Abstract
Kidney function monitoring using creatinine-based GFR estimation is a routine part of clinical practice. Emerging evidence has shown that cystatin C may improve classification of GFR for defining chronic kidney disease (CKD) in certain clinical populations, and assist in understanding the complications of CKD. In this review and update, we summarize the overall literature on cystatin C, critically evaluate recent high-impact studies, highlight the role of cystatin C in recent kidney disease guidelines, and suggest a practical approach for clinicians to incorporate cystatin C into practice. We conclude by addressing frequently asked questions related to implementing cystatin C use in a clinical setting.
Keywords: cystatin C, GFR estimation, chronic kidney disease
Introduction
Cystatin C has been available as a measure of kidney function for many years. However, its clinical use worldwide remains very limited compared with that of serum creatinine. During the past decade, cystatin C has been used extensively as a research tool for understanding how kidney function affect health outcomes, particularly within the presumed normal range of kidney function (glomerular filtration rate [GFR] >60mL/min/1.73m2). In the last 2 years, several studies have catalyzed broader interest in cystatin C as a clinical test of kidney function. These studies have had immediate impact upon the 2012 KDIGO (Kidney Disease: Improving Global Outcomes) clinical practice guideline relating to the evaluation and management of chronic kidney disease (CKD).1 In this review and update, we will summarize three recent high-impact papers that have changed perspectives on the clinical utility of cystatin C. We will describe the 2012 KDIGO guideline statements that relate directly to cystatin C, and we will illustrate several clinical situations in which a cystatin-C-based GFR estimate (eGFRcys) may be helpful. We will conclude with responses to frequently asked questions regarding cystatin C.
Background
Creatinine and Cystatin C as Filtration Markers
Endogenous filtration markers have been used as tests of kidney function for over 70 years, with serum creatinine the most widely applied marker. Since creatinine is primarily filtered through the glomerulus, it may be used to estimate GFR when its generation and renal elimination are at steady state. The limitation of creatinine as a marker of GFR is that its generation is highly heterogeneous across individuals and its tubular secretion may vary across populations.2 As an end-product of creatine generation by muscle turnover, creatinine production increases in proportion with muscle mass, physical activity, dietary meat consumption, and better overall health status.3 Since these characteristics are not readily measurable, creatinine generation is approximated clinically by using the demographic characteristics of age, sex, race, and/or body mass in equations to estimate GFR. The development of equations that estimate GFR from serum creatinine has dramatically altered clinical practice.
Dr. Anders Grubb initially identified the potential of cystatin C as an alternative filtration marker in an attempt to overcome known limitations of serum creatinine. Similar to creatinine, cystatin C is freely filtered at the glomerulus, but it is metabolized in the proximal tubules so its clearance cannot be calculated.4 The primary advantage of cystatin C is that its generation appears to be more uniform across populations. It is not a product of muscle mass, but rather appears to be produced by all nucleated cells and released constitutively to the bloodstream. Therefore, the relationship of serum cystatin C to directly measured GFR appears to be less influenced by demographic characteristics and health status than creatinine. In certain clinical settings, however, cystatin C may be biased as a marker of kidney function, such as among patients with rapid cell turnover, uncontrolled thyroid disease, or corticosteroid use.5
Cystatin C has been investigated as a marker of GFR for over 25 years and has consistently been found to have a higher correlation with standard measures of GFR when compared with creatinine (Figure 1).6,7 The addition of demographic coefficients to creatinine, however, substantially improves GFR estimation, whereas cystatin C is only minimally improved by this adjustment. This reflects the greater influence by demographic factors on creatinine. With incorporation of demographic factors, several cross-sectional studies have found that creatinine-and cystatin C-based GFR estimating equations have similar effectiveness for predicting measured GFR. It is unclear, however, whether participants in these GFR studies are adequately representative of the general population.
Figure 1.
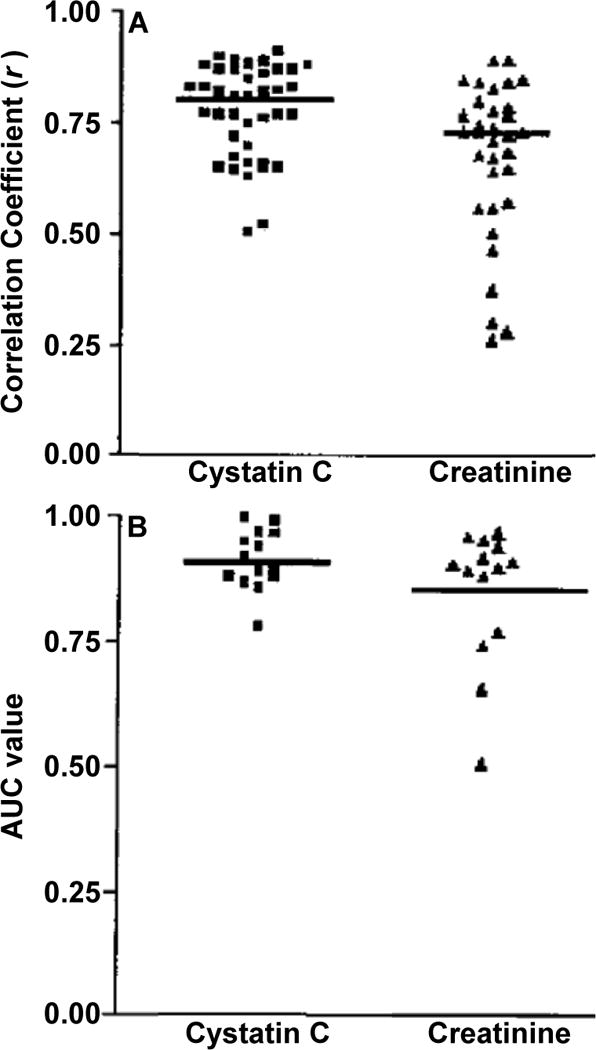
Cystatin C versus Creatinine as markers of GFR. (A) Scatter plot of correlation coefficients (r) for 1/Cys C and 1/Cr with measured GFR. Horizontal line represents the cumulative mean r of all studies analyzed. (B) Scatter plot of ROC-plot AUC values for Cys C and Cr. Horizontal line represents the cumulative mean of all 14 data sets. Adapted and reproduced from Dharnidharka et al7 with permission of the National Kidney Foundation.
Creatinine and Cystatin C to Study Adverse Outcomes Associated with Decreased GFR
Many studies have also compared associations of creatinine and cystatin C with longitudinal complications of kidney disease, such as cardiovascular disease, heart failure, end-stage renal disease (ESRD), and death. In comparison with the GFR measurement studies, these epidemiological studies with clinical outcomes have had a much broader range of participants based on age and health status, including those with chronic diseases. In these settings, cystatin C has demonstrated much stronger associations than eGFRcr with cardiovascular disease, hypertension, infection risk, heart failure, frailty, and all-cause mortality.8–13 In several of these studies, such as those evaluating the outcome of all-cause mortality, eGFRcr has a reverse J-shaped association with clinical outcomes, and has minimal associations with adverse outcomes above 60 mL/min/1.73 m2.14 In contrast, cystatin C has had a linear association across the GFR range, including among persons with GFR levels 60–90 mL/min/1.73 m2, a group that we have described as “preclinical kidney disease”.15
There is a notable discrepancy between GFR studies, in which creatinine and cystatin C GFR estimating equations have similar performance, versus prognosis studies, in which cystatin C is a superior predictor relative to creatinine. This distinction between the two groups of studies has generated substantial confusion about the role of cystatin C, although this difference is almost certainly due to the characteristics of the populations that have been studied. GFR measurement studies consistently recruit relatively healthy persons who are more likely to have predictable muscle mass, a requirement for optimal performance of eGFRcr. For example, the CKD population in GFR studies is much younger and has fewer comorbid diseases than the overall kidney disease population; and participants without CKD tend to be either middle-aged volunteers or prospective kidney donors.16,17 In contrast, the prognosis studies have included a higher proportion of elderly persons and a greater prevalence of chronic diseases that make creatinine less reliable than cystatin C. The alternative viewpoint to explain these disparate findings is that cystatin C has a direct link to adverse outcomes that is independent of its role as a marker of GFR, such as being a manifestation of inflammation or adiposity. This theory is unsupported by epidemiological evidence, as associations of cystatin C with adverse outcomes in the general population are statistically independent of these proposed alternative pathways, and remain much stronger than creatinine. Conversely, in advanced CKD where levels of cystatin C and creatinine are highest, eGFRcr, eGFRcys, and measured GFR all have very similar associations with mortality.18 These results are conclusive that cystatin C is unlikely to have substantial associations with adverse outcomes outside of its role as a marker of GFR.
Findings From the Recent Literature
The extensive literature and controversy, as summarized in the background section, have led to a great interest in understanding the clinical role of cystatin C. Three recent, high-impact papers have brought cystatin C much closer to widespread clinical use. In addition, these studies have brought some reconciliation between the findings from measured GFR and prognostic studies.
CKD Reclassification by Cystatin C in the REGARDS Cohort
In this longitudinal analysis from the REGARDS (Reasons for Geographic and Racial Differences in Stroke) cohort that appeared in JAMA,19 the investigators compared the association of reduced eGFR (<60 mL/min/1.73 m2) defined by creatinine (using the 2009 CKD Epidemiology Collaboration [CKD-EPI] equation) and/or cystatin C (calculated with the 2008 CKD-EPI equation without demographic coefficients) with longitudinal risk for all-cause mortality or ESRD. REGARDS is a population-based cohort of adults aged 45 and older (mean age of 65) with representation from 48 states in the US. The 26,643 participants were categorized into four mutually exclusive groups: CKD by both eGFRcr and eGFRcys, eGFRcr only, eGFRcys only, or neither. Among 2904 participants with eGFRcr <60 mL/min/1.73 m2, 29% (n=849) had eGFRcys >60 mL/min/1.73 m2. Compared with persons with preserved eGFR by both measures, the group with reduced eGFRcr only had no significant increase for mortality or ESRD (Table 1). Conversely, 5% (n=1378) of the cohort had reduced eGFRcys only; these persons had a two-fold mortality risk and a nearly six-fold ESRD risk compared with the reference group. Persons with reduced eGFR by both markers (8%, n=2055) had a two-fold adjusted mortality risk, but a 26-fold risk of ESRD compared with the reference group. The addition of cystatin C significantly improved classification of CKD as a determinant of both outcomes, with net-reclassification improvements (NRI) of 13.3% for mortality and 6.4% for ESRD (both p-values <0.001).
Table 1.
Comparison of Reduced GFR Using Creatinine and Cystatin C with Longitudinal Risks of Death and ESRD
| CKD Defined By | No. | Annual Rate per 1000 persons | Addjusted HR* |
|---|---|---|---|
| All-Cause Mortality | |||
| Neither | 22361 | 10.9 (10.9–11.0) | 1.0 (reference) |
| Creatinine only | 849 | 15.4 (14.9–15.9) | 0.9 (0.7–1.1) |
| Cystatin C only | 1378 | 47.0 (45.8–48.2) | 2.1 (1.9–2.5) |
| Both | 2055 | 57.8 (56.6–59.1) | 2.1 (1.9–2.4) |
| ESRD | |||
| Neither | 22361 | 0.2 (0.1–0.3) | 1.0 (reference) |
| Creatinine only | 849 | 0.5 (0.1–2.2) | 2.5 (0.6–10.9) |
| Cystatin C only | 1378 | 2.2 (1.3–3.8) | 5.8 (2.8–12.1) |
| Both | 2055 | 15.8 (13.5–18.6) | 26.1 (14.9–45.7) |
Note: Data from REGARDS (Reasons for Geographic and Racial Differences in Stroke) Study over 4.6 years of follow-up. Values in parentheses are 95% confidence intervals.
Mortality model adjusts for age, race, sex, income, educational attainment, hypertension, diabetes, prevalent cardiovascular disease, smoking status, BMI, waist circumference, and log albumin-to-creatinine ratio. ESRD model adjusts for age, race, sex, hypertension, diabetes, and log(albumin-creatinine ratio).
Abbreviation: HR, hazard ratio; CKD, chronic kidney disease; GFR, glomulerar filtration rate; ESRD, end-stage renal disease.
The impact of this study is that it demonstrated that eGFRcys improves CKD definition and risk stratification relative to eGFRcr as determined by longitudinal risks for two major complications of CKD.
New CKD-EPI Equations that Incorporate Cystatin C
In this cross-sectional analysis published in NEJM,20 the CKD-EPI collaborators combined patient-level data from 13 cohorts that used several methodologies to measure GFR; 5,352 persons were included in the development data set and 1,119 in the validation set. The investigators utilized newly established international reference standard for cystatin C to develop new GFR-estimating equations. This standardization overcomes a major limitation of prior cystatin C literature, as cystatin C concentrations may have varied by manufacturer and been susceptible to drift.21,22 In general, participants were selected for GFR measurement based upon having known kidney disease or being healthy volunteers. The mean age was 47 in the development sample and 50 in the validation group; mean creatinine was 1.6 mg/dL, mean cystatin C was 1.4 mg/L, and mean measured GFR was 68 ml/min/1.73m2. The authors developed two new equations involving cystatin C. The 2012 CKD-EPI cystatin C equation did not require a race coefficient and included smaller coefficients for age and sex, as compared with the 2009 CKD-EPI creatinine equation. The 2012 CKD-EPI creatinine-cystatin C equation was also presented and included creatinine, cystatin C, age, sex, and race (Box 1).
Box 1. CKD-EPI Equations that Incorporate Creatinine, Cystatin C, or Both.
2009 CKD-EPI creatinine (Levey et al16)
eGFR = 141 × min(SCr/K,1)α× max(SCr/K,1)−1209 × 0.993age [× 1.018 if female] [x 1.159 if black]
If female: K = 0.7, α =−0.329
If male: K = 0.9, α =−0.411
2012 CKD-EPI cystatin C (Inker et al20)
eGFR = 133 × min(SCysC/0.8, 1)−0.499 × max(SCysC/0.8, 1)−1328 × 0.996age [ × 0.932 if female]
2012 CKD-EPI creatinine-cystatin C (Inker et al20)
eGFR = 135 × min(SCr/K, 1)−α × max(SCr/K, 1)−0601 × min(SCysC/0.8, 1)−0375 × max(SCysC/0.8, 1)− 0.711 × 0.995age [ × 0.969 if female ] [ × 1.08 if black ]
If female: K = 0.7, α =−0.248
If male: K = 0.9, α =−0.207
SCysC, serum cystatin C; SCr, serum creatinine; min, minimum; max; maximum; eGFR, estimated glomerular filtration rate; CKD-EPI, Chronic Kidney Disease Epidemiology Collaboration
The 2012 CKD-EPI cystatin C and 2009 CKD-EPI creatinine equations had similar performance in this setting for the outcome of measured GFR. The best GFR estimation resulted from the combined creatinine-cystatin C equation. Near the CKD threshold (GFR 45–75 ml/min/1.73m2), the combined equation meaningfully improved staging of CKD (GFR <60 ml/min/1.73m2) relative to the 2009 creatinine equation. Among 277 participants in the validation set with measured GFR of 45–74 ml/min/1.73m2, 21% (n=58) were reclassified above or below the CKD border of 60 ml/min/1.73m2 by eGFRcr-cys relative to eGFRcr. Among these reclassifications, 74% (43 of 58) were accurate, yielding a net reclassification improvement of 19% (95% CI, 9%–30%). The limitation of this study was that it lacked ethnic diversity and elderly participants.
The contribution of this study is that it offers state of the art cystatin C equations that are based upon the cystatin C reference standard. The combined creatinine-cystatin C equation appears to be the optimal GFR estimate, whereas the 2012 CKD-EPI cystatin C equation has the advantage of not requiring a coefficient for black race, which is a unique attribute among the CKD-EPI equations.
GFR-estimating Equations in Elders: the Berlin Initiative Study
As reported in the Annals of Internal Medicine,23 the investigators from the Berlin Initiative Study (BIS) of elderly adults measured GFR by iohexol clearance in a subset of 610 participants. As in the study introducing the CKD-EPI cystatin C equations, this was a cross-sectional study designed to evaluate GFR-estimating equations in comparison with a measured GFR outcome. BIS is unique, however, for measuring GFR in an elderly cohort; mean age was 78.5, mean creatinine was 1.0 mg/dL, and mean cystatin C was 1.15 mg/L. A major finding of this study was that cystatin C had a much stronger association with GFR than creatinine (Figure 2). The addition of age and sex to creatinine greatly improved GFR prediction, but age and sex added little value to cystatin C. In this elderly cohort, the best GFR estimation was derived from a combined creatinine-cystatin C equation that was developed by the investigators; however, a cystatin C only equation was clearly superior to a creatinine only equation.
Figure 2.
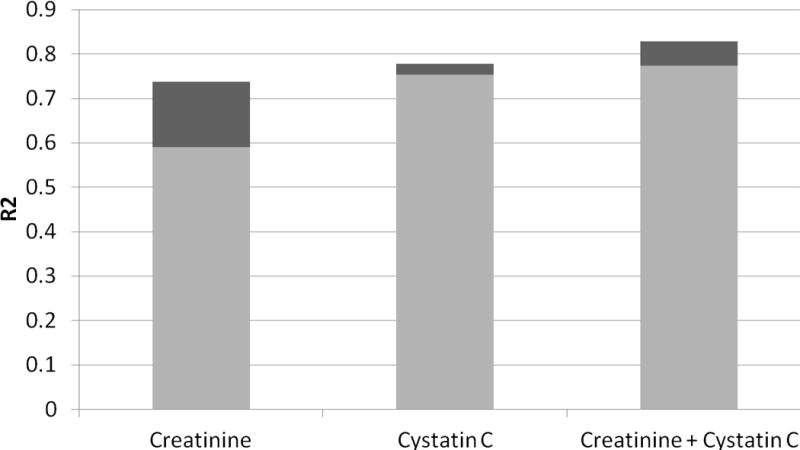
Proportion of GFR Variance Explained by the Filtration Markers (creatinine and cystatin C) and Demographic Factors (age and sex, shown in dark grey) in the Berlin Initiative Study.
With regard to impact, this study in community-based elderly persons demonstrated superiority of cystatin C relative to creatinine for GFR prediction. These findings are consistent with prior literature demonstrating that cystatin C has much stronger associations with adverse outcomes than creatinine in older adults, suggesting that the prognostic superiority of cystatin C is attributable to its approximation of kidney function.
2012 KDIGO Guideline for Evaluation and Management of CKD
The working group of the recently released 2012 KDIGO CKD guidelines included several suggestions and recommendations that relate to cystatin C (Box 2). The guideline authors were influenced in part by the JAMA and NEJM articles described in the previous section. Both of these recent studies showed that cystatin C can improve CKD classification based on the eGFR threshold of 60 ml/min/1.73m2. The KDIGO guidelines suggest measuring cystatin C in patients with CKD defined solely by eGFRcr 45–60 ml/min/1.73m2 but without other manifestations of CKD, such as an albumin-creatinine ratio >30 mg/g. Those with eGFRcys >60 ml/min/1.73m2, approximately one third of this population, should be considered to not have CKD, whereas those with eGFRcys <60 ml/min/1.73m2 have confirmed CKD. Although this may appear to be a small indication for the use of cystatin C, the population with eGFR 45–59 ml/min/1.73m2 without albuminuria currently accounts for 9.9 million people in the United States, or approximately 30% of the overall CKD population.24 Clinicians can thus use cystatin C to reassure the low risk subset of patients with eGFRcr <60 ml/min/1.73m2 and to prioritize care to CKD patients with higher risk of complications. Although the guideline workgroup reported enthusiasm for this indication of cystatin C, it was reported as a “suggestion” rather than “recommendation” due to concerns about the availability and cost of cystatin C worldwide.
Box 2. Statements Within the 2012 KDIGO CKD Guidelines that Relate to Cystatin C.
1.4.3.2: We suggest using additional tests (such as cystatin C or a clearance measurement) for confirmatory testing in specific circumstances when eGFR based on serum creatinine is less accurate. (2B)
1.4.3.5: We suggest measuring cystatin C in adults with eGFRcreat 45–59 mL/min/1.73 m2 who do not have markers of kidney damage if confirmation of CKD is required. (2C)
If eGFRcys/eGFRcreat-cys is also <60 mL/min/1.73 m2, the diagnosis of CKD is confirmed.
If eGFRcys/eGFRcreat-cys is ≥60 mL/min/1.73 m2, the diagnosis of CKD is not confirmed.
1.4.3.6: If cystatin C is measured, we suggest that health professionals (2C):
use a GFR estimating equation to derive GFR from serum cystatin C rather than relying on the serum cystatin C concentration alone.
understand clinical settings in which eGFRcys and eGFRcreat-cys are less accurate.
1.4.3.7: We recommend that clinical laboratories that measure cystatin C should (1B):
measure serum cystatin C using an assay with calibration traceable to the international standard reference material.
report eGFR from serum cystatin C in addition to the serum cystatin C concentration in adults and specify the equation used whenever reporting eGFRcys and eGFRcreat-cys.
report eGFRcys and eGFRcreat-cys in adults using the 2012 CKD-EPI cystatin C and 2012 CKD-EPI creatinine-cystatin C equations, respectively, or alternative cystatin C-based GFR estimating equations if they have been shown to improve accuracy of GFR estimates compared to the 2012 CKD-EPI cystatin C and 2012 CKD-EPI creatinine-cystatin C equations.
When reporting serum cystatin C:
We recommend reporting serum cystatin C concentration rounded to the nearest 100th of a whole number when expressed as conventional units (mg/L).
When reporting eGFRcys and eGFRcreat-cys:
We recommend that eGFRcys and eGFRcreat-cys be reported and rounded to the nearest whole number and relative to a body surface area of 1.73 m2 in adults using the units mL/min/1.73 m2.
We recommend eGFRcys and eGFRcreat-cys levels less than 60 mL/min/1.73 m2 should be reported as “decreased.”
4.4.2: Where precision is required for dosing (due to narrow therapeutic or toxic range) and/or estimates may be unreliable (e.g., due to low muscle mass), we recommend methods based upon cystatin C or direct measurement of GFR. (1C)
Note: Level 1 corresponds to a recommendation statement of “we recommend”; Level 2, to a statement of “we suggest”; the quality of supporting evidence is graded from A to D, with letter grades corresponding to high, moderate, low, and very low quality of evidence, respectively. Abbreviations: KDIGO, Kidney Disease: Improving Global Outcomes; eGFR, estimated glomerular filtration rate; eGFRcreat, creatinine-based estimated glomerular filtration rate; eGFRcys, cystatin C-based estimated glomerular filtration rate; eGFRcys-creat, cystatin C- and creatinine-based estimated glomerular filtration rate
Reproduced with permission of KDIGO from the KDIGO 2012 Clinical Practice Guideline for the Evaluation and Management of Chronic Kidney Disease.1
The guidelines also include a suggestion for additional tests of kidney function in specific circumstances when eGFR based on serum creatinine may be less accurate. The two options are serum cystatin C or a direct measurement of GFR. For medication dosing, this statement is strengthened into a recommendation for GFR measurement methods using either cystatin C or direct clearance methods for dosing of potentially toxic medications.
Finally, the guidelines give several recommendations to clinical laboratories. When cystatin C is measured, the methods should be traceable to international reference material and should report a GFR estimate in addition to cystatin C concentration. At present, the 2012 CKD-EPI cystatin C equation appears to be the optimal choice in most populations.
CKD Screening using Cystatin C
Screening for CKD has been a topic of great controversy, since most studies question whether population screening is cost-effective.25,26 However, screening in high-risk populations, such as in patients with diabetes, directly guides clinical management.27,28 Although cystatin C appears to improve CKD classification compared with creatinine, the KDIGO guidelines have only endorsed its use for improving the specificity of CKD diagnosis, rather than the sensitivity of CKD detection. Clearly, eGFRcys also identifies additional persons with CKD who are otherwise missed by eGFRcr, and these patients have elevated risk for CKD complications. The challenge of a screening strategy using cystatin C is that it would apply to a substantially greater population and thus would have lower yield per test compared with the strategy for confirming CKD. The cost effectiveness of cystatin C screening is beyond the scope of this review, but it depends upon factors such as the cost of the test, frequency of abnormal results, and the utility of earlier detection of CKD. The value of a timely CKD diagnosis made through cystatin C screening could potentially be realized through improved medication dosing, risk stratification for procedures, or more intensive interventions to prevent kidney function decline. We believe there are three potential strategies that could be used for cystatin C screening: persons with borderline eGFRcr, persons at high risk for CKD, and persons with conditions known to make creatinine insensitive for detecting CKD (e.g. unpredictable muscle mass).
One screening strategy would involve measuring cystatin C in all adults with eGFRcr 60–90 ml/min/1.73 m2 without albuminuria. In our work, we have found that 14% of persons in this category have eGFRcys <60 ml/min/1.73 m2, so the number needed to screen (NNS) would be approximately seven to detect a new case of CKD. However, 32.3% of the U.S. population is in this eGFRcr subgroup, thus requiring an enormous number of cystatin C tests. If the screening strategy is narrowed to eGFRcr 60–74 ml/min/1.73 m2 without albuminuria, then screening is reduced to 10.9% of the U.S. population, among whom 23% will have eGFRcys <60 ml/min/1.73 m2 (NNS=4). We believe that this population with eGFRcr 60–74 ml/min/1.73 m2 would be an appropriate target for cystatin C screening; the yield is high, and an earlier diagnosis of CKD can be made in one-fourth of persons screened.
Another potential strategy for CKD screening with cystatin C would be to target persons with established kidney disease or chronic diseases known to cause kidney disease. The most extensive literature is in the setting of diabetes; studies have found eGFRcys to correspond with measured GFR much better than eGFRcr in the normal range.29 A recent study found that patients with CKD defined by cystatin C had substantially higher risk for progression to ESRD compared with patients with CKD defined by creatinine.30 Other chronic conditions associated with high prevalence of CKD include cardiovascular disease, heart failure, and hypertension. In kidney transplant recipients, a recent multi-center study of 670 patients with GFR measured by inulin clearance found both eGFRcys (R2 = 0.67) and eGFRcr-cys (R2 = 0.70) to be superior to eGFRcr (R2 = 0.60). The net reclassification index for improving categorization of eGFR stages was 22.5% (95% CI, 10.2%–34.9%) for eGFRcys and 18.8% (95% CI, 8.6%–28.9%) for eGFRcr-cys. Therefore, the authors state that cystatin C might be even more valuable in kidney transplantation than in the general CKD population.31 These findings are notable, as corticosteroid use is common in kidney transplant patients, which might be expected to have biased the cystatin C concentrations.
A third potential target for cystatin C screening is among persons with conditions affecting muscle mass, which may lead to a bias in creatinine overestimating eGFR. For example, Segarra and colleagues31a found that cystatin C-based GFR equations were more accurate than the CKD-EPI equation in a study of hospitalized patients who were noted to have malnutrition and who had GFR measured by iohexol clearance. They found the eGFRcr to be accurate among patients without malnutrition. In the setting of HIV infection, muscle mass is unpredictable and several studies have demonstrated lower GFR estimates from cystatin C relative to creatinine.32–34 In addition, cystatin C has a much stronger association with mortality than creatinine in this setting.35 Chronic liver disease and cirrhosis are characterized by low GFR when measured by clearance methods, despite normal creatinine levels. Cystatin C appears to be somewhat more sensitive than creatinine for detecting reduced GFR in this population, although it also appears to overestimate GFR.36–40 Other populations in which creatinine may be insensitive to capture reduced kidney function include frail elders and persons with malignancy. In contrast, in a cohort of healthy middle-aged adults in Tromso, Norway, cystatin C had no advantage when compared to creatinine. This demonstrates that the particular advantage of cystatin C is among persons and populations in which creatinine production is variable and unpredictable. To optimize the yield of cystatin C screening, individual patient-based prediction tools are needed to estimate the likelihood of occult CKD (eGFR <60 ml/min/1.73 m ) based on the patient’s eGFRcr and clinical characteristics.
In addition to patient characteristics that might warrant kidney function screening with cystatin C, certain clinical scenarios may drive the need for more accurate GFR estimation. KDIGO recommendation 4.4.2 indicates the need for optimal GFR estimation for patients undergoing treatments in which risk and benefits may be influenced by kidney function (Box 2). The requirement for procedures such as chemotherapy, surgery, or angiography is a situation in which determining kidney function may be important for moderating the risk for complications.
A more extensive literature concerns the safety and pharmacokinetics of chemotherapeutics, in which several studies have found cystatin C to predict serum concentrations of the specific agent better than creatinine.41–44 The impact of accurate GFR estimation on medication safety is an important topic for future investigation in other settings.
In summary, the value of screening with cystatin C to detect occult CKD depends upon the prevalence of reduced eGFR in that population, the likelihood that creatinine would overestimate actual GFR, and the implications for treatments.
Frequently Asked Questions
In the final section of this review, we respond to common question related to the clinical use of cystatin C.
What if my lab cannot measure cystatin C?
Serum or plasma cystatin C is now an automated test that can be measured on any automated chemistry platform without the requirement for extra blood. The clinical lab must become familiar with the assay, as with any new test. Many labs have not taken this step yet because the clinical chemistry personnel may not perceive a high demand for cystatin C. As a result, it may only be available currently as a “send-out” test. The 2012 KDIGO guidelines will likely increase demand for cystatin C, and we encourage clinicians to request cystatin C from their clinical labs.
Is cystatin C too expensive for routine measurement?
Because cystatin C is automated, the labor costs are minimal and the primary costs are the reagents. Currently, our hospital’s clinical lab purchases cystatin C reagents at $4 per cystatin C test, which is 20 times the cost of a creatinine test using the Jaffe reaction ($0.20), and about 3 times the cost of an enzymatic creatinine assay ($1.50). However, this cost is less than or equivalent to the cost of several tests that nephrologists and cardiologists frequently order: C-reactive protein ($2.75), parathyroid hormone ($3.25), 25-hydroxyvitamin D ($7), troponin T ($10), and B-type natriuretic peptide ($15). Although cost may differ in other settings, we believe this cost for cystatin C does not preclude its selected use as a secondary test of kidney function in settings with adequate healthcare resources for the indications described above.
Which company’s cystatin C assay should I use?
The most important issue is that chemistry labs use a cystatin C measurement method that is validated against the international standard reference material.45 This standardization should eliminate prior issues of assay drift that were noted a few years ago.22,46 Two companies that have well-validated cystatin C assays are Gentian and Siemens; these products may be marketed by other vendors. In addition to the reference standard, the decision should be made based on cost and feasibility for the chemistry lab.
Which equation should we use?
As stated above, the new CKD-EPI cystatin C equations currently appear to be the best available GFR estimating equations using cystatin C. However, these equations have not been evaluated in large segments of the population, such as non-black/non-white ethnicities, elderly persons, and persons with certain comorbidities such as liver disease. Although it is unclear whether the CKD-EPI cystatin C equations are ideal in all populations, they appear at present to be the most broadly applicable cystatin C equations.
The choice of the combined creatinine-cystatin C equation versus the cystatin C only equation depends upon the diagnostic strategy. If the goal is to choose the optimal GFR estimate for either screening or a specific clinical indication, then the combined eGFRcr-cys should be used. This strategy is widely used in parts of Sweden, where the use of cystatin C as a filtration marker originated.47,48 On the other hand, if cystatin C is used as a secondary test based on the results of eGFRcr, then the eGFRcys should be used so that creatinine is not in both the screening and verification steps of a GFR estimating strategy. eGFRcr-cys should not be used if the eGFRcr and eGFRcys differ by more than 40%.48 The approach in Sweden is to compare the eGFRcr and eGFRcys: if they are within 40% of one another, then their average is the best estimate; if the difference is >40%, then clinicians should choose the estimate least likely to be biased based on clinical characteristics.
Is it important to monitor changes in eGFR using cystatin C?
In our literature review and research experience, we have generally found that changes in eGFRcr and eGFRcys are well correlated in the ambulatory setting. In one study of elders, changes in eGFRcys were of higher magnitude than eGFRcr.49 Cystatin C may have advantages over creatinine for detecting acute changes in eGFR in the hospital setting for patients in intensive care; however, minimal difference was observed for patients undergoing cardiac surgery.50,51 Overall, we do not believe that routine use of cystatin C is warranted for monitoring acute changes in kidney function.
Conclusions
For many years, cystatin C has been known to have a higher correlation with measured GFR and much stronger associations with adverse outcomes compared with creatinine. Recent advances have facilitated the use of cystatin C as a clinical measure of kidney function: an international reference standard, broadly applicable GFR equations, and a guideline-endorsed indication for confirming CKD among persons with eGFR 45–59 ml/min/1.73 m2. Future work must develop strategies for implementing cystatin C into clinical care with the goal of optimizing the diagnosis, staging, and treatment of CKD.
Acknowledgments
Support: none.
Financial Disclosure: The authors declare that they have no relevant financial interests.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.KDIGO Clinical Practice Guideline for the Evaluation and Management of Chronic Kidney Disease. Kidney Int Suppl. 2013;3:1–150. [Google Scholar]
- 2.Levey AS, de Jong PE, Coresh J, et al. The definition, classification, and prognosis of chronic kidney disease: a KDIGO Controversies Conference report. Kidney Int. 2010 Jul;80(1):17–28. doi: 10.1038/ki.2010.483. [DOI] [PubMed] [Google Scholar]
- 3.Stevens LA, Coresh J, Greene T, Levey AS. Assessing kidney function-measured and estimated glomerular filtration rate. N Engl J Med. 2006 Jun 8;354(23):2473–2483. doi: 10.1056/NEJMra054415. [DOI] [PubMed] [Google Scholar]
- 4.Kyhse-Andersen J, Schmidt C, Nordin G, et al. Serum cystatin C, determined by a rapid, automated particle-enhanced turbidimetric method, is a better marker than serum creatinine for glomerular filtration rate. Clin Chem. 1994 Oct;40(10):1921–1926. [PubMed] [Google Scholar]
- 5.Knight EL, Verhave JC, Spiegelman D, et al. Factors influencing serum cystatin C levels other than renal function and the impact on renal function measurement. Kidney Int. 2004 Apr;65(4):1416–1421. doi: 10.1111/j.1523-1755.2004.00517.x. [DOI] [PubMed] [Google Scholar]
- 6.Levey AS, Coresh J, Greene T, et al. Using standardized serum creatinine values in the modification of diet in renal disease study equation for estimating glomerular filtration rate. Ann Intern Med. 2006 Aug 15;145(4):247–254. doi: 10.7326/0003-4819-145-4-200608150-00004. [DOI] [PubMed] [Google Scholar]
- 7.Dharnidharka VR, Kwon C, Stevens G. Serum cystatin C is superior to serum creatinine as a marker of kidney function: a meta-analysis. Am J Kidney Dis. 2002 Aug;40(2):221–226. doi: 10.1053/ajkd.2002.34487. [DOI] [PubMed] [Google Scholar]
- 8.Menon V, Shlipak MG, Wang X, et al. Cystatin C as a risk factor for outcomes in chronic kidney disease. Ann Intern Med. 2007 Jul 3;147(1):19–27. doi: 10.7326/0003-4819-147-1-200707030-00004. [DOI] [PubMed] [Google Scholar]
- 9.Sarnak MJ, Katz R, Stehman-Breen CO, et al. Cystatin C concentration as a risk factor for heart failure in older adults. Ann Intern Med. 2005 Apr 5;142(7):497–505. doi: 10.7326/0003-4819-142-7-200504050-00008. [DOI] [PubMed] [Google Scholar]
- 10.Shlipak MG, Sarnak MJ, Katz R, et al. Cystatin C and the risk of death and cardiovascular events among elderly persons. N Engl J Med. 2005 May 19;352(20):2049–2060. doi: 10.1056/NEJMoa043161. [DOI] [PubMed] [Google Scholar]
- 11.Sarnak MJ, Katz R, Fried LF, et al. Cystatin C and aging success. Arch Intern Med. 2008 Jan 28;168(2):147–153. doi: 10.1001/archinternmed.2007.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Odden MC, Chertow GM, Fried LF, et al. Cystatin C and measures of physical function in elderly adults: the Health, Aging, and Body Composition (HABC) Study. Am J Epidemiol. 2006 Dec 15;164(12):1180–1189. doi: 10.1093/aje/kwj333. [DOI] [PubMed] [Google Scholar]
- 13.Dalrymple LS, Katz R, Kestenbaum B, et al. The risk of infection-related hospitalization with decreased kidney function. Am J Kidney Dis. 2011 Mar;59(3):356–363. doi: 10.1053/j.ajkd.2011.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Matsushita K, van der Velde M, Astor BC, et al. Association of estimated glomerular filtration rate and albuminuria with all-cause and cardiovascular mortality in general population cohorts: a collaborative meta-analysis. Lancet. 2010 Jun 12;375(9731):2073–2081. doi: 10.1016/S0140-6736(10)60674-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shlipak MG, Katz R, Sarnak MJ, et al. Cystatin C and prognosis for cardiovascular and kidney outcomes in elderly persons without chronic kidney disease. Ann Intern Med. 2006 Aug 15;145(4):237–246. doi: 10.7326/0003-4819-145-4-200608150-00003. [DOI] [PubMed] [Google Scholar]
- 16.Levey AS, Greene T, Kusek J, Beck G. A simplified equation to predict glomerular filtration rate from serum creatinine [Abstract] Journal of the American Society of Nephrology. 2000;11:155A. [Google Scholar]
- 17.Levey AS, Stevens LA, Schmid CH, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009 May 5;150(9):604–612. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tangri N, Inker LA, Tighiouart H, et al. Filtration markers may have prognostic value independent of glomerular filtration rate. J Am Soc Nephrol. 2011 Feb;23(2):351–359. doi: 10.1681/ASN.2011070663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Peralta CA, Shlipak MG, Judd S, et al. Detection of chronic kidney disease with creatinine, cystatin C, and urine albumin-to-creatinine ratio and association with progression to end-stage renal disease and mortality. JAMA. 2011 Apr 20;305(15):1545–1552. doi: 10.1001/jama.2011.468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Inker LA, Schmid CH, Tighiouart H, et al. Estimating glomerular filtration rate from serum creatinine and cystatin C. N Engl J Med. 2012 Jul 5;367(1):20–29. doi: 10.1056/NEJMoa1114248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shlipak MG, Weekley CC, Li Y, Hansson LO, Larsson A, Whooley M. Comparison of cardiovascular prognosis by 3 serum cystatin C methods in the Heart and Soul Study. Clin Chem. 2011 May;57(5):737–745. doi: 10.1373/clinchem.2010.158915. [DOI] [PubMed] [Google Scholar]
- 22.Larsson A, Hansson LO, Flodin M, Katz R, Shlipak MG. Calibration of the Siemens cystatin C immunoassay has changed over time. Clin Chem. 2011 May;57(5):777–778. doi: 10.1373/clinchem.2010.159848. [DOI] [PubMed] [Google Scholar]
- 23.Schaeffner ES, Ebert N, Delanaye P, et al. Two novel equations to estimate kidney function in persons aged 70 years or older. Ann Intern Med. 2012 Oct;157(7):471–481. doi: 10.7326/0003-4819-157-7-201210020-00003. [DOI] [PubMed] [Google Scholar]
- 24.Foley RN, Wang C, Snyder JJ, Collins AJ. Cystatin C levels in U.S. adults, 1988–1994 versus 1999–2002: NHANES. Clin J Am Soc Nephrol. 2009 May;4(5):965–972. doi: 10.2215/CJN.05281008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Manns B, Hemmelgarn B, Tonelli M, et al. Population based screening for chronic kidney disease: cost effectiveness study. BMJ. 2010;341:c5869. doi: 10.1136/bmj.c5869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Boulware LE, Jaar BG, Tarver-Carr ME, Brancati FL, Powe NR. Screening for proteinuria in US adults: a cost-effectiveness analysis. JAMA. 2003 Dec 17;290(23):3101–3114. doi: 10.1001/jama.290.23.3101. [DOI] [PubMed] [Google Scholar]
- 27.National Kidney Foundation. KDOQI Clinical Practice Guideline for Diabetes and CKD: 2012 Update. Am J Kidney Dis. 2012 Nov;60(5):850–886. doi: 10.1053/j.ajkd.2012.07.005. [DOI] [PubMed] [Google Scholar]
- 28.Executive summary: Standards of medical care in diabetes-2012. Diabetes Care. 2012 Jan;35(Suppl 1):S4–S10. doi: 10.2337/dc12-s004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Perkins BA, Nelson RG, Ostrander BE, et al. Detection of renal function decline in patients with diabetes and normal or elevated GFR by serial measurements of serum cystatin C concentration: results of a 4-year follow-up study. J Am Soc Nephrol. 2005 May;16(5):1404–1412. doi: 10.1681/ASN.2004100854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Krolewski AS, Warram JH, Forsblom C, et al. Serum concentration of cystatin C and risk of end-stage renal disease in diabetes. Diabetes Care. 2012 Nov;35(11):2311–2316. doi: 10.2337/dc11-2220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Masson I, Maillard N, Tack I, et al. GFR Estimation Using Standardized Cystatin C in Kidney Transplant Recipients. Am J Kidney Dis. 2012 Nov 7; doi: 10.1053/j.ajkd.2012.09.010. [DOI] [PubMed] [Google Scholar]
- 31a.Segarra A, de la Torre J, Ramos N, Quiroz A, Garjau M, Torres I, Azancot MA, López M, Sobrado A. Clin J Am Soc Nephrol. 2011 Oct;6(10):2411–20. doi: 10.2215/CJN.01150211. Assessing glomerular filtration rate in hospitalized patients: a comparison between CKD-EPI and four cystatin C-based equations. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Estrella MM, Parekh RS, Abraham A, et al. The impact of kidney function at highly active antiretroviral therapy initiation on mortality in HIV-infected women. J Acquir Immune Defic Syndr. 2010 Oct;55(2):217–220. doi: 10.1097/QAI.0b013e3181e674f4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Odden MC, Scherzer R, Bacchetti P, et al. Cystatin C level as a marker of kidney function in human immunodeficiency virus infection: the FRAM study. Arch Intern Med. 2007 Nov 12;167(20):2213–2219. doi: 10.1001/archinte.167.20.2213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jones CY, Jones CA, Wilson IB, et al. Cystatin C and creatinine in an HIV cohort: the nutrition for healthy living study. Am J Kidney Dis. 2008 Jun;51(6):914–924. doi: 10.1053/j.ajkd.2008.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Choi A, Scherzer R, Bacchetti P, et al. Cystatin C, albuminuria, and 5-year all-cause mortality in HIV-infected persons. Am J Kidney Dis. 2010 Nov;56(5):872882. doi: 10.1053/j.ajkd.2010.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Finkenstedt A, Dorn L, Edlinger M, et al. Cystatin C is a strong predictor of survival in patients with cirrhosis: is a cystatin C-based MELD better? Liver Int. 2012 Sep;32(8):1211–1216. doi: 10.1111/j.1478-3231.2012.02766.x. [DOI] [PubMed] [Google Scholar]
- 37.Kim DJ, Kang HS, Choi HS, et al. Serum cystatin C level is a useful marker for the evaluation of renal function in patients with cirrhotic ascites and normal serum creatinine levels. Korean J Hepatol. 2011 Jun;17(2):130–138. doi: 10.3350/kjhep.2011.17.2.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Poge U, Gerhardt T, Stoffel-Wagner B, Klehr HU, Sauerbruch T, Woitas RP. Calculation of glomerular filtration rate based on cystatin C in cirrhotic patients. Nephrol Dial Transplant. 2006 Mar;21(3):660–664. doi: 10.1093/ndt/gfi305. [DOI] [PubMed] [Google Scholar]
- 39.Sharawey MA, Shawky EM, Ali LH, Mohammed AA, Hassan HA, Fouad YM, Cystatin C. a predictor of hepatorenal syndrome in patients with liver cirrhosis. Hepatol Int. 2011 Mar 12; doi: 10.1007/s12072-011-9266-y. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 40.Ahn HS, Kim YS, Kim SG, et al. Cystatin C is a good predictor of hepatorenal syndrome and survival in patients with cirrhosis who have normal serum creatinine levels. Hepatogastroenterology. 2011 Jun;59(116):1168–1173. doi: 10.5754/hge11616. [DOI] [PubMed] [Google Scholar]
- 41.Bolke E, Schieren G, Gripp S, et al. Cystatin C – a fast and reliable biomarker for glomerular filtration rate in head and neck cancer patients. Strahlenther Onkol. 2011 Mar;187(3):191–201. doi: 10.1007/s00066-010-2203-5. [DOI] [PubMed] [Google Scholar]
- 42.Schmitt A, Gladieff L, Lansiaux A, et al. A universal formula based on cystatin C to perform individual dosing of carboplatin in normal weight, underweight, and obese patients. Clin Cancer Res. 2009 May 15;15(10):3633–3639. doi: 10.1158/1078-0432.CCR-09-0017. [DOI] [PubMed] [Google Scholar]
- 43.Stabuc B, Vrhovec L, Stabuc-Silih M, Cizej TE. Improved prediction of decreased creatinine clearance by serum cystatin C: use in cancer patients before and during chemotherapy. Clin Chem. 2000 Feb;46(2):193–197. [PubMed] [Google Scholar]
- 44.Benohr P, Grenz A, Hartmann JT, Muller GA, Blaschke S. Cystatin C-a marker for assessment of the glomerular filtration rate in patients with cisplatin chemotherapy. Kidney Blood Press Res. 2006;29(1):32–35. doi: 10.1159/000092485. [DOI] [PubMed] [Google Scholar]
- 45.Grubb A, Blirup-Jensen S, Lindstrom V, Schmidt C, Althaus H, Zegers I. First certified reference material for cystatin C in human serum ERM-DA471/IFCC. Clin Chem Lab Med. 2010 Nov;48(11):1619–1621. doi: 10.1515/CCLM.2010.318. [DOI] [PubMed] [Google Scholar]
- 46.Voskoboev NV, Larson TS, Rule AD, Lieske JC. Analytic and clinical validation of a standardized cystatin C particle enhanced turbidimetric assay (PETIA) to estimate glomerular filtration rate. Clin Chem Lab Med. 2012;50(9):1591–1596. doi: 10.1515/cclm-2012-0063. [DOI] [PubMed] [Google Scholar]
- 47.Grubb A. Non-invasive estimation of glomerular filtration rate (GFR). The Lund model: Simultaneous use of cystatin C- and creatinine-based GFR-prediction equations, clinical data and an internal quality check. Scand J Clin Lab Invest. 2010 Apr;70(2):65–70. doi: 10.3109/00365511003642535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Grubb A, Nyman U, Bjork J. Improved estimation of glomerular filtration rate (GFR) by comparison of eGFRcystatin C and eGFRcreatinine. Scand J Clin Lab Invest. 2011 Feb;72(1):73–77. doi: 10.3109/00365513.2011.634023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Shlipak MG, Katz R, Kestenbaum B, et al. Rate of kidney function decline in older adults: a comparison using creatinine and cystatin C. Am J Nephrol. 2009;30(3):171–178. doi: 10.1159/000212381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Herget-Rosenthal S, Marggraf G, Husing J, et al. Early detection of acute renal failure by serum cystatin C. Kidney Int. 2004 Sep;66(3):1115–1122. doi: 10.1111/j.1523-1755.2004.00861.x. [DOI] [PubMed] [Google Scholar]
- 51.Spahillari A, Parikh CR, Sint K, et al. Serum cystatin C- versus creatinine-based definitions of acute kidney injury following cardiac surgery: a prospective cohort study. Am J Kidney Dis. 2012 Dec;60(6):922–929. doi: 10.1053/j.ajkd.2012.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]


