Abstract
Upon entrance into the host, fungi encounter a myriad of host effector products and microenvironments that they sense and adapt to for survival. Alterations of the structure and composition of the cell wall is a major fungal adaptation mechanism to evade these environments. Here we discuss recent findings of host-microenvironmental induced fungal cell wall changes, including structure, composition, and protein content, and their effects on host immune responses. A take home message from these recent studies is an emerging understanding of how integration of multiple signals, of both fungal and host responses to dynamic infection site microenvironments, determines outcomes of infection. A challenge moving forward is to further understand these mechanisms and harness them for therapeutic benefit.
Introduction
Fungi encounter diverse microenvironments in mammalian hosts, including macro and micro nutrient limitation, pH changes, oxygen levels, antifungal drugs, and host derived proteins/effectors. An important response to these microenvironments is modulation of a major defensive apparatus, the fungal cell wall (CW). CW’s of fungi are dynamic structures that fluctuate not only during growth, but in direct response to environmental stimuli and stress [1]. While the underlying mechanisms are not fully understood, they are important because detection of CW components drives immune responses through host pattern recognition receptor (PRRs) sensing of pathogen associated molecular patterns (PAMPs) [2]. Responses between the fungus and host may lead to a protective response or host mediated damage. For example, CW components can lead to host damage, such as in immune reconstitution syndrome (IRIS) [3], or they can suppress protective responses, such as with the recently described Aspergillus fumigatus CW component galactosaminogalactan [4]. These interactions have traditionally been depicted as one-dimensional and there is much unknown about how host microenvironments affect this relationship at the initiation of the interaction and throughout the course of infection during antifungal therapy. Here, we discuss recent observations illustrating how host microenvironments affect CW dynamics of fungi associated with human disease (Figure 1).
Figure 1.
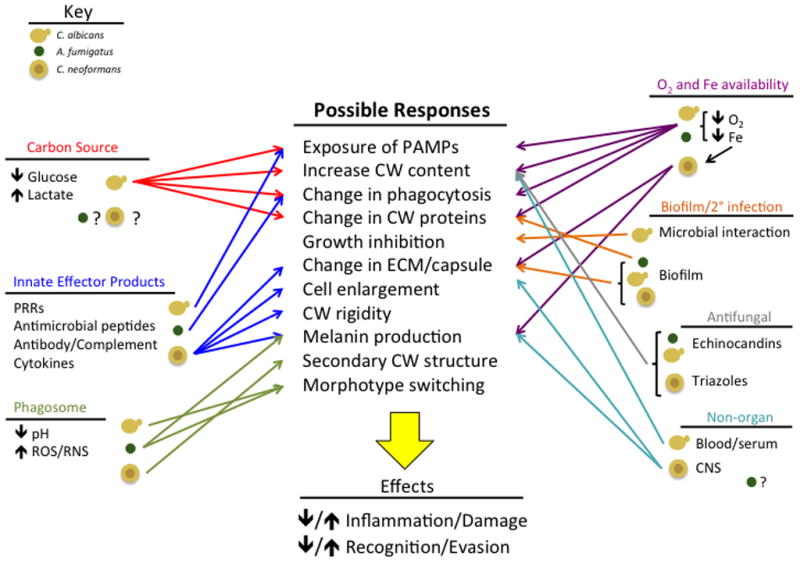
Potential Responses of the Fungal Cell wall to Signals encountered in infection site microenvironments. The diagram presents the various signals that are known to occur in vivo during fungal pathogenesis and the potential responses of the fungal cell wall in Aspergillus fumigatus, Candida albicans, and Cryptococcus neoformans. The diagram highlights the complexity of in vivo microenvironments and the number of signals that the invading fungus must integrate into the subsequent response. A challenge moving forward is to understand these in vivo microenvironments and how signal integration leads to disease outcome.
Carbon source
Carbon sources utilized by fungi in vivo remain to be fully defined and vary with host niche and fungus. Most host environments are sugar limited causing the fungi to rely on alternative energy sources, such as amino and organic acids [5,6]. However, most in vitro analyses of the fungal cell wall (and growth) occur in sugar, usually glucose, rich conditions. Growth of A. fumigatus on glucose alone was found to influence the effect of antifungal inhibitory properties [7]. The importance of the variety and dynamism of these in vivo nutrient sources on the fungal CW is now being understood. Lactate, a non-fermentable carbon source, produced at sites of infection has strong effects on the CW of C. albicans. Growth on lactate induced a decrease in the CW mass, increased expression of CW remodeling proteins, and increases in CW synthase gene transcripts [6,8]. Additionally, the lactate grown CW was more resistant to osmotic stress, CW perturbing agents, and antifungal drugs [8]. Lactate induced changes in C. albicans affected the yeasts interaction with the immune response, with decreased phagocytosis and more efficient killing and evading of macrophages [9]. There is less known about the effects of carbon source on CW and PAMP changes for other fungi even though nutrient limiting conditions were found in vivo in infection transcriptomic data [10]. Utilization of different carbon sources for A. fumigatus and C. neoformans are involved in virulence, but whether there is contribution from CW changes and immune recognition is unknown [11,12]. With regard to mechanism of carbon source induced CW changes, differences in ubiquitination targets between fungi may lead to metabolic flexibility that is likely critical for fungal survival in mammalian hosts [13].
Immune Effector Products
During infection, fungi encounter immune effector products. An exciting emerging area of fungal pathogenesis research is the observation that immune effector products such as PRRs, antifungal/microbial peptides, and antibody/complement alter fungal CW composition, metabolism, and virulence. Binding of murine soluble PRR Ptx3, causes remodeling of the CW and alterations in the inflammatory response with C. albicans and increased phagocytosis of A. fumigatus conidia [14]. Remodeling of CWs by antimicrobial peptides, such as LL-37, was prevented in C. albicans through reduction of Xogp1 activity [15,16]. For C. neoformans, an antimicrobial peptide from a cattle tick causes defects in melanization and capsule formation, leading to decreased survival [17]. One resulting hypothesis is that fungal interactions with immune cells, through PRRs, trigger CW remodeling as an immune evasion strategy.
One immune evasion technique that C. neoformans utilizes is the formation of enlarged, multinucleated “titan” yeast cells that cannot be phagocytosed. An unknown effector product in the lung stimulates the formation of these cells by signaling through the G-coupled receptor Grp5 and activating CW and cell structure remodeling [18]. Moreover, antibody binding to C. neoformans caused changes, different between serotypes and strains, in the CW diameter, capsule size, and overall rigidity of the CW [19–21]. Additionally, use of antibody alone caused inhibition of capsule formation and growth in C. neoformans and C. albicans, respectively, but the changes induced in the CW or capsule by antibody binding are unknown. Recently the cytokine IL-17A was observed to bind to fungal cells and induce significant changes in fungal transcriptomes that affected responses to the host [22]. Thus, changes in CW and how they affect the immune response in relation to immune effector products is an exciting area of study, particularly considering their involvement in vaccine and therapeutic strategies.
Internal Cell Environment
Advances in understanding how internal host cell environments, such as the phagosome, are involved in recognizing fungi has highlighted the exposure of CW PAMPs in this mechanism. Fungi have mechanisms for sensing these attacks, which results in CW restructuring and morphological changes. One mechanism utilized by C. albicans and A. fumigatus involves the morphological switch from yeast/conidia to hyphae. The phagosome contains several factors that can alter the CW including low pH, low nitrogen, reactive oxygen and nitrogen species, and digestive enzymes (upon lysosome fusion). For C. albicans, this switch involves sensing lower pH as a trigger to begin remodeling, but also prevention of phagolysosome formation [23]. A. fumigatus conidia are protected from the low pH and ROS by melanin, however, how these conditions affect the CW are unknown [24]. In response to acidic conditions, C. neoformansforms a “secondary” CW, with increased chitin and β-glucan, underneath the original CW in order to prevent autolysis [25]. For resistance to oxidative stress in C. neoformans, capsule formation is required, but the effect on the CW is unknown [26].
Oxygen/Iron Availability
Oxygen and iron are required for maintaining fungal growth and survival. Within the host, these compounds are limited at sites of infection [27,28]. Fungal responses to low oxygen (hypoxia) and low iron have, for the most part, similar outcomes in transcriptional and proteomic alterations [29–31]. The response to hypoxia and iron starvation affects the CW and membrane of fungi, as both oxygen and iron are required for enzymes involved in their synthesis. Recent studies have demonstrated that the transcriptional and translational response of A. fumigatus to hypoxia involves the upregulation of a subset of genes and proteins involved in CW synthesis, including glycolysis, β-glucan, and chitin [30,32]. This led to overall changes in the structure of the CW and component distribution causing an increased inflammatory response by host phagocytes [32]. Similar results were demonstrated in C. albicans exposed to hypoxic conditions, with increased transcript levels of CW biosynthesis, remodeling, precursor metabolism (glycolysis), and protein genes [33,34]. Intriguingly, in C. neoformans there is minimal change for glycolytic transcriptional responses under hypoxic conditions and its affects on CW dynamics has not been studied [35]. In the case of CW remodeling, responses to low iron largely mimic those found with hypoxia, except for C. neoformans [29,34]. In C. neoformans, low iron, unlike the known hypoxic responses, results in increased capsule and melanin production in the CW [36].
Biofilm/Secondary infection
Considering recent advances in our understanding of mammalian microbiomes, it seems clear that fungi will often encounter other microbes during fungal pathogenesis. There are diseases or disease states that are characterized as being comprised of multiple microbes, such as biofilm formation or cystic fibrosis. Under biofilm conditions A. fumigatus induces expression of genes involved in rodlet production, leading to immune evasion [37]. Biofilm conditions for several fungi also result in increased expression and production of extracellular matrix proteins, that provide biofilm CWs with resistance to host and drug effectors [37–40]. Within these co-habitating microbial environments, bacteria exist and the effects of these microbe-microbe interactions, through soluble and non-soluble compounds, on the CW are unknown. For example, Pseudomonas aeruginosa produces soluble compounds, phenazines, that cause inhibition of hyphal growth and hyphal death in C. albicans [41]. At low concentrations, phenazines also impact colony morphology and block aerobic respiration. The effect of phenazines on the CW of C. albicans has yet to be determined. The interaction of CW components with bacteria affect not only the response of the microbes to each other, but also how the immune system responds [42]. Interactions between A. fumigatus and C. neoformans with the microbiomes they encounter during infection are understudied, but is an exciting area for future investigation. It will be important to determine whether microbe-microbe interactions in the host induce microbial defense responses that subsequently alter host immune efficacy. It seems clear that these dynamics will be critical for understanding commensalism, microbial pathogenesis, and many human diseases [43].
Other Host Niches
Increased host damage during infection by fungiis often caused by dissemination. Growth of C. albicans on media containing blood or serum resulted in alterations in the CW, including increased mannan [44,45]. For C. neoformans, serum affects the half-life of the CW component GXM, however, GXM branching also contributes [46]. Extracts from serum, specifically phospholipids, also cause an increase in capsule size [47]. A. fumigatus and other molds are known to be angioinvasive. However, affects from growth in blood and serum on the CW are undefined. Within the brain and CNS C. neoformans can utilize L-DOPA as a precursor for CW melanin production [48]. L-DOPA also induces genes that are involved in stress responses, but the overall affects of the CNS on CW structure are not known.
Antifungal drugs can change the microenvironment that the fungi/host occupies through modulation of the CW and plasma membrane. Echinocandins cause increased chitin levels, which confer echinocandin resistance [49,50]. Treatment of A. fumigatus with echinocandins resulted in increased β-glucan exposure and an increased inflammatory response [51,52]. Additionally, biofilm formation in C. albicans caused an increase in the secretion of β-glucan into the extracellular matrix, which acts to sequester antifungal drugs [53]. C. neoformans treated with the triazole drug fluconazole resulted in increased transcripts of genes involved in plasma membrane and CW synthesis, CW maintenance, and stress [54].
Conclusions
Recent studies have shown the dynamic nature of the fungal CW in response to microenvironments encountered in vivo. However, much remains to be learned regarding the significance of these dynamics for the outcome of infections. Studies to investigate the mechanisms underlying these observed in vivo dynamics have great promise to answer this important question. Signal transduction pathways regulating CW dynamics and composition are now being identified and characterized building on previous observations identifying a cell wall integrity (CWI) pathway [55]. For C. neoformans, RIM101 is involved in responding to host microenvironments and recently was observed to control proper remodeling of the CW and in turn, capsule attachment [56]. Null mutants were determined to cause severe disease because unshielded PAMPs resulted in increased inflammation. Another signaling cascade, the MAP kinase pathway involved in heat shock, was observed to control long term viability and thermotolerance through CW remodeling in C. albicans [57]. C. albicans also controls cell wall remodeling through the catalytic protein kinase A subunit Tpk1, which has roles in biofilm formation, CW integrity (CWI), and repression of cell surface proteins [58]. Underlying all these CW changes are mechanisms linked to fungal metabolism. Mitochondrial function has recently been linked to CWI, where a mitochondrial outermembrane protein SAM37 is required for proper CW structure and virulence [59]. One important issue that is now being addressed is the importance of strain differences in host responses, where different wild types of the same species have different responses in the host [20]. Ultimately, the complexity of the in vivo microenvironments remains a challenge in defining in vivo relevant mechanisms. While, reductionism is critical to understand mechanism, it is clear for example, that host receptor costimulation contributes to disease outcome [60]. What remains a significant but exciting challenge is understanding how microenvironment “fungal costimulation” (aka multiple signal integration) impacts fungal metabolism and CW dynamics and how these responses ultimately affect the outcome of fungal infections. An intriguing idea is to alter specific signals driving CW and fungal metabolic changes that are detrimental to the host through modulation of in vivo microenvironments to change the signal to a host beneficial response.
Highlights.
Fungal cell wall composition and structure are influenced by microenvironments.
In vivo microenvironments remain to be fully defined.
How the complex nature of in vivo microenvironments is sensed by fungi to alter the cell wall is an emerging area of investigation.
Fungal signal transduction cascades integrating host derived signals are being identified.
Manipulation of in vivo microenvironment is a potential avenue to improve fungal infection outcomes
Acknowledgments
KMS is currently supported by a training grant fellowship from National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases (5T32DK007301). RAC is currently supported by a grant from the National Institutes of Health, National Institute of Allergy and Infectious Diseases (R01AI81838).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Latge JP. Tasting the fungal cell wall. Cell Microbiol. 2010;12:863–872. doi: 10.1111/j.1462-5822.2010.01474.x. [DOI] [PubMed] [Google Scholar]
- 2.Gupta AO, Singh N. Immune reconstitution syndrome and fungal infections. Curr Opin Infect Dis. 2011;24:527–533. doi: 10.1097/QCO.0b013e32834ab20a. [DOI] [PubMed] [Google Scholar]
- 3.Cunha C, Carvalho A, Esposito A, Bistoni F, Romani L. DAMP signaling in fungal infections and diseases. Front Immunol. 2012;3:286. doi: 10.3389/fimmu.2012.00286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *4.Fontaine T, Delangle A, Simenel C, Coddeville B, van Vliet SJ, van Kooyk Y, Bozza S, Moretti S, Schwarz F, Trichot C, et al. Galactosaminogalactan, a new immunosuppressive polysaccharide of Aspergillus fumigatus. PLoS Pathog. 2011;7:e1002372. doi: 10.1371/journal.ppat.1002372. Important paper describing the discovery of a potent immunosuppressive polysaccharide found in the cell wall of Aspergillus fumigatus. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barelle CJ, Priest CL, Maccallum DM, Gow NA, Odds FC, Brown AJ. Niche-specific regulation of central metabolic pathways in a fungal pathogen. Cell Microbiol. 2006;8:961–971. doi: 10.1111/j.1462-5822.2005.00676.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *6.Ene IV, Heilmann CJ, Sorgo AG, Walker LA, de Koster CG, Munro CA, Klis FM, Brown AJ. Carbon source-induced reprogramming of the cell wall proteome and secretome modulates the adherence and drug resistance of the fungal pathogen Candida albicans. Proteomics. 2012;12:3164–3179. doi: 10.1002/pmic.201200228. Excellent study showing the impact of carbon sources on the cell wall proteome and secretome that affects virulence properties and responses to therapy. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Clavaud C, Beauvais A, Barbin L, Munier-Lehmann H, Latge JP. The composition of the culture medium influences the beta-1,3-glucan metabolism of Aspergillus fumigatus and the antifungal activity of inhibitors of beta-1,3-glucan synthesis. Antimicrob Agents Chemother. 2012 doi: 10.1128/AAC.05661-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ene IV, Adya AK, Wehmeier S, Brand AC, MacCallum DM, Gow NA, Brown AJ. Host carbon sources modulate cell wall architecture, drug resistance and virulence in a fungal pathogen. Cell Microbiol. 2012;14:1319–1335. doi: 10.1111/j.1462-5822.2012.01813.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ene IV, Cheng SC, Netea MG, Brown AJ. Growth of Candida albicans Cells on the Physiologically Relevant Carbon Source Lactate Affects Their Recognition and Phagocytosis by Immune Cells. Infect Immun. 2013;81:238–248. doi: 10.1128/IAI.01092-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *10.Kronstad J, Saikia S, Nielson ED, Kretschmer M, Jung W, Hu G, Geddes JM, Griffiths EJ, Choi J, Cadieux B, et al. Adaptation of Cryptococcus neoformans to mammalian hosts: integrated regulation of metabolism and virulence. Eukaryot Cell. 2012;11:109–118. doi: 10.1128/EC.05273-11. Study elegantly shows substantial changes in the cell wall in response to carbon sources found in vivo during fungal pathogenesis. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Xue C. Cryptococcus and beyond--inositol utilization and its implications for the emergence of fungal virulence. PLoS Pathog. 2012;8:e1002869. doi: 10.1371/journal.ppat.1002869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Grahl N, Dinamarco TM, Willger SD, Goldman GH, Cramer RA. Aspergillus fumigatus mitochondrial electron transport chain mediates oxidative stress homeostasis, hypoxia responses and fungal pathogenesis. Mol Microbiol. 2012;84:383–399. doi: 10.1111/j.1365-2958.2012.08034.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sandai D, Yin Z, Selway L, Stead D, Walker J, Leach MD, Bohovych I, Ene IV, Kastora S, Budge S, et al. The evolutionary rewiring of ubiquitination targets has reprogrammed the regulation of carbon assimilation in the pathogenic yeast Candida albicans. MBio. 2012;3 doi: 10.1128/mBio.00495-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tierney L, Linde J, Muller S, Brunke S, Molina JC, Hube B, Schock U, Guthke R, Kuchler K. An Interspecies Regulatory Network Inferred from Simultaneous RNA-seq of Candida albicans Invading Innate Immune Cells. Front Microbiol. 2012;3:85. doi: 10.3389/fmicb.2012.00085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tsai PW, Yang CY, Chang HT, Lan CY. Human antimicrobial peptide LL-37 inhibits adhesion of Candida albicans by interacting with yeast cell-wall carbohydrates. PLoS One. 2011;6:e17755. doi: 10.1371/journal.pone.0017755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tsai PW, Yang CY, Chang HT, Lan CY. Characterizing the role of cell-wall beta-1,3-exoglucanase Xog1p in Candida albicans adhesion by the human antimicrobial peptide LL-37. PLoS One. 2011;6:e21394. doi: 10.1371/journal.pone.0021394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Silva FD, Rossi DC, Martinez LR, Frases S, Fonseca FL, Campos CB, Rodrigues ML, Nosanchuk JD, Daffre S. Effects of microplusin, a copper-chelating antimicrobial peptide, against Cryptococcus neoformans. FEMS Microbiol Lett. 2011;324:64–72. doi: 10.1111/j.1574-6968.2011.02386.x. [DOI] [PubMed] [Google Scholar]
- 18.Okagaki LH, Wang Y, Ballou ER, O’Meara TR, Bahn YS, Alspaugh JA, Xue C, Nielsen K. Cryptococcal titan cell formation is regulated by G-protein signaling in response to multiple stimuli. Eukaryot Cell. 2011;10:1306–1316. doi: 10.1128/EC.05179-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McClelland EE, Nicola AM, Prados-Rosales R, Casadevall A. Ab binding alters gene expression in Cryptococcus neoformans and directly modulates fungal metabolism. J Clin Invest. 2010;120:1355–1361. doi: 10.1172/JCI38322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **20.McClelland EE, Casadevall A. Strain-related differences in antibody-mediated changes in gene expression are associated with differences in capsule and location of binding. Fungal Genet Biol. 2012;49:227–234. doi: 10.1016/j.fgb.2012.01.006. Exciting study that observes modulation of fungal metabolism by binding of host antibodies. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yano M, Gohil S, Coleman JR, Manix C, Pirofski LA. Antibodies to Streptococcus pneumoniae capsular polysaccharide enhance pneumococcal quorum sensing. MBio. 2011;2 doi: 10.1128/mBio.00176-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zelante T, Iannitti RG, De Luca A, Arroyo J, Blanco N, Servillo G, Sanglard D, Reichard U, Palmer GE, Latge JP, et al. Sensing of mammalian IL-17A regulates fungal adaptation and virulence. Nat Commun. 2012;3:683. doi: 10.1038/ncomms1685. [DOI] [PubMed] [Google Scholar]
- 23.Gow NA, van de Veerdonk FL, Brown AJ, Netea MG. Candida albicans morphogenesis and host defence: discriminating invasion from colonization. Nat Rev Microbiol. 2012;10:112–122. doi: 10.1038/nrmicro2711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Heinekamp T, Thywissen A, Macheleidt J, Keller S, Valiante V, Brakhage AA. Aspergillus fumigatus melanins: interference with the host endocytosis pathway and impact on virulence. Front Microbiol. 2012;3:440. doi: 10.3389/fmicb.2012.00440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Farkas V, Takeo K, Macekova D, Ohkusu M, Yoshida S, Sipiczki M. Secondary cell wall formation in Cryptococcus neoformans as a rescue mechanism against acid-induced autolysis. FEMS Yeast Res. 2009;9:311–320. doi: 10.1111/j.1567-1364.2008.00478.x. [DOI] [PubMed] [Google Scholar]
- 26.Zaragoza O, Chrisman CJ, Castelli MV, Frases S, Cuenca-Estrella M, Rodriguez-Tudela JL, Casadevall A. Capsule enlargement in Cryptococcus neoformans confers resistance to oxidative stress suggesting a mechanism for intracellular survival. Cell Microbiol. 2008;10:2043–2057. doi: 10.1111/j.1462-5822.2008.01186.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Weinberg ED. Iron availability and infection. Biochim Biophys Acta. 2009;1790:600–605. doi: 10.1016/j.bbagen.2008.07.002. [DOI] [PubMed] [Google Scholar]
- 28.Grahl N, Puttikamonkul S, Macdonald JM, Gamcsik MP, Ngo LY, Hohl TM, Cramer RA. In vivo hypoxia and a fungal alcohol dehydrogenase influence the pathogenesis of invasive pulmonary aspergillosis. PLoS Pathog. 2011;7:e1002145. doi: 10.1371/journal.ppat.1002145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sosinska GJ, de Groot PW, Teixeira de Mattos MJ, Dekker HL, de Koster CG, Hellingwerf KJ, Klis FM. Hypoxic conditions and iron restriction affect the cell-wall proteome of Candida albicans grown under vagina-simulative conditions. Microbiology. 2008;154:510–520. doi: 10.1099/mic.0.2007/012617-0. [DOI] [PubMed] [Google Scholar]
- 30.Barker BM, Kroll K, Vodisch M, Mazurie A, Kniemeyer O, Cramer RA. Transcriptomic and proteomic analyses of the Aspergillus fumigatus hypoxia response using an oxygen-controlled fermenter. BMC Genomics. 2012;13:62. doi: 10.1186/1471-2164-13-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Blatzer M, Barker BM, Willger SD, Beckmann N, Blosser SJ, Cornish EJ, Mazurie A, Grahl N, Haas H, Cramer RA. SREBP coordinates iron and ergosterol homeostasis to mediate triazole drug and hypoxia responses in the human fungal pathogen Aspergillus fumigatus. PLoS Genet. 2011;7:e1002374. doi: 10.1371/journal.pgen.1002374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *32.Shepardson KM, Ngo LY, Aimanianda V, Latge JP, Barker BM, Blosser SJ, Iwakura Y, Hohl TM, Cramer RA. Hypoxia enhances innate immune activation to Aspergillus fumigatus through cell wall modulation. Microbes Infect. 2012 doi: 10.1016/j.micinf.2012.11.010. Study is first to describe to oxygen mediated changes in polysaccharide composition of the fungal cell wall and its impacts on the inflammatory response. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Setiadi ER, Doedt T, Cottier F, Noffz C, Ernst JF. Transcriptional response of Candida albicans to hypoxia: linkage of oxygen sensing and Efg1p-regulatory networks. J Mol Biol. 2006;361:399–411. doi: 10.1016/j.jmb.2006.06.040. [DOI] [PubMed] [Google Scholar]
- 34.Synnott JM, Guida A, Mulhern-Haughey S, Higgins DG, Butler G. Regulation of the hypoxic response in Candida albicans. Eukaryot Cell. 2010;9:1734–1746. doi: 10.1128/EC.00159-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chun CD, Liu OW, Madhani HD. A link between virulence and homeostatic responses to hypoxia during infection by the human fungal pathogen Cryptococcus neoformans. PLoS Pathog. 2007;3:e22. doi: 10.1371/journal.ppat.0030022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.O’Meara TR, Alspaugh JA. The Cryptococcus neoformans capsule: a sword and a shield. Clin Microbiol Rev. 2012;25:387–408. doi: 10.1128/CMR.00001-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gibbons JG, Beauvais A, Beau R, McGary KL, Latge JP, Rokas A. Global transcriptome changes underlying colony growth in the opportunistic human pathogen Aspergillus fumigatus. Eukaryot Cell. 2012;11:68–78. doi: 10.1128/EC.05102-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Robertson EJ, Casadevall A. Antibody-mediated immobilization of Cryptococcus neoformans promotes biofilm formation. Appl Environ Microbiol. 2009;75:2528–2533. doi: 10.1128/AEM.02846-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **39.Taff HT, Nett JE, Zarnowski R, Ross KM, Sanchez H, Cain MT, Hamaker J, Mitchell AP, Andes DR. A Candida biofilm-induced pathway for matrix glucan delivery: implications for drug resistance. PLoS Pathog. 2012;8:e1002848. doi: 10.1371/journal.ppat.1002848. Important study detailing the role of Candida biofilms and drug resistance. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Vialas V, Perumal P, Gutierrez D, Ximenez-Embun P, Nombela C, Gil C, Chaffin WL. Cell surface shaving of Candida albicans biofilms, hyphae, and yeast form cells. Proteomics. 2012;12:2331–2339. doi: 10.1002/pmic.201100588. [DOI] [PubMed] [Google Scholar]
- *41.Morales DK, Grahl N, Okegbe C, Dietrich LE, Jacobs NJ, Hogan DA. Control of Candida albicans Metabolism and Biofilm Formation by Pseudomonas aeruginosa Phenazines. MBio. 2013;4 doi: 10.1128/mBio.00526-12. Elegant study detailing the impact of microbe-microbe interactions on fungal metabolism and biofilm formation. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Brand A, Barnes JD, Mackenzie KS, Odds FC, Gow NA. Cell wall glycans and soluble factors determine the interactions between the hyphae of Candida albicans and Pseudomonas aeruginosa. FEMS Microbiol Lett. 2008;287:48–55. doi: 10.1111/j.1574-6968.2008.01301.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Iliev ID, Funari VA, Taylor KD, Nguyen Q, Reyes CN, Strom SP, Brown J, Becker CA, Fleshner PR, Dubinsky M, et al. Interactions between commensal fungi and the C-type lectin receptor Dectin-1 influence colitis. Science. 2012;336:1314–1317. doi: 10.1126/science.1221789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lowman DW, Ensley HE, Greene RR, Knagge KJ, Williams DL, Kruppa MD. Mannan structural complexity is decreased when Candida albicans is cultivated in blood or serum at physiological temperature. Carbohydr Res. 2011;346:2752–2759. doi: 10.1016/j.carres.2011.09.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kruppa M, Greene RR, Noss I, Lowman DW, Williams DL. C. albicans increases cell wall mannoprotein, but not mannan, in response to blood, serum and cultivation at physiological temperature. Glycobiology. 2011;21:1173–1180. doi: 10.1093/glycob/cwr051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cordero RJ, Frases S, Guimaraes AJ, Rivera J, Casadevall A. Evidence for branching in cryptococcal capsular polysaccharides and consequences on its biological activity. Mol Microbiol. 2011;79:1101–1117. doi: 10.1111/j.1365-2958.2010.07511.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hohl TM, Van Epps HL, Rivera A, Morgan LA, Chen PL, Feldmesser M, Pamer EG. Aspergillus fumigatus triggers inflammatory responses by stage-specific beta-glucan display. PLoS Pathog. 2005;1:e30. doi: 10.1371/journal.ppat.0010030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Eisenman HC, Chow SK, Tse KK, McClelland EE, Casadevall A. The effect of L-DOPA on Cryptococcus neoformans growth and gene expression. Virulence. 2011;2:329–336. doi: 10.4161/viru.2.4.16136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *49.Lee KK, Maccallum DM, Jacobsen MD, Walker LA, Odds FC, Gow NA, Munro CA. Elevated cell wall chitin in Candida albicans confers echinocandin resistance in vivo. Antimicrob Agents Chemother. 2012;56:208–217. doi: 10.1128/AAC.00683-11. Clinically important study showing how elevated levels of cell wall chitin lead to echinocandin drug resistance in vivo. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Walker LA, Gow NA, Munro CA. Elevated chitin content reduces the susceptibility of Candida species to caspofungin. Antimicrob Agents Chemother. 2013;57:146–154. doi: 10.1128/AAC.01486-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hohl TM. Stage-specific innate immune recognition of Aspergillus fumigatus and modulation by echinocandin drugs. Med Mycol. 2008:1–7. doi: 10.1080/13693780802078131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hohl TM, Feldmesser M, Perlin DS, Pamer EG. Caspofungin modulates inflammatory responses to Aspergillus fumigatus through stage-specific effects on fungal beta-glucan exposure. J Infect Dis. 2008;198:176–185. doi: 10.1086/589304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Nett JE, Sanchez H, Cain MT, Ross KM, Andes DR. Interface of Candida albicans biofilm matrix-associated drug resistance and cell wall integrity regulation. Eukaryot Cell. 2011;10:1660–1669. doi: 10.1128/EC.05126-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Florio AR, Ferrari S, De Carolis E, Torelli R, Fadda G, Sanguinetti M, Sanglard D, Posteraro B. Genome-wide expression profiling of the response to short-term exposure to fluconazole in Cryptococcus neoformans serotype A. BMC Microbiol. 2011;11:97. doi: 10.1186/1471-2180-11-97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Levin DE. Regulation of cell wall biogenesis in Saccharomyces cerevisiae: the cell wall integrity signaling pathway. Genetics. 2011;189:1145–1175. doi: 10.1534/genetics.111.128264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **56.O’Meara TR, Holmer SM, Selvig K, Dietrich F, Alspaugh JA. Cryptococcus neoformans Rim101 Is Associated with Cell Wall Remodeling and Evasion of the Host Immune Responses. MBio. 2013;4 doi: 10.1128/mBio.00522-12. An important study that details how a fungal transcription factor associated with the cell wall impacts and drives the interaction with host immune responses. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Leach MD, Budge S, Walker L, Munro C, Cowen LE, Brown AJ. Hsp90 Orchestrates Transcriptional Regulation by Hsf1 and Cell Wall Remodelling by MAPK Signalling during Thermal Adaptation in a Pathogenic Yeast. PLoS Pathog. 2012;8:e1003069. doi: 10.1371/journal.ppat.1003069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Fanning S, Xu W, Beaurepaire C, Suhan JP, Nantel A, Mitchell AP. Functional control of the Candida albicans cell wall by catalytic protein kinase A subunit Tpk1. Mol Microbiol. 2012;86:284–302. doi: 10.1111/j.1365-2958.2012.08193.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *59.Qu Y, Jelicic B, Pettolino F, Perry A, Lo TL, Hewitt VL, Bantun F, Beilharz TH, Peleg AY, Lithgow T, et al. Mitochondrial sorting and assembly machinery subunit Sam37 in Candida albicans: insight into the roles of mitochondria in fitness, cell wall integrity, and virulence. Eukaryot Cell. 2012;11:532–544. doi: 10.1128/EC.05292-11. Important study linking the mitochondria with cell wall integrity and virulence. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **60.da Sousa MG, Reid DM, Schweighoffer E, Tybulewicz V, Ruland J, Langhorne J, Yamasaki S, Taylor PR, Almeida SR, Brown GD. Restoration of pattern recognition receptor costimulation to treat chromoblastomycosis, a chronic fungal infection of the skin. Cell Host Microbe. 2011;9:436–443. doi: 10.1016/j.chom.2011.04.005. Beautiful and clinically relevant study showing the importance and complexity of costimulation in the immune response to fungal pathogens. Is foundational for future studies on the mechanisms and outcomes of costimulation. [DOI] [PMC free article] [PubMed] [Google Scholar]


