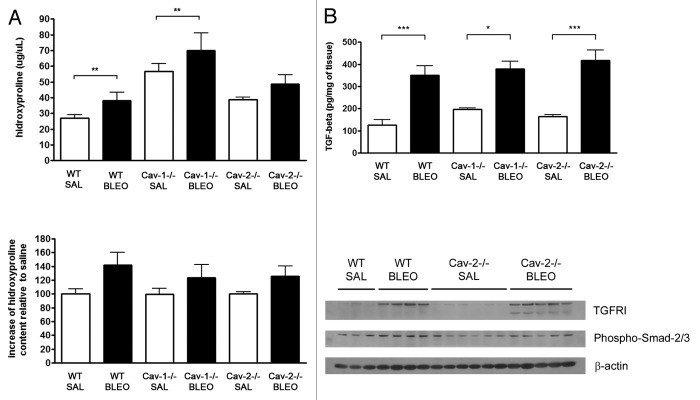
To evaluate whether lung injury was due to alterations in the fibrotic response in Cav-2−/− mice, we determined the hydroxyproline content of the right caudal lobe of the lung, as an indication of collagen deposition in the pulmonary tissue. Although basal levels of hydroxyproline were higher in Cav-1−/− mice, the increase in hydroxyproline deposition observed following bleomycin instillation was similar among the different genotypes (Fig. 3A, P = ns). In addition, we also determined the levels of TGF-β in lungs of all genotypes. As shown in Figure 3B, the increase in TGF-β levels observed after bleomycin treatment were similar in all the genotypes tested (P = ns). Moreover, expressions of the TGF-β receptor I as well as the activation of Smad2/3 were similar in both wild-type and Cav-2−/− mice subjected to bleomycin instillation (Fig. 3B). Therefore, we did not find any alterations in the TGF-β-signaling pathway after bleomycin treatment in lungs of Cav-2−/− mice.
Figure 3. Fibrosis is not altered in bleomycin-treated Cav-2−/− mice. (A) The collagen content is similar in lungs of bleomycin-treated wild-type and Cav-2−/− mice. Hydroxyproline content of the right lung was determined and expressed as the total amount per lobe (upper panel) and as a percentage of the hydroxyproline content in saline-treated mice (lower panel) (**P < 0.01, n = 5–7 for each group). (B) The expression and activation of members of the TGF-β signaling pathway are not altered in Cav-2−/− mice. Upper panel: TGF-β levels were determined in lysates from the right lung of mice instilled with saline or bleomycin for 14 d (*P < 0.05, ***P < 0.001, n = 5–9 for each group). Lower panel: Expressions of the TGF-β receptor I and the phosphorylated form of Smad2/3 were detected by immunoblot analyses of lung lysates from wild-type and Cav-2−/− mice instilled intratracheally with saline or bleomycin and were shown to be present in similar levels in wild-type and Cav-2−/− mice. β-actin is shown as a control for equal protein loading. Each group consists of 3–6 mice (WT SAL, n = 3; WT BLEO, n = 4; Cav-2−/−, n = 6, and Cav-2−/−, n = 5).
Apoptosis incidence is higher in Cav-2−/− mice after bleomycin instillation
Bleomycin is a strong inducer of apoptosis, and apoptosis is a stimulus for the fibrotic response, including the proliferation and accumulation of cells in the lungs.13 We determined the incidence of apoptotic cells after instillation of saline or bleomycin in lung sections of all genotypes. As shown in Figure 4, Cav-2−/− mice treated with bleomycin show an increased number of apoptotic cells (~2-fold) as compared with their wild-type and Cav-1−/− counterparts (P < 0.05).
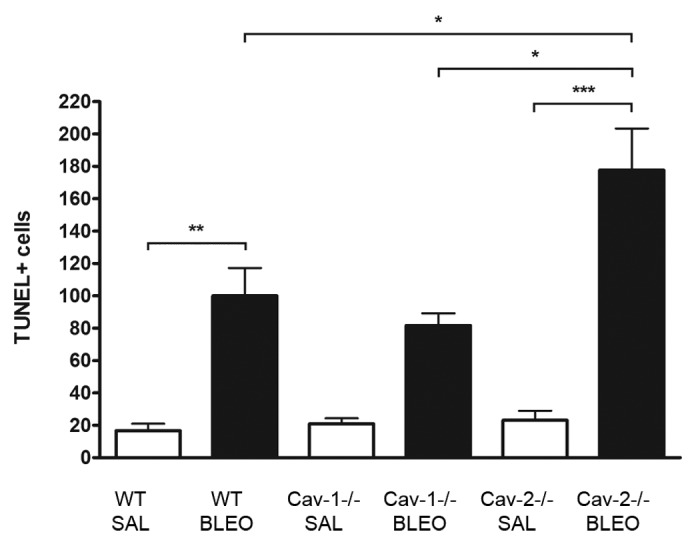
Figure 4. Lungs of bleomycin-treated Cav-2−/− mice exhibit an increased incidence of apoptotic cells as compared with WT and Cav-1−/− mice. Whole sections of paraffin-embedded left lungs of wild-type, Cav-1−/− and Cav-2−/− mice treated with saline or bleomycin were used for detection of apoptosis by the TUNEL technique. Data were expressed as a percentage of the WT+Bleo group (* p < 0.05, ** p < 0.01, *** p < 0.001, n = 4 to 11 for each group)
We also determined the expression of proteins involved in proliferation in lungs of wild-type and Cav-2−/− mice treated or not with bleomycin. Interestingly, we detected an increase in Cyclin D1 expression in bleomycin-treated mice, which is higher in Cav-2−/− mice in comparison to wild-type mice. In contrast, we detected an increase in the expression of the CDK (cyclin-dependent kinase) inhibitor p27 in bleomycin-treated wild-type mice, but not in Cav-2−/− mice (Fig. 5).
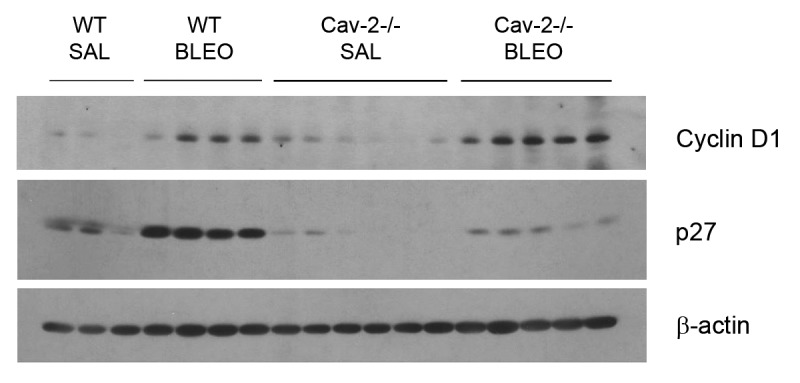
Figure 5. Lungs of bleomycin-treated Cav-2−/− mice exhibit altered expression of cyclin D1 and p27, indicating increased proliferation. Lysates from the right lung of wild-type and Cav-2−/− mice treated with saline or bleomycin were submitted to SDS-PAGE. Cyclin D1 and p27 were detected with specific antibodies. β-actin is shown as a control for equal protein loading. Each group consists of 3–6 mice (WT SAL, n = 3; WT BLEO, n = 4; Cav-2−/−, n = 6, and Cav-2−/−, n = 5).
Thus, Cav-2 modulates the fibrotic response by a mechanism that involves signal transduction pathways implicated in the control of apoptosis and proliferation, rather than the induction of molecules of the extracellular matrix, such as collagen. Since Cav-1−/− mice also lack Cav-2 expression and are less severely affected by bleomycin treatment as compared with Cav-2−/− mice (which still express Cav-1), we suggest that the balance of Cav-1 and Cav-2 determines the outcome of bleomycin-induced injury.
Discussion
Bleomycin is an antibiotic largely used in chemotherapy protocols. Nevertheless, an important side-effect of bleomycin is the development of lung fibrosis. Hence, bleomycin has been largely used as a prototypic inducer of lung fibrosis in animal studies. It has been shown that bleomycin activates the TGF-β signaling pathway leading to collagen deposition. Furthermore, many studies demonstrated that it induces apoptosis, particularly within lung epithelial cells, which triggers the fibrogenic response. Interestingly, apoptosis inhibitors are able to block the deposition of collagen in the pulmonary tissue.13
Here, we observed that Cav-2−/− mice are more sensitive to bleomycin-induced lung injury than wild-type and/or Cav-1−/− mice. Bleomycin-induced lung injury is characterized by alveolar septa thickening, increase in cellularity and disorganization of the lung parenchyma, collagen deposition, and induction of apoptosis. Interestingly, collagen production, TGF-β levels, and the activation of the TGF-β signaling pathway are not altered in bleomycin-treated Cav-2−/− mice in comparison to their wild-type counterparts. In contrast, proliferation markers, such as Cyclin D1 and p27 are altered in bleomycin-treated Cav-2−/− mice. Indeed, we showed that expression of Cyclin D1 is increased after bleomycin instillation, and this effect is more pronounced in the lungs of Cav-2−/− mice. Moreover, bleomycin treatment increases the expression of the CDK inhibitor p27 in the lungs of wild-type mice, but not in those of Cav-2−/− mice. These results indicate a higher incidence of proliferating cells in lungs of bleomycin-treated Cav-2−/− mice. Downregulation of p27 and the subsequent upregulation of Cyclin D1 expression are also implicated in IPF, another model of lung fibrosis,14 and in the proliferation of distinct pulmonary cell types such as fibroblasts15 and alveolar type II cells.16 Cav-2 is also implicated in the regulation of proliferation. It has been shown that the expression of Cav-2 counteracts the effects of miR-199a-3p, a microRNA that mediates proliferation and survival of tumor cells.17 According to this idea, are reports of thickening of the alveolar septa and proliferation of endothelial cells in lungs of Cav-2−/− mice.5,6
In addition, we noticed that lungs of bleomycin-treated mice present dead cells with condensed nuclei and well-preserved membranes, compatible with apoptotic cell death. Bleomycin is a toxic compound that may provoke distinct mechanisms of cell death according to the number of molecules internalized. Indeed, while thousands of molecules of bleomycin induce mitotic death, which is characterized by enlarged polynucleated cells, millions of molecules of bleomycin lead to apoptosis.18 Interestingly, it is also known that bleomycin does not cross the cell membrane efficiently. Some studies indicated that bleomycin binds to a protein receptor on the plasma membrane, which is then endocytosed.19,20 Here, we detected an increase in the number of apoptotic cells after bleomycin treatment in lungs of Cav-2−/− mice as compared with wild-type mice. We did not notice any enlarged polyploid cells in our preparations. These results indicate that the cells are exposed to high quantities of bleomycin. Interestingly, Schmuel et al. have presented evidences that Cav-2 may act as an inhibitory modulator of endocytosis of the M1 muscarinic receptor in epithelial cells.21 Moreover, our recent results show that, in contrast to wild-type mice, intestinal epithelial cells of Cav-2−/− mice display big vacuoles in the cell cytoplasm after intraperitoneal injection of LPS.22 Therefore, a more efficient uptake of bleomycin molecules by the pulmonary epithelial cells could explain the higher incidence of apoptotic cells in lungs of Cav-2−/− mice. Altogether, our results suggest that the increased damage observed in bleomycin-treated Cav-2−/− mice results from alterations in the apoptotic and proliferation processes.
Finally, we conclude that the balance of Cav-1 and Cav-2 expressions determines the outcome of tissue repair after bleomycin-induced lung injury. Interestingly, although Cav-2 expression is absent from lung tissues of Cav-1−/− mice, the pulmonary damage observed in these mice is comparable to the damage observed in wild-type mice. These results are in accordance with our results with LPS-induced sepsis in Cav-1−/− and Cav-2−/− mice. Indeed, a lack of Cav-2 expression is correlated with a higher sensitivity to LPS. In contrast, Cav-1−/− mice show a similar response to LPS as wild-type mice. Moreover, several mechanisms involved in the sepsis response are induced in opposite ways in Cav-1−/− and Cav-2−/− mice. For instance, induction of iNOS, production of NO, increase in permeability are more prominent in LPS-treated Cav-2−/− mice, whereas it is less induced in LPS-treated Cav-1−/− in comparison to wild-type mice.22 Since Cav-1−/− mice do not express Cav-2 and Cav-2−/− mice display a 50% reduction of Cav-1 expression, we conclude that it is not only the absence of Cav-2 that promote these effects, but rather the absence of Cav-2 in the presence of Cav-1.
Materials and Methods
Materials
The bleomycin and phosphatase inhibitors were purchased from Sigma Chemical Co. Protease inhibitors are from Roche, the LSAB2 system kit from Dako Cytomation and the TACS2 Tdt-blue label in situ apoptosis detection kit from Trevigen, Inc. Antibodies were as follows: β-actin (cat# A1978, Sigma), Cav-2 (cat# 610684, BD-PharMingen), phospho(Tyr19)-Cav-2 (cat# PA1-060, Affinity Bioreagents), phospho(Ser23)-Cav-2 (cat# PA1-25625, Affinity Bioreagents), cyclin D1 (cat# sc-717, Santa Cruz Biotechnology), p27 (cat# sc-1641, Santa Cruz Biotechnology), phospho(Ser423/425) Smad2/3 (cat# sc-11769, Santa Cruz Biotechnology), Smad 7 (cat# sc-11392, Santa Cruz Biotechnology), and TGF-βRI (cat# sc-398, Santa Cruz Biotechnology).
Animal studies
All animals were housed and maintained in a pathogen-free environment/barrier facility at the Kimmel Cancer Center at Thomas Jefferson University. Mice were kept on a 12 h light/dark cycle with ad libitum access to chow diet and water. Cav-1−/− and Cav-2−/− mice were generated as previously described.6,23
Only 12-wk-old male mice in the C57Bl/6J genetic background were used for these experiments. Experimental procedures were approved by the Institutional Animal Care and Use Committee (IACUC) at Thomas Jefferson University.
Bleomycin-induced lung fibrosis
Mice were anesthetized with a mixture of 5 mg/kg xylazine and 50 mg/kg ketamine. A dose of 1 U/kg of bleomycin in 50 μL sterile saline solution was administered intra-tracheally followed by 300 μL of air to assure the spreading of the drug to the distal sites of the lungs. Control animals were given an equal volume of sterile saline only. Fourteen days after administration of saline or bleomycin, mice were euthanized by CO2 inhalation.
Immunoblot analyses
For protein isolation from lung tissue, the right cranial lobe of the lung was isolated, promptly frozen in liquid nitrogen, and stored at –80 °C. The frozen tissue samples were then homogenized in an appropriate volume of lysis buffer (10 mmol/L Tris, pH 7.5, 150 mmol/L NaCl, 1% Triton X-100, and 60 mmol/L n-octyl-glucoside), containing protease and phosphatase inhibitors. Tissue lysates were then centrifuged at 12000 × g for 10 min (at 4 °C) to remove insoluble debris. Protein concentrations were analyzed using the BCA reagent (Pierce), and the volume required for 50 µg of protein was determined. Samples were then separated by sodium dodecyl sulfate (SDS)-PAGE (12% acrylamide) and transferred to nitrocellulose. All subsequent wash buffers contained 10 mmol/L Tris, pH 8.0, 150 mmol/L NaCl, 0.05% Tween 20, which was supplemented with 5% nonfat dry milk (Carnation) for the blocking solution, and 1% bovine serum albumin for the antibody diluent. Primary antibodies were used at a 1:1000 dilution. Horseradish peroxidase-conjugated secondary antibodies (anti-mouse 1:6000 dilution [Pierce] or anti-rabbit 1:5000 [Transduction Laboratories]) were used to visualize bound primary antibodies with the Supersignal chemiluminescence substrate (Pierce). When phospho-specific antibody probes were used, nonfat dry milk was omitted from the blocking and primary antibody solutions.
Histology
Fourteen days after bleomycin instillation, mice were sacrificed and the left lobe of the lung was collected, perfused with 10% buffered neutral formalin, and placed in 10% buffered neutral formalin for fixation. Twenty-four hours later, formalin was substituted for 70% ethanol. The tissue was paraffin-embedded, sectioned and stained with hematoxylin and eosin (H&E). Histological sections were examined with a Zeiss phase contrast microscope. Injured areas were measured using the ImageJ software.
Hydroxyproline assay
The right caudal lungs obtained from mice were used for a hydroxyproline assay. The lungs were weighted and hydrolyzed in 6N HCl for 16 h before hydroxyproline content determination, as previously described.24
TGF-β ELISA
Protein samples derived from the right cranial lobe of the lung were prepared as described above. TGF-β was detected using the TGF-β1 Emax immunoassay system (Promega) according to the manufacturer’s instructions.
In situ apoptosis staining (TUNEL) assay
Paraffin-embedded sections were deparaffinized, and rehydrated with decreasing concentrations of ethanol. Apoptosis was detected by the in situ TUNEL (terminal deoxynucleotidyl transferase biotin-dUTP nick end labeling) labeling procedure using the TACS2 Tdt-blue label in situ apoptosis detection kit. In brief, sections were treated with 50 μL of a proteinase K solution for 20 min and quenched in freshly prepared 3% hydrogen peroxide in methanol for 4 min at room temperature. Then, sections were labeled with the TdT dNTP mix at 37°C for 1 h in a humidified chamber. The color reaction was developed with the Streptavidin-HRP solution for 10 min, followed by incubation with the blue label solution for 3 min at room temperature. After counterstaining for 1 min with the nuclear dye fast red, sections were dehydrated and mounted with mounting medium (Surgipath Inc).
Statistical analysis
All values are expressed as mean ± SEM. Data sets were examined by one-way analysis of variance (ANOVA), and individual group means were compared with the Student unpaired t test. A P value of < 0.05 was considered significant.
Acknowledgments
This work was supported by grants from the National Institutes of Health. Cecilia J de Almeida was supported by a Fogarty International Training Grant (1D43TW007129). Jean-Francois Jasmin was supported by an American Lung Association Biomedical Research Grant. Also, these developments were made possible through the resources of Thomas Jefferson University.
Glossary
Abbreviations:
- CDK
cyclin-dependent kinase
- ERK
extracellular signal-regulated kinases
- iNOS
inducible nitric oxide synthase
- IPF
idiopathic pulmonary fibrosis
- LPS
lipopolysaccharide
- MEK
MAPK/ERK kinase
- NO
nitric oxide
- TGF-β
tumor growth factor-β
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Footnotes
Previously published online: www.landesbioscience.com/journals/cc/article/25335
References
- 1.Scherer PE, Lewis RY, Volonte D, Engelman JA, Galbiati F, Couet J, et al. Cell-type and tissue-specific expression of caveolin-2. Caveolins 1 and 2 co-localize and form a stable hetero-oligomeric complex in vivo. J Biol Chem. 1997;272:29337–46. doi: 10.1074/jbc.272.46.29337. [DOI] [PubMed] [Google Scholar]
- 2.Song KS, Scherer PE, Tang Z, Okamoto T, Li S, Chafel M, et al. Expression of caveolin-3 in skeletal, cardiac, and smooth muscle cells. Caveolin-3 is a component of the sarcolemma and co-fractionates with dystrophin and dystrophin-associated glycoproteins. J Biol Chem. 1996;271:15160–5. doi: 10.1074/jbc.271.25.15160. [DOI] [PubMed] [Google Scholar]
- 3.Cohen AW, Hnasko R, Schubert W, Lisanti MP. Role of caveolae and caveolins in health and disease. Physiol Rev. 2004;84:1341–79. doi: 10.1152/physrev.00046.2003. [DOI] [PubMed] [Google Scholar]
- 4.Breuza L, Corby S, Arsanto JP, Delgrossi MH, Scheiffele P, Le Bivic A. The scaffolding domain of caveolin 2 is responsible for its Golgi localization in Caco-2 cells. J Cell Sci. 2002;115:4457–67. doi: 10.1242/jcs.00130. [DOI] [PubMed] [Google Scholar]
- 5.Drab M, Verkade P, Elger M, Kasper M, Lohn M, Lauterbach B, et al. Loss of caveolae, vascular dysfunction, and pulmonary defects in caveolin-1 gene-disrupted mice. Science. 2001;293:2449–52. doi: 10.1126/science.1062688. [DOI] [PubMed] [Google Scholar]
- 6.Razani B, Wang XB, Engelman JA, Battista M, Lagaud G, Zhang XL, et al. Caveolin-2-deficient mice show evidence of severe pulmonary dysfunction without disruption of caveolae. Mol Cell Biol. 2002;22:2329–44. doi: 10.1128/MCB.22.7.2329-2344.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tourkina E, Gooz P, Pannu J, Bonner M, Scholz D, Hacker S, et al. Opposing effects of protein kinase Calpha and protein kinase Cepsilon on collagen expression by human lung fibroblasts are mediated via MEK/ERK and caveolin-1 signaling. J Biol Chem. 2005;280:13879–87. doi: 10.1074/jbc.M412551200. [DOI] [PubMed] [Google Scholar]
- 8.Wang XM, Zhang Y, Kim HP, Zhou Z, Feghali-Bostwick CA, Liu F, et al. Caveolin-1: a critical regulator of lung fibrosis in idiopathic pulmonary fibrosis. J Exp Med. 2006;203:2895–906. doi: 10.1084/jem.20061536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tourkina E, Richard M, Gööz P, Bonner M, Pannu J, Harley R, et al. Antifibrotic properties of caveolin-1 scaffolding domain in vitro and in vivo. Am J Physiol Lung Cell Mol Physiol. 2008;294:L843–61. doi: 10.1152/ajplung.00295.2007. [DOI] [PubMed] [Google Scholar]
- 10.Del Galdo F, Sotgia F, de Almeida CJ, Jasmin JF, Musick M, Lisanti MP, et al. Decreased expression of caveolin 1 in patients with systemic sclerosis: crucial role in the pathogenesis of tissue fibrosis. Arthritis Rheum. 2008;58:2854–65. doi: 10.1002/art.23791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Linge A, Morishima N, Kasper M, Barth K. Bleomycin induces caveolin-1 and -2 expression in epithelial lung cancer A549 cells. Anticancer Res. 2007;27(3A):1343–51. [PubMed] [Google Scholar]
- 12.Koslowski R, Barth K, Augstein A, Tschernig T, Bargsten G, Aufderheide M, et al. A new rat type I-like alveolar epithelial cell line R3/1: bleomycin effects on caveolin expression. Histochem Cell Biol. 2004;121:509–19. doi: 10.1007/s00418-004-0662-4. [DOI] [PubMed] [Google Scholar]
- 13.Uhal BD. Apoptosis in lung fibrosis and repair. Chest. 2002;122(Suppl):293S–8S. doi: 10.1378/chest.122.6_suppl.293S. [DOI] [PubMed] [Google Scholar]
- 14.Moodley YP, Scaffidi AK, Misso NL, Keerthisingam C, McAnulty RJ, Laurent GJ, et al. Fibroblasts isolated from normal lungs and those with idiopathic pulmonary fibrosis differ in interleukin-6/gp130-mediated cell signaling and proliferation. Am J Pathol. 2003;163:345–54. doi: 10.1016/S0002-9440(10)63658-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wu SH, Wu XH, Lu C, Dong L, Chen ZQ. Lipoxin A4 inhibits proliferation of human lung fibroblasts induced by connective tissue growth factor. Am J Respir Cell Mol Biol. 2006;34:65–72. doi: 10.1165/rcmb.2005-0184OC. [DOI] [PubMed] [Google Scholar]
- 16.Zhang F, Nielsen LD, Lucas JJ, Mason RJ. Transforming growth factor-beta antagonizes alveolar type II cell proliferation induced by keratinocyte growth factor. Am J Respir Cell Mol Biol. 2004;31:679–86. doi: 10.1165/rcmb.2004-0182OC. [DOI] [PubMed] [Google Scholar]
- 17.Shatseva T, Lee DY, Deng Z, Yang BB. MicroRNA miR-199a-3p regulates cell proliferation and survival by targeting caveolin-2. J Cell Sci. 2011;124:2826–36. doi: 10.1242/jcs.077529. [DOI] [PubMed] [Google Scholar]
- 18.Tounekti O, Pron G, Belehradek J, Jr., Mir LM. Bleomycin, an apoptosis-mimetic drug that induces two types of cell death depending on the number of molecules internalized. Cancer Res. 1993;53:5462–9. [PubMed] [Google Scholar]
- 19.Pron G, Belehradek J, Jr., Mir LM. Identification of a plasma membrane protein that specifically binds bleomycin. Biochem Biophys Res Commun. 1993;194:333–7. doi: 10.1006/bbrc.1993.1824. [DOI] [PubMed] [Google Scholar]
- 20.Pron G, Mahrour N, Orlowski S, Tounekti O, Poddevin B, Belehradek J, Jr., et al. Internalisation of the bleomycin molecules responsible for bleomycin toxicity: a receptor-mediated endocytosis mechanism. Biochem Pharmacol. 1999;57:45–56. doi: 10.1016/S0006-2952(98)00282-2. [DOI] [PubMed] [Google Scholar]
- 21.Shmuel M, Nodel-Berner E, Hyman T, Rouvinski A, Altschuler Y. Caveolin 2 regulates endocytosis and trafficking of the M1 muscarinic receptor in MDCK epithelial cells. Mol Biol Cell. 2007;18:1570–85. doi: 10.1091/mbc.E06-07-0618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.de Almeida CJ, Witkiewicz AK, Jasmin JF, Tanowitz HB, Sotgia F, Frank PG, et al. Caveolin-2-deficient mice show increased sensitivity to endotoxemia. Cell Cycle. 2011;10:2151–61. doi: 10.4161/cc.10.13.16234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Razani B, Combs TP, Wang XB, Frank PG, Park DS, Russell RG, et al. Caveolin-1-deficient mice are lean, resistant to diet-induced obesity, and show hypertriglyceridemia with adipocyte abnormalities. J Biol Chem. 2002;277:8635–47. doi: 10.1074/jbc.M110970200. [DOI] [PubMed] [Google Scholar]
- 24.Stegemann H, Stalder K. Determination of hydroxyproline. Clin Chim Acta. 1967;18:267–73. doi: 10.1016/0009-8981(67)90167-2. [DOI] [PubMed] [Google Scholar]