Abstract
Mucopolysaccharidosis type IVA (MPS IVA) was described in 1929 by Luis Morquio from Uruguay and James Brailsford from England, and was later found as an autosomal recessive lysosomal storage disease. MPS IVA is caused by mutations in the gene encoding the enzyme, N-acetylgalactosamine-6-sulfate sulfatase (GALNS). Reduced GALNS activity results in impaired catabolism of two glycosaminoglycans (GAGs), chondroitin-6-sulfate (C6S) and keratan sulfate (KS). Clinical presentations of MPS IVA reflect a spectrum of progression from a severe ”classical” phenotype to a mild “attenuated” phenotype. More than 180 different mutations have been identified in the GALNS gene, which likely explains the phenotypic heterogeneity of the disorder.
Accumulation of C6S and KS manifests predominantly as short stature and skeletal dysplasia (dysostosis multiplex), including atlantoaxial instability and cervical cord compression. However, abnormalities in the visual, auditory, cardiovascular, and respiratory systems can also affect individuals with MPS IVA. Diagnosis is typically based on clinical examination, skeletal radiographs, urinary GAG, and enzymatic activity of GALNS in blood cells or fibroblasts. Deficiency of GALNS activity is a common assessment for the laboratory diagnosis of MPS IVA; however, with recently increased availability, gene sequencing for MPS IVA is often used to confirm enzyme results. As multiple clinical presentations are observed, diagnosis of MPS IVA may require multi-system considerations.
This review provides a history of defining MPS IVA and how the understanding of the disease manifestations has changed over time. A summary of the accumulated knowledge is presented, including information from the International Morquio Registry. The classical phenotype is contrasted with attenuated cases, which are now being recognized and diagnosed more frequently. Laboratory based diagnoses of MPS IVA are also discussed.
Keywords: Mucopolysaccharidosis IVA, MPS IVA, Morquio A, GALNS, laboratory diagnosis
Introduction
Mucopolysaccharidosis type IVA (MPS IVA), commonly referred to as Morquio A syndrome, is an autosomal recessive lysosomal storage disease (1). MPS IVA was first described in 1929 by Luis Morquio (2), a pediatrician from Uruguay, and James Brailsford (3) a radiologist in England. The incidence of MPS IVA in the general population is estimated to be 1:201,000 (4) and ranges among various populations from 1 in 76,000 live births in Northern Ireland (5) to 1 in 640,000 live births in Western Australia (6). The concept of a founder’s effect may account for the regional differences that are observed (7–11).
MPS IVA is caused by mutations in the gene encoding the enzyme N-acetylgalactosamine-6-sulfate sulfatase (GALNS, EC 3.1.6.4) (12). Reduced GALNS activity results in impaired catabolism of 2 glycosaminoglycans (GAGs), chondroitin-6-sulfate (C6S) (13) and keratan sulfate (KS) (14). The chondrocytes are predominantly responsible for KS synthesis. As in other MPS disorders, these undegraded GAGs accumulate in the lysosomes of cells, which, in turn, interferes with cellular function. The tissue distribution of the particular GAGs, which accumulate in patients with MPS IVA, as in other MPS disorders, is reflected in the clinical presentation of the disease. In patients with MPS IVA, KS and C6S accumulation typically results in short stature and skeletal dysplasia (15). Bone deformity is the most common initial manifestation (16–18) of skeletal dysplasia. Additional compromised systems include the visual system, auditory system, cardiovascular system, and respiratory system (19). The central nervous system is not believed to have significant manifestations of GAG accumulation and normal intelligence appears to be preserved (15). However, patients have a high risk of developing neurological complications caused by a combination of odontoid hypoplasia, incomplete ossification of the anterior and posterior rings of the atlas, and deposition of GAGs in the anterior extradural space. This results in atlantoaxial subluxation and spinal cord compression (SCC), with cervical myelopathy, consequential quadriparesis or even death (19;20).
Quantitative and qualitative blood and urine KS assays were established in two major methods; ELISA (21) and tandem mass spectrometry (22) and were successively applied to MPS IVA patients (23;24).
There is a wide spectrum of disease progression among individuals with MPS IVA. A high degree of genetic heterogeneity is likely responsible for this phenotypic variety. More than 180 different mutations have been identified in the GALNS gene (7–10;25–28). Clinical presentations of severely affected patients have been reported as severe (29;30), or classical (28–30) phenotypes. Less severe forms of MPS IVA have been reported as mild (30;31) or attenuated (7;9) phenotypes. As well, an intermediate subtype of MPS IVA has been proposed (29;30;32). Onset of disease symptoms commonly occurs prior to 1 year of age in severely affected (rapidly progressing) patients or as late as the second decade of life in less severely affected (mild) patients (33). Diagnosis is typically based on clinical examination, skeletal radiographs, and the enzymatic activity of GALNS in blood cells or fibroblasts (16;17;34).
Once diagnosed, MPS IVA requires a multi-disciplinary approach to patient care (17;19). While management of skeletal manifestations and the associated neurological complications is critically important, management of other organ systems, including visual, auditory, cardiovascular, and respiratory systems, are also important to assure quality of life of individuals with MPS IVA. With the goal of describing the natural history of disease progression and improving recognition, diagnosis, and management of MPS IVA, a review of the historical and current clinical experience of patients with MPS IVA is presented.
Historical Review of Defining Mucopolysaccharidosis IVA
The symptoms associated with MPS IVA were first described in 1929 by Dr. Luis Morquio (2) in sibling cases in Uruguay, and James Brailsford (3) in a patient in England at approximately the same time. However, Whiteside and Chomeley (1952) (35) proposed that symptoms similar to those observed by Morquio and Brailsford were reported as early as 1913 (36) and McKusick et al (1972) (37) have theorized that the first cases of Morquio syndrome were likely reported in a French Canadian brother and sister by Osler (38) (1897) as cases of achondroplasia. Morquio described the symptoms as an osseous dystrophy while subsequent reports have referred to the symptoms described by Morquio (35). Brailsford, followed by others (35;39) referred to the condition as chondroosteodystrophy and osteochondrodystrophia deformans (40). In a tribute to both Morquio and Brailsford, the symptoms have also been referred to as Morquio-Brailsford disease (39–41).
In 1961, Maroteaux and Lamy (42) reported elevated levels of the acid mucopolysaccharide in the urine of patients with Morquio’s disease. In 1963, Robins et al (43) proposed that Morquio syndrome was caused by an abnormality in mucopolysaccharide metabolism (mucopolysaccharidosis) and in their seminal description and categorization of the mucopolysaccharidoses in 1965, McKusick et al (44) classified Morquio syndrome as MPS IV. Langer and Carey (41) reported abnormal levels of the mucopolysaccharide, keratosulphate, in patients with Morquio-Brailsford disease.
In 1974, Matalon et al. (1) found that patients with Morquio syndrome were deficient in the enzyme chondroitin sulfate N-acetylhexosamine sulphate sulfatase and Singh et al (1976) (45) found that a deficiency in the enzyme GALNS resulted in impaired degradation of chondroitin-6-sulfate in patients with Morquio syndrome. Additional descriptions of a defect in galactosamine-6-sulfate sulfatase involving degradation of KS further suggested a similar association with MPS IVA (46). In 1976, Obrien et al (1976) described a case with mild clinical manifestations similar to Morquio syndrome; however, the patient was deficient in acid β-galactosidase. In the follow-up study, Arbisser et al (1977) (48) described a similar case of mild MPS IVA-like symptoms in a patient with β-galactosidase deficiency, proposing an alternate description of the disease as MPS IVB. Since the description of MPS IVB in 1977 (48), MPS IV has been categorized as two types, which include MPS IVA (GALNS deficiency) and MPS IVB (β-galactosidase deficiency). The clinical manifestation of β-galactosidase deficiency include platyspondyly of the vertebrae, significant dysplasia of the long bones (femoral epiphyses), atlanto-occipital instability, distal epiphyses, genu valgum, gait abnormalities, and corneal clouding. Distinct from MPS IVA, patients with MPS IVB show normal or near normal stature with normal neck development and absence of hearing loss and hepatomegaly (47;48).
As the clinical manifestations of the disease prior to description of MPS IVB in 1977 simply refer to the affliction as Morquio syndrome or MPS IV, it is difficult to differentiate published clinical descriptions as types A or B. Morquio A syndrome is recognized as MPS IVA in the Online Mendelian Inheritance in Man® (OMIM®) database as entry #253000.
Obrien et al (47) have suggested that a patient with type B had skeletal dysplasia of the long bones and extremities with a more normal stature. As well, GM-1 β-galactosidase activity may be sufficient to prevent peripheral nervous system disorders seen in MPS IVA. Arbisser et al (48) have described the symptoms of MPS IVB as being mild (less severe) relative to those observed in patients with MPS IVA; however, varying severities of MPS IVA have been proposed (7;16;17;23;29–31;50). The diagnosis and classification of MPS IVA disease severity will be discussed later in this manuscript.
In a summary of literature up to the time of delineation of types A and B, a number of extensive reviews of the clinical symptoms to consider in the diagnosis of MPS IV have been published (35;41;43;44) . Since that time, a number of reviews of the clinical manifestations particular to the diagnoses of MPS IVA have been published (16–19). As well, biochemical analyses for diagnosis based on the enzymatic activity of GALNS in leukocytes or fibroblasts (7;17;18;34) have been described. Diagnoses of MPS IVA have been confirmed by molecular analysis (10;23). More recently, diagnostic algorithms, which incorporate clinical, biochemical, and molecular evaluations, have been proposed for MPS IVA (51).
Clinical manifestations of MPS IVA vary from a severe form characterized by severe systemic bone dysplasia present at birth to a less severe form characterized by less significant bone involvement in patients diagnosed in adulthood. Patients with the severe form of the disease often do not survive beyond the second or third decade of life due primarily to cervical instability and pulmonary compromise (16;17;19). A high degree of genetic heterogeneity is likely responsible for this phenotypic variance with approximately 180 different mutations having been identified in the GALNS gene (10) (unpublished data). Clinical presentations of severely affected patients have been reported as severe or classical (28–30) phenotypes. Less severe forms of MPS IVA have been reported as mild (30;31;52) or attenuated (7;9) phenotypes. As well, an intermediate subtype of MPS IVA has been proposed (29;30;32). Onset of disease symptoms commonly occurs prior to 1 year of age in patients with the severe form of MPS IVA or as late as the second decade of life in patients with mild MPS IVA (16).
A very mild form of MPS IVA was first described in 1981 (29), in which the patient was over 150 cm tall, and did not have many of the characteristic features, including pectus.carinatum, genu valugum, laxity of joints, severe corneal clouding, and facial changes. Additional reports of patients with mild MPS IVA indicate these patients may survive 50–60 years (11;16;17). However, to date, most reported phenotypes have typically appeared to be more severe (16;17). Montaño et al. (16) proposed that 68.4% of patients could be categorized as having the severe form of MPS IVA while 9.8% were categorized as mild and 15.1% were categorized as intermediate. While the clinical phenotype for categorizing severity has not been clearly established, height based on age may be the most objective measurement. In this study, height was compared to age-matched normal growth charts developed by the Centers for Disease Control (CDC) and patients were defined as having severe, intermediate or mild MPS IVA if final height was below 120 cm, between 120 and 140 cm, or above 140 cm, respectively. Children with severe MPS IVA show a reduced growth rate beginning at approximately 18 months of age and growth will stop at approximately 7 or 8 years of age; however, some patients with attenuated MPS IVA may continue growing into adolescence and exceed 140 cm in height (18;53).
Patients with severe MPS IVA will exhibit initial symptoms prior to 1 year of age and are typically diagnosed before 5 years of age. Montaño et al (16) have reported that the major symptoms in determining severity can be derived from degree of skeletal involvement such as short stature, odontoid dysplasia, pectus carinatum, genu valgum, kyphoscoliosis, and hypermobility of joints and abnormal gait. While symptoms appear less severe or delayed in patients with attenuated MPS IVA, the disease progression in these individuals will eventually lead to the symptoms seen in younger patients with more severe disease (19;54). An alternative criterion for defining severity, based phenotype, was subsequently proposed when standard growth charts for each gender of MPS IVA patients became available (53). The MPS IVA growth charts define a more accurate phenotypic classification for both genders.
Although the severity of MPS IVA in affected patients has been discussed in the context of various observations, a unified definition of severity has not been proposed. A true measure of disease severity may require multivariate considerations of this disease with such a vast spectrum of clinical manifestations. However, regardless of severity, MPS IVA is a progressive systemic disease that will eventually result in morbidity and mortality and early diagnosis is important in order to improve the quality of life in these patients (16;17).
Description of Patients with Mucopolysaccharidosis IVA
A. The International Morquio Registry
The International Morquio A Registry started by International Morquio Organization and Carol Ann Foundation (www.morquio.com) and was designed to study clinical outcomes of patients with MPS IVA. The Registry included patient reported data from questionnaires regarding family history, diagnosis, initial and present MPS IVA symptoms, birth length and weight, current height and weight, surgical history, physical activities, and serious problems. The characteristics of patients in the International Morquio A Registry have been previously reported (16;17). These data provided an effective characterization of the manifestations of MPS IVA and enhance the understanding of the variability, progression, and natural history of MPS IVA among a large group of patients. Previously unpublished data collected through the end of 2011 have been recently compiled from 399 patients in the International Morquio A Registry and are presented (see (16) for a description of the data collection methods).
Table 1 shows the demographics of patients enrolled in the International Morquio A Registry. A summary of the characteristics of patients in The International Morquio A Registry are summarized in Table 2. The most common initial symptoms reported by patients included bone deformity (genu valgum, kyphosis, pectus carinatum), short stature and abnormal gait. Current symptoms reported in ≥ 50% of MPS IVA patients included short stature, bone deformity, joint instability and laxity, difficulty with joint movement, and abnormal gait.
Table 1.
Demographics of Patients in the International Morquio A Registry
| Statistics | Males | Females | |
|---|---|---|---|
| Number enrolled | n (%) | 209 (52.4) | 190 (47.6) |
| Age at enrollment | Mean (SD) | 17.0 (12.3) | 19.2 (13.9) |
| Median | 11.6 | 15.2 | |
| Range | 1–74 | 0–68 | |
| Age distribution | |||
| ≥18years | n (%) | 68 (32.5) | 81 (42.6) |
| <18 years | n (%) | 141 (67.5.1) | 109 (57.4) |
| Ethnicity | n | ||
| Caucasian | n (%) | 127 (60.7) | 124 65.3) |
| Hispanic | n (%) | 39 (18.7) | 29 (15.3) |
| Black | n (%) | 4 (1.9) | 1 (0.5) |
| Asian | n (%) | 33 (15.8) | 32 (16.8) |
| Other | n (%) | 6 (2.9) | 4 (2.1) |
Results based on data available as of the end of 2011 from 399 patients in the International Morquio A Registry.
Table 2.
Initial Characteristics of Patients in the International Morquio A Registry
| Status | Value |
|---|---|
| Mean age at enrollment (years) | 17.4 |
| Mean age of onset (years) | 2.2 |
| Mean age at diagnosis (years) | 4.9 |
| Age at diagnosis (%) | |
| < 1 year | 9 |
| 1–3 years | 34 |
| 3–5 years | 33 |
| 5–10 years | 16 |
| > 10 years | 8 |
| Phenotype (N, %)a | |
| Severe | 288 (72.2) |
| Mild | 82 (20.6) |
| Unknown | 28 (7.2) |
| Most common initial symptoms (%) | Short stature (49.9) |
| Genu valgum (45.1) | |
| Kyphosis (44.4) | |
| Pectus carinatum (43.6) | |
| Abnormal gait (37.8) | |
| Most common current symptoms (%) | Short stature (84.7) |
| Genu valgum (78.7) | |
| Pectus carinatum (71.4) | |
| Kyphosis (70.4) | |
| Abnormal gait (64.4) | |
| Laxity of wrist joints (63.2%) | |
| Patients with ≥1 surgery (%) | 222 (55.6) |
| Most frequent surgery (%) | Cervical spine surgery (51) |
| Walk endurance (maximum distance) | |
| <200 meters (%) | 63.2 |
| ≥200 meters (%) | 13.1 |
| Require walking aids (%) | 23.7 |
| Wheelchair usage (%) | 37.3 |
| Mean (SD) birth weight (kg) | |
| M | 3.50 (0.66) |
| F | 3.42 (0.61) |
| Mean (SD) weight of patients > 18 years (kg) |
|
| M | 43.02 ± 18.02 |
| F | 36.7 ± 14.5 |
| Mean (SD) birth length (cm) | |
| M | 52.0 ± 4.7 |
| F | 51.9 ± 4.5 |
| Mean (SD) height of patients > 18 years (cm) |
|
| M | 120.4 ± 22.5 |
| F | 114.8 ± 20.5 |
Phenotypic severity based on height
Results based on data available as of the end of 2011 from 399 patients in the International Morquio A Registry.
Patients with a severe phenotype accounted for nearly 3/4 of the 399 MPS IVA patients registered in International Morquio Registry (Table 2). The age at presentation with symptoms of MPS IVA is variable, as are the presenting signs and disease complications. The age at onset of the main signs and symptoms of the disease was 2.2 years; however, diagnosis was delayed by nearly 3 years, with a mean age of 4.9 years at diagnosis. It is important to note that individuals who are diagnosed with a less severe form of the disease may still have symptoms and complications that lead to significant morbidity and disability, and may present with less severe to moderate physical disabilities. Although the clinical course for the more severely affected patients is relatively predictable, there is considerable variability in the clinical phenotype and progression of the more attenuated form of the disease.
Survival to adulthood was common and in patients with less severe symptoms of MPS IVA, life expectancy can be near normal. Less severely affected females have given birth to children; however, delivery by Cesarean section was required in all cases. Patients with MPS IVA can usually be clinically distinguished from patients with other MPS diseases as they have additional skeletal manifestations derived from a unique epiphyseal chondrodysplasia and laxity of joints. Patients with a severe phenotype show typical skeletal manifestations of MPS IVA (kyphosis, pectus carinatum) by 1 year of age, followed by gibbus deformity of the back, which may become clinically obvious at approximately 14 months of age. Genu valgum and abnormal gait may become clinically obvious by 2 years of age. At that time, it is common to observe the beginnings of bone abnormalities in x-rays, particularly within the hip, ovoid vertebrae, and as widening of the ribs.
The current signs and symptoms observed in over 60% of patients enrolled in the International Morquio A Registry were short stature, genu valgum, kyphosis, pectus carinatum, abnormal gait, laxity of joints, hip deformity, and difficulty of joint movement. High mortality and morbidity rates are related to the occurrence of cervical myelopathy and dysplasia in patients with severe MPS IVA, and these symptoms will eventually occur in virtually all patients with MPS IVA (16;17;54). Many patients with MPS IVA walk with a waddling gait or stand and walk with their knees and hips flexed.
Orthopedic complications were the most critical issues for MPS IVA patients with over half of the patients enrolled in the International Morquio A Registry having surgical procedures. The common orthopedic interventions were cervical spinal cord decompression/fusion, osteotomy, and hip reconstruction and replacement. On average, by the age of 10 years, MPS IVA patients often require major surgical operations in the neck, hip, knee, or leg regions (16;17). Surgeries for skeletal, joint and ENT problems were common and wheelchairs were used more frequently than walk aids. Height in children was typically below the 3rd percentile of CDC growth charts and approximately 1/3 of pediatric and adult patients were at a healthy weight with the remaining 2/3 tending to be overweight.
B. Musculoskeletal Observations of Patients with Mucopolysaccharidosis IVA
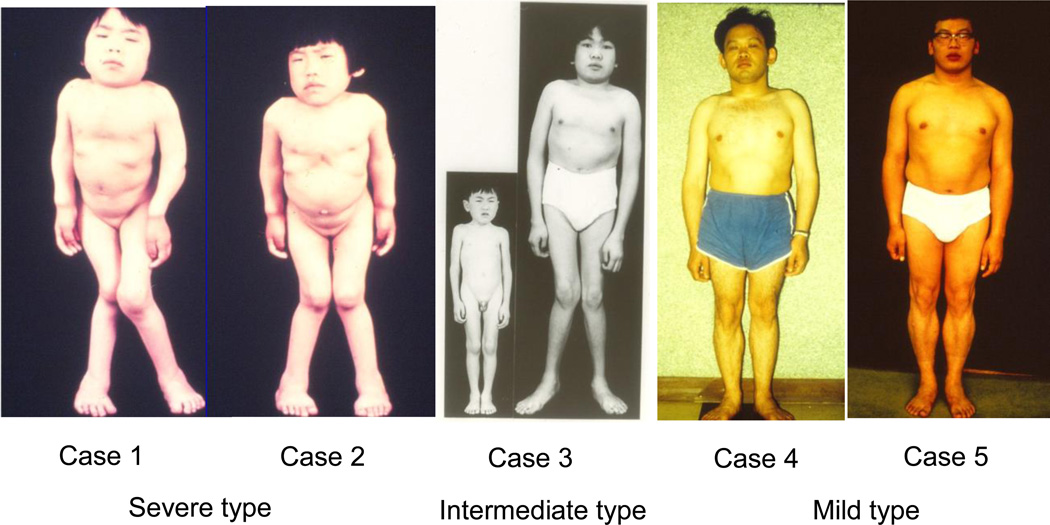
Data from the International Morquio A Registry further emphasize that the key symptoms found in patients with MPS IVA include musculoskeletal abnormalities and limited growth. Understanding and detecting the musculoskeletal manifestations is key to initial and early diagnosis. Examples of skeletal observations in patients with severe (classical), intermediate and mild MPS IVA are provided in Table 3. Figure 1 also provides photographic examples of the musculoskeletal characteristics of the same patients with severe, intermediate and mild types of MPS IVA.
Table 3.
Summary of Subtypes of Five Patients with Mucopolysaccharidosis IVA.
| MPS IV Subtype |
|||||
|---|---|---|---|---|---|
| Severea | Intermediatea | Mildb | |||
| Patients (year) | Sisters (1971) | 1 Boy (1971) | Brothers (1981) | ||
| Case Number | 1 | 2 | 3 | 4 | 5 |
| Age, Years | 12 | 8 | 6 | 29 | 25 |
| Height, cm | 98 | 89 | 105.2 | 147 | 157 |
| Genu valgum | (+) | (+) | (−)→(+) | (−) | (−) |
| Gene Mutation | P125L/P125L | R94G/N204K | N204K/N204K | ||
Orii et al. 1971
Orii et al., 1981
Figure 1.
Spectrum of disease progression among MPS IVA-affected individuals. Copy right permission from Gifu University.
In Figure 1, Cases 1 and 2 (29) are children of parents that were from a consanguineous marriage. Two sisters, aged 12 (Case 1) and 8 (Case 2), classified as having severe MPS IVA, show the skeletal deformities typical of MPS IVA. They are 98 and 89 cm tall respectively, with skeletal manifestations of pectus carinatum, genu valgum, and atlantoaxial subluxation, as well laxity of joints. Both exhibit diffuse corneal clouding as non-skeletal features. In Case 1, abnormal deformity in the ribs was observed at birth, and the patient had kyphosis (with abnormal gait) at 15 months of age. Abnormal gait was also noticed in Case 2 at 15 months of age. Both patients were homozygotes for p.P125L mutation (27). Both of these patients died suddenly in their sleep prior to the age of 20 years, possibly due to acute atlantoaxial subluxation.
Case 3 is a male described as having a variant form of MPS IVA (29). At 5 months of age, adduction of both thumbs was observed and at 18 months of age pectus carinatum was noted. At the age of 3 years, the patient had kyphosis and abnormal gait was observed at the age of 5 years. He was clinically diagnosed as having MPS IVA at 5.5 years of age. At the age of 6 years the patient was referred to Sapporo Medical College Hospital; he was 105.2 cm tall and had corneal clouding, some vacuoles containing vesicles and electron-dense granules in Kupffer cells, and less severe skeletal dysplasia. This atypical case did not have genu valgum, obliquity of the lower ends of the radius or ulna, or KS excretion in the urine and differed from the criteria described by Maroteaux and Lamy (55). At 15 years of age, the patient had keratansulfaturia and was diagnosed enzymatically as intermediate MPS IVA. At 18 years of age, the patient was 135 cm tall with less severe skeletal deformities of pectus carinatum, genu valgum, laxity of joints. The genotype of the patient is p.R94G/p.N204K (56). Case 3 is currently 48 years of age, and has a gait abnormalities and atlantoaxial subluxation. The patient is able to drive a car and works in the software industry.
Cases 4 and 5 were classified as having attenuated MPS IVA (31) and are offspring of consanguineous.brothers. Two brothers aged 29 (Case 4) and 25 (Case 5), are 147 and 157 cm tall, respectively. Skeletal features related to MPS IVA include short neck, short trunk, pectus carinatum, less severe skeletal deformities of thoracolumbar gibbus, less severe platyspondyly with anterior unossified wedging of the vertebral bodies compared with Cases 1 and 2; they did not have genu valgum or wrist laxity were observed. Both cases exhibit diffuse corneal clouding. Gait disturbances were observed in both brothers with hip joint pain at ages of 9 and 10 years, respectively. Both patients underwent osteotomy at age of 13 years. The GALNS enzyme activities in fibroblasts derived from both cases were 16–26% of normal controls (52). The genotypes of both cases are p.N204K/p.N204K (28). Case 4 died of myocardial infarction at the age of 52 years. Case 5 is currently 57 years of age. He had lower extremity deep vein thrombosis and obesity at 41 years, and was noticed chronic membranous glomerulonephritis at 49 years. His condition continues to deteriorate and he has undergone hemodialysis since 52 years of age but is currently stable.
C. Growth in Patients with MPS IVA
Most children with MPS IVA show poor growth and become physically handicapped due to the multisystem involvement of the disease. For children with MPS IVA, periodic assessments of growth are essential for monitoring disease activity and for acting proactively to prevent complications. The growth retardation of individuals with severe MPS IVA starts in early childhood and growth may stop by approximately 8 years of age (16). However, some patients with mild or intermediate phenotypes continue growing into adolescence or may continue to normal height (18;30;31;53) . Unlike other types of MPS, current criteria used to determine the clinical severity of a patient with MPS IVA are partly based on growth rates and final height (18;53).
Children with severe MPS IVA show a reduced growth rate beginning at approximately 18 months of age and growth will stop at approximately 7 or 8 years of age. The mean final height of boys and girls with classic MPS IVA has been shown to be −8.81 standard deviations (SD) and −8.48 SD, respectively, when compared with the mean height for healthy controls. However, some patients with attenuated MPS IVA continue growing into their teens and may reach or exceed 140 cm (18;53).
Height and weight data collected through the end of 2011 from the International Morquio A Registry compared measurements from 190 females and 209 males to reference growth curves of healthy children provided by the CDC. Height (n = 2,105) and weight (n = 1,844) measurements from 190 females and 209 males with MPS IVA were collected in the International Morquio A Registry (16) and compared to reference growth curves of healthy children provided by the CDC. The mean birth lengths of males and females with MPS IVA were 52.0 (n = 165) and 51.9 cm (n = 136), respectively, corresponding to +0.75 SD for boys and +0.98 SD for girls relative to the CDC growth charts. At 1 year of age, the mean heights for males and females were 78.1 and 78.3 cm, respectively, corresponding to +0.85 SD for males and +1.6 SD for females, remaining slightly higher than normal. At 2 years of age, the mean height of males and females with MPS IVA closely corresponded to that of the normal population; however, after the age of 2 years, the mean height of both genders began to fall markedly below the −2 SD value. The mean height for males and females at 18 years of age was 120.4 (n = 78) and 114.8 cm (n = 62), resulting in a difference of −55.8 and −48.3 cm, respectively, compared to the mean height for the age-matched controls. These values correspond to −8.0 SD and −7.7 SD of the height for normal healthy males and females.
Mean birth weights for males and females were 3.5 (n=189) and 3.4 (n=163) kg, respectively. The mean body weights for males and females at 18 years of age and older were 40.7 and 36.7 kg (n=74), respectively while normal healthy males and females at 18 years old had mean body weights of 67.2 and 56.2 kg, respectively. The weight of children with MPS IVA up to age 12 years was within the −2 SD of the normal children. However, the mean weight of males and females with MPS IVA at 18 years of age was 36.5 (n = 96) and 35.2 kg (n = 108), respectively. These values corresponded to −3.5 SD and −3.1 SD of the weight for age-matched normal males and females.
Age-dependent Z-score curves of the mean heights and weights were determined and compared with the aged-matched controls. The Z-score of the mean weight of patients with MPS IVA to the age matched control was higher than that of the mean height through almost all ages in both genders. From birth to 1 or 2 years of age, Z-scores of the weight for male and female patients remained positive, while Z-scores of the height stayed positive until 1 year of age. After 1 year of age, Z-scores of height were markedly reduced, indicating early slowing of height growth compared with weight. These findings resulted in mean body mass indexes (BMI) of males and females over 18 years of age of 27.4 and 27.7 kg/m2, respectively. These values corresponded to +1.3 SD and +1.9 SD of the BMI for age matched normal males and females, respectively, suggesting that the majority of adult patients with MPS IVA are overweight. A BMI above the 95th percentile, indicating an individual is obese (34.7 and 34.4 kg/m2 for males and females, respectively) was observed in 6.8% of the males and 5.4% of the females with MPS IVA that were 18 years of age and older.
Diagnosis and Management of Patients with MPS IVA
A. Clinical Recognition and Management
Clinical recognition and management of skeletal manifestations and the associated spinal complications is critically important for MPS IVA patients. However, as MPS IVA affects multiple organ systems, the recognition and management of other aspects of the disease cannot be ignored. Dysfunctions in the visual, auditory, cardiovascular, and respiratory systems can severely affect quality of life in patients with MPS IVA and may also contribute to decreased longevity. In addition to the skeletal manifestations, a review of clinical recognition and management of extra-skeletal symptoms of MPS IVA is presented along with recommended assessments and interventions.
With increasing age, joint laxity and skeletal deformities become increasingly evident, and typically require support by orthopedic interventions (17). Genu valgum can be corrected with temporary medial hemi-epiphysiodesis of the femoral and tibial growth plates with the caveat that this procedure should be postponed as long as possible as relapse of this deformity may occur during continued childhood growth (57). It is also important to note that if a procedure is excessively delayed, the patient may not show enough post-procedure growth and the procedure could fail. If the epiphyses are already closed, an osteotomy is an option. In the adult patient who remains mobile, hip replacement may become necessary. Lastly, physiotherapy may be beneficial for all patients to preserve joint function.
Early detection of SCC is important in order to prevent further development of SCC and myelomalacia, which indicates irreversible damage of the spinal cord. Periodic neurological examinations should be performed from approximately 5 years of age in patients that are diagnosed at a young age. The degree of functional impairment of the spinal cord can be assessed by neurophysiological examinations such as somatosensory-evoked potentials (SSEP). In addition, magnetic resonance imaging (MRI) studies of the cranio-cervical junction as well as simple radiological examinations in flexion and extension positions of the neck are strongly recommended. The SSEP and radiological examinations should be repeated every 2 years. If there are any clinical and/or radiological signs of SCC, a surgical decompression may be required. In order to stabilize the cranio-cervical junction, a fusion operation using an onlay bone graft may follow the decompression. Weakness of the legs and bladder and anal incontinence are symptoms of compression in the lower spine that can also require surgical correction.
Any surgical procedures requiring sedation that are performed on patients with MPS IVA require an anesthesiologist who has extensive experience in anaesthesia of patients with MPS diseases and is familiar with the complications that may arise from the intubation of children and adults affected by these diseases. Because of the risk of SCC due to atlanto-axial subluxation during the intubation, hyperflexion of the neck should be prevented. In case of airway obstruction, fiberoptic intubation may be applied (58;59).
In most patients with MPS IVA, the eye is affected as well (60). Typically, corneal clouding is usually less severe, and in some cases retinopathy may be observed (61). In patients with significant corneal clouding, penetrating keratoplasty provides good results with a clear donor cornea. The success of the graft, however, is limited as accumulation of GAGs in host keratinocytes may lead to recurrence of opacification.
As MPS IVA progresses in patients, cardiac and pulmonary complications typically arise, even in cases categorized as mild. Echocardiographic investigations have revealed moderate mitral and aortic regurgitation and valve thickening (62). Valve replacement is necessary in rare cases, appearing to affect the aortic valve more frequently (63;64); however, endocarditis prophylaxis may be required in some cases. Echocardiography should be repeated every 2 to 3 years, depending on initial findings. Recurrent infections of the upper airway and hearing problems are common among patients with MPS IVA, which require frequent examinations by an otolaryngologist. Prophylactic antibiotics may be useful to manage recurrent infections due to abnormalities in the ears, nose, and throat (ENT) and corrective procedures such as adenoidectomy or tonsillectomy may become necessary. As the disease progresses, patients may experience mixed hearing loss and may require tympanostomy tube insertion and/or hearing aids (19). While hearing loss may be postponed in mild cases, eventual hearing loss is common (19).
The cause of restrictive lung disease in patients with MPS IVA is likely multifactorial; however, thoracic deformity (pectus carinatum, rib deformity) appears to be a primary cause. The resulting poor clearance of airway secretions commonly leads to recurrent pneumonia. Pulmonary function tests (PFT) such as forced vital capacity (FVC) and forced expiratory volume in 1 second (FEV1) should be performed regularly to assess changes in lung volume and obstruction. Pulmonary hypertension, which may be caused by obstructive sleep apnea, is observed in patients with MPS IVA as commonly as observed in other mucopolysaccharidoses. Patients may complain of nocturnal dyspnea, in which case polysomnography can be used to assess sleep disturbances. Continuous positive airway pressure (CPAP) to retain the patency of the airway has been shown to be beneficial for patients with nocturnal sleep apnea (17;65).
Abnormalities of the teeth are very common in patients with MPS IVA (66). The teeth of patients with MPS IVA are typically small and the enamel is thin with a greyish color and the cusps of the permanent teeth are sharpened (17). Because of high risk of dental caries, regular conservative dental therapy is important in patients with MPS IVA.
The data of the International Morquio A registry have revealed that <50% of patients with MPS IVA are diagnosed prior to 5 years of age (16). A delayed diagnosis of a serious chronic disorder poses a severe psychological burden on the parents of patients, who have believed that they had a healthy child. When the parents have been informed about the diagnosis of their child, it is recommended that they be referred to a clinical geneticist for appropriate genetic counseling. To diminish this burden the whole family may seek psychological assistance. Connecting parents to resources, support, and advocacy groups, such as an MPS Society, is recommended.
B. Radiographic Observations
As reported by Montaño et al. (16), musculoskeletal manifestations are a significant observation in patients with MPS IVA. An important component of the review of musculoskeletal manifestations in patients with MPS IVA patients is dysostosis multiplex (DM). The diagnosis of DM is often non specific and can be determined radiologically. The radiographic expression of DM is similar to almost the entire group of mucopolysaccharidoses and mucolipidoses diseases as well as other similar disorders. The diagnosis of DM in patients with MPS IVA is typically based on the radiological findings presented in Table 4.
Table 4.
Radiological Findings of Patients with MPS IVA.
| Skeletal Region | Potential Radiological Findings of Dysostoses Multiplex |
|---|---|
| Skull | Typically normal |
| Possible abnormal J-shaped sella turca | |
| Thickened diploic space | |
| Chest/Thorax | Pectus carinatum (pigeon chest) |
| Funnel Chest | |
| Clavicles (Collar bone) | Short, thick |
| Ribs | Paddle/oar shaped (not common in MPS IVA) |
| Spine | Superiorly notched, inferiorly beaked vertebral bodies |
| Middle beaked vertebral bodies (most common in MPS IVA) | |
| Posterior scalloping of vertebrae | |
| Kyphosis | |
| Pelvis/Hips | Rounded iliac wings |
| Inferiorly tapered ilia | |
| Coxa valga | |
| Long bones | Generalized mildly hypoplastic epiphyses |
| Hypoplastic fragmented capital femoral epiphyses | |
| Long narrow femoral necks | |
| Genu valgum | |
| Hypoplastic distal ulnae/radial tilt | |
| Thick short diaphyses | |
| Tarsal bones with irregular contours | |
| Hands | Metacarpals, proximal pointing (often persistent proximal rounding in patients with MPS IVA) |
| Metacarpals, thick/short/thin cortices (typically mild in patients with MPS IVA) |
|
| Carpal bones, irregular/Hypoplastic | |
| Hand bone age compared to chronological age shows delayed bone development |
|
| Carpal bone age compared to chronological carpal bone age shows very delayed maturation in carpal bones [epiphyseal- carpal disassociation] |
|
| Scaphoid bone extremely delayed ossification or not radiographically present |
Universal platyspondyly and beaking of the vertebral bodies with thoracolumbar kyphosis are commonly observed as the most distinct skeletal changes in patients with MPS IVA. Skeletal radiographs of some patients with MPS IVA at birth have shown small abnormalities in the lower lumbar vertebrae followed by progressive bone dysplasia characteristic of MPS IVA. Therefore the characteristic findings of platyspondyly and anterior beaking of the vertebral bodies might be detectable at an early age. Progression of the anterior beaking, progressive kyphosis, platyspondyly, and irregularities of the vertebral bodies are characteristic MPS IVA (67).
Laboratory Diagnoses of MPS IVA
A. Biochemical Diagnoses of MPS IVA and Challenges
Once a high degree of clinical suspicion has been established, further biochemical testing is required. Historically, total urine GAGs (uGAGs) testing have been performed which, if positive, was followed by measurement of GALNS enzyme activity. However, as urine based testing is considered only a screen and can have false negatives, it is recommended that enzymatic or molecular testing be used to confirm or rule out the diagnosis of MPS IVA.
Measurement of uGAGs can be performed quantitatively and qualitatively. Both are recommended as part of the evaluation of patient for MPS IVA. Quantitative uGAG analyses measure the total amount of GAG in the sample per unit creatinine. The normal range is age dependent and values decrease with age, reaching a plateau in adolescence (68;69). This testing is considered as a screen for any MPS disorder (51;68) as the reported value includes all GAG species and does not allow for delineation of the specific MPS subtype. While a gross elevation of quantitative uGAGs is normally specific for an MPS disorder, borderline or slightly elevated values are common in affected patients, especially those with MPS IVA (34;68;70;71).
Qualitative urine-based testing methods separate the various GAG species and therefore, provide a starting path for differential diagnosis. For these procedures, the GAGs are first isolated from the urine and then separated by thin layer chromatography or electrophoresis (72–75). A GAG specific stain such as dimethylmethylene blue is then used to visualize the GAGs and their relative position on the gel or plate is used for identification (76). The specific diagnostic GAG in patients with MPS IVA, KS, can be difficult to separate and visualize by these methods and false negatives can occur. The positive identification of KS, unlike other types of MPS, is generally specific for MPS IVA, MPS IVB, or rarely other LSD conditions; however, qualitative methods do not give a specific value for the amount of KS.
Detection of specific abnormal GAG levels has been described using tandem mass spectrometry based methods first by Oguma et al (22;77), followed by others (24;67;78–83). The measurements of blood and urine KS specifically have been accomplished using an inhibition ELISA (21) or a sandwich ELISA (34;84), supplied by Seikagaku Co (Tokyo, Japan), and tandem mass spectrometry (22;24;80;81; 83; 85). Blood and urine KS levels correlate with clinical severity in MPS IVA patients (24;34;79;80). The results of these research studies show that blood and urine KS measurements may potentially be used as a biomarker for assessing clinical severity at an early stage and monitoring therapeutic effects (82). Currently, there is no commercial ELISA assay available to measure KS; however, tandem mass spectrometry methods of measuring KS are in use (22;24).
Regardless of the test used, uGAGs and/or KS based testing should be considered as a screening tool, which has a lower sensitivity and specificity than enzyme based testing. Patients with a strong clinical indication, regardless of total uGAGs and/or blood and urine KS results, may move straight to enzyme based testing or further follow up testing. In addition, patients with less severe MPS IVA and older patients have a greater chance of false negative in total uGAGs or even blood and urine KS measurements. Blood KS levels can be measured using dried blood spot with tandem mass spectrometry (22) and may be applicable for newborn screening for severe type of MPS IVA patients. A pilot study of newborn screening for MPS including MPS IVA by measuring specific GAGs on dried blood spots (DBS) has been initiated by an international consortium supported by an NIH grant (Montaño and Tomatsu, unpublished).
Measurement of the activity of GALNS is a common laboratory assessment for the diagnosis of MPS IVA. The enzyme is active in leukocytes and fibroblasts and either sample type is acceptable for diagnosis. Use of DBS has recently been described and can be effective in areas where transportation delays or difficulties in shipping blood or fibroblasts may impact sample quality. However, the GALNS enzyme is particularly sensitive to temperature and recommended guidelines for shipping samples and interpretation of results should be observed (86).
Measurement of enzyme activity is normally performed using an artificial radioactive (87;88) or fluorogenic substrate (88). To measure the activity, the amount of signal (substrate) is measured per unit of time and is standardized for the amount of sample used (typically per mg of protein lysate in the leukocyte or fibroblast pellet). Lack of or significant reduction in the amount of substrate released compared consistent with a deficiency of the enzyme. However, all results should be compared to the reported normal range of enzyme activity, which can vary between laboratories and methodologies. Recently, a substrate has been designed, which allows the enzyme activity to be monitored via tandem mass spectrometry (89;90). While not currently available to clinical laboratories, this methodology shows great promise and may provide a significant advance for the diagnosis of MPS IVA.
Although GALNS is most commonly associated with MPS IVA, this is not the only disorder, which can lead to decreased GALNS activity. Some additional causes are provided in Table 5. Patients with mucolipidoses II (MLII, also known as I-cell disease, OMIM# 252500) and III (ML III, 252600) have reduced levels of a number of lysosomal hydrolases including GALNS when measured in fibroblasts. Symptoms of MLII and MLIII result from N-acetylglucosamine-1-phosphotransferase (GlcNAc-phosphotransferase; EC 2.7.8.17) deficiency, which catalyzes the addition of a mannose 6-phosphate (M6P) targeting signal on a large number of lysosomal hydrolases. In patients with MLII or MLIII, enzymes are mis-localized to the extracellular space and therefore, intracellular concentrations are very low. Finding normal fibroblast activity of a second M6P targeted enzyme (eg β-hexosaminidase, α-iduronidase, β-galactosidase) can be used to rule out this disorder. Patients with I-cell disease will have normal uGAG levels but may show increased urine oligosaccharides. Measurements of various plasma enzymes can also be used as a screen for I-cell disease as the plasma activity level of the mis-targeted enzymes are grossly increased. It is important to note that leukocyte enzyme activity levels are generally normal in I-cell disease and therefore only when GALNS is measured in fibroblasts should a concern for a ML II or ML III be raised. Additionally, GALNS levels will be low in patients with multiple sulfatase deficiency (MSD, OMIM# 272200), a disease resulting in abnormally low activity of all sulfatase enzymes. As with I cell disease measurement of a second enzyme, another sulfatase, is recommended to rule out this disorder. Potentially the most common cause of false positive enzyme results is degradation of the sample during shipment if a DBS or peripheral blood sample is used. As with the previous two examples, measurement of a second enzyme is recommended.
Table 5.
Sources of Deficient GALNS Activity not related to MPS IVA
| Source of Deficiency | Cause | Confirmation of Alternative Diagnosis/Cause |
|---|---|---|
| Patient has I-cell disease (specific to measurement of GALNS in fibroblasts) |
Mutations in GNPTAB or GNPTG gene. Improper trafficking of numerous lysosomal enzymes. |
Measurement of a second enzyme that uses M6P targeting (β-hexosaminidase, α-iduronidase, alpha mannosidase, etc) or measurement of various plasma enzymes |
| Patient has multiple sulfatase deficiency (all sample types) |
Mutations in SUMF1 gene. Inability to activate sulfatase active site. |
Measurement of a second sulfatase (iduronate 2 sulfatase, arylsulfatase A or B) |
| Sample has degraded during shipment |
Sample has been impacted by event that leads to low enzyme activity for numerous enzymes. |
Measurement of a second enzyme which is found to be deficient. Assay in a second sample may be considered. |
While measurement of GALNS activity is common for the laboratory diagnosis of MPS IVA, recently there has been increased availability of MPS IVA gene sequencing, confirmation of the enzyme studies with the identification of one or more pathogenic mutations is recommended and is necessary to facilitate carrier studies and in many cases prenatal analysis. Molecular testing can be beneficial for confirming enzyme testing, especially in patients whose clinical phenotype is less severe. However, two clearly pathogenic mutations are not always found, and at times, only a single mutation may be identified.
While laboratory studies are a vital part of the diagnosis of MPS IVA, any laboratory result, which does not agree with the clinical assessment and other supporting evidence, should be questioned. Consultation with the laboratory staff and director about concern or questions may be required. Many laboratories will perform repeat testing to confirm prior results and use of a second laboratory may be suggested when a diagnosis is in question.
B. Genetic Diagnosis of MPS IVA
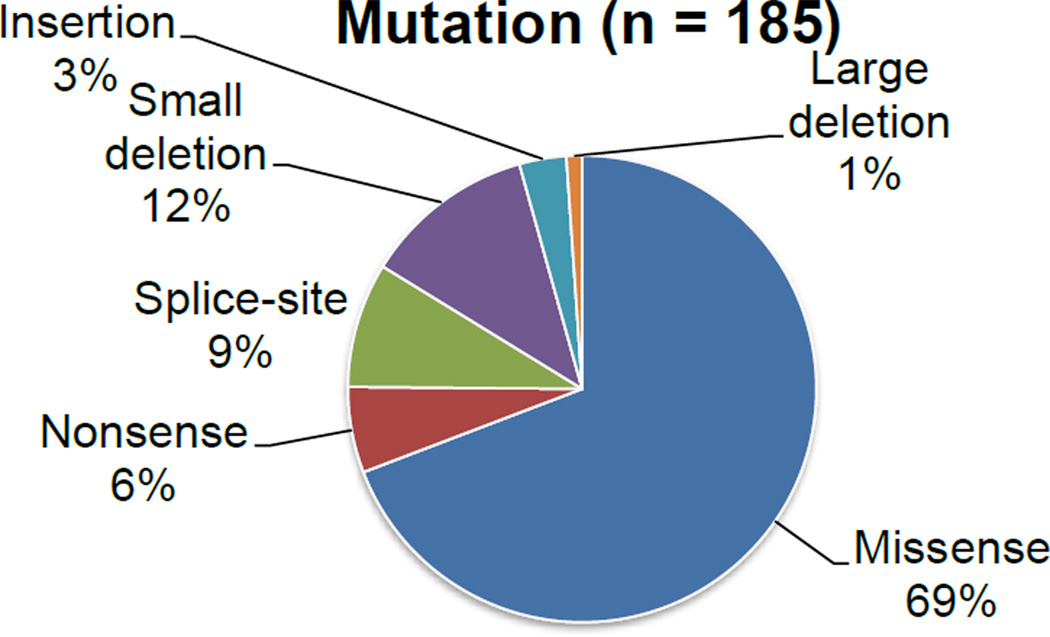
Mutation of the GALNS gene in patients with MPS IVA results in impaired catabolism of two GAGs, KS (91) and C6S (13). The human gene encoding GALNS has been mapped to chromosome arm 16q24.3 (92;93), spanning approximately 50 kb and containing 13 introns and 14 exons. A single-splice product, 1566-bp GALNS mRNA, codes for a 522-amino acid precursor. After the cleavage of a 21–amino acid N-terminal signal peptide and glycosylation, it becomes 40- and 15-kDa subunits of mature active enzyme (12;94;95). The GALNS gene is a member of the sulfatase gene family, of which 13 sulfatase genes in humans have been cloned. All sulfatase gene products are closely related, showing 20–35% similarity at the amino acid level. The C79 residue of human GALNS is conserved among all sulfatase proteins from many species. The post-translational modification of the highly conserved cysteine residue is required for catalytically active sulfatases (96). Characterization of sulfatase proteins by structural analysis and homology comparisons confirmed C79 as an active site residue (97). There have been 180 different mutations of the GALNS gene reported, resulting in a spectrum of disease phenotypes. These mutations have been identified in over 400 mutant alleles in a total group of 250 patients by a variety of molecular techniques (8–10;26–28). The mutations are distributed along the entire gene and all types of mutations are found (missense, nonsense, splice site mutations, small deletions and others).
By cataloguing current literature, missense mutations were found to be the most prevalent (69%) among GALNS mutations (Figure 2). The 10 most frequent mutations accounting for over 7 mutant alleles are represented by single nucleotide changes except for one deletion. They make up 35% of all described mutations. The 16 mutations accounting for 6, 5 or 4 mutant alleles account for 19% of all mutations. The remaining 46% of mutations occur less than 3 times in the total population, suggesting that molecular heterogeneity in GALNS mutations is marked. The most prevalent c.1156C>T transitional mutation at CpG dinucleotide (p.R386C) was found in 9% of patients from various ethnic groups origin (9;10;32;98;99).
Figure 2.
Occurrences of Genetic Mutation Types in Patients with MPS IVA Based on Literature Review.
In an assessment of the genetic composition of 9 MPS IVA patients in Latin America (32), all 9 patients were homozygous for 1 of 4 different mutations (c.1156C, c.224C, c.500A, c.346G) and developed a severe type of MPS IVA with similar clinical signs and symptoms. The p.I113F and p.T312S mutations are of great interest since these mutations were specific to British, Irish, and Australian (100;101) populations and account for 18 and 14% of all mutations, respectively (101). These two founder mutations are associated with a 3–6-fold increase of the incidence of MPS IVA in Northern Ireland compared to other ethnic populations. The p.I113F mutation defines a severe phenotype while the p.T312S mutation defines an attenuated phenotype (101). Thus, some mutations are clearly correlated with a specific clinical phenotype. Based on a review of missense mutations in the current literature, 69% of mutations were defined as severe, 19% were as attenuated and other 12% were as undefined.
Emerging Developments in Our Understanding of MPS IVA Clinical Manifestations
MPS IVA has historically been understood as a disease caused by pathogenic storage of KS in cartilage and connective tissue, leading to skeletal dysplasia and visual, auditory, cardiovascular, and respiratory compromise (15;19). However, this model does not fully account for all of the clinical observations in MPS IVA (102). Recent studies and observations have lead to novel theories regarding the pathogenesis behind MPS IVA and the resulting clinical manifestations. Although it has been generally assumed that MPS IVA does not directly affect the central nervous system (CNS), a contemporary publication challenged this assumption by presenting subtle neuroimaging, neurochemical, and neurocognitive abnormalities indicating the potential for CNS involvement in MPS IVA (103). A complex interplay between lysosomal, mitochondrial, and calcium trafficking dysfunction was proposed as a hypothetical mechanism (103). Anecdotally, patients with MPS IVA tend to have skeletal muscle weakness and slightly lower than average core temperatures (unpublished data). If calcium trafficking, and hence mitochondrial functioning, is affected in MPS IVA as proposed by Davison et al (103), it could also be a possible explanation behind these clinical observations. Calcium abnormalities have been noted in other lysosomal storage diseases (104;105) and their role in the pathogenesis of these diseases has been described in the context of impaired autophagy and accumulation of dysfunctional mitochondria (106–108). Furthermore, impaired autophagy and mitochondrial dysfunction have been reported in fibroblasts from patients with MPS VI (109). This pathogenic cascade may well be present in many lysosomal storage diseases including the mucopolysaccharidoses and MPS IVA specifically. Further research is needed to substantiate the anecdotal clinical observations and to investigate the roles of calcium trafficking and mitochondrial dysfunction, or alternative mechanisms, in the pathogenesis of MPS IVA.
Conclusions
The symptoms of patients reported to have MPS IVA related disease (chondro osteo dystrophy, osteochondrodystrophia deformans, Morquio’s syndrome, Morquio-Brailsford disease, Morquio-Ulrich’s disease) have been described for at least 80 years. A historical review of these reports based on case reports or limited sample sizes indicates that MPS IVA affects multiple organ systems, with skeletal dysplasia and growth being the most severely affected. In addition, other organ systems including visual, auditory, cardiovascular, and respiratory systems are also affected. Similar descriptions of the symptoms of MPS IVA have recently been demonstrated in a survey of a large population (399 patients), confirming previous observations.
While many of the signs and symptoms of MPS IVA are well described, others such as joint laxity and lack of strength are not. These have been reviewed in this report, but the lack of understanding of these findings also illustrates the possibility that additional unaddressed symptoms may occur in this multi-organ disease. It is also important to note that some symptoms reported by patients with MPS IVA can clinically and radiographically resemble other disorders, and differential diagnosis requires enzymatic or molecular diagnosis.
The age at diagnosis, natural progression of the disease, and severity of symptoms is determined by the severity of the disease, which may be associated with specific genetic mutations. Although the severity of MPS IVA in affected patients has been discussed in the context of various observations, particularly height, a unified definition of severity has not been proposed. It is possible that defining criteria for the early diagnosis of MPS IVA in less severely affected children may be required in order to initiate early management to alleviate the effects seen later in life.
With the goal of describing the expected natural history of disease progression and improving recognition, diagnosis, and management of MPS IVA, a review of the historical and current clinical experience of patients with MPS IVA has been presented, along with some possible treatment options. In addition, contemporary laboratory assessments and well defined algorithms for diagnoses will further improve early diagnoses. Understanding the symptoms and progressive nature of MPS IVA will provide a solid basis for evaluating the efficacy of treatment modalities (110).
Highlights.
International experts discussed diagnosis of MPS IVA.
Historical Review of the Clinical Presentation was summarized.
Clinical to lab data on MPS IVA was summarized.
Updated information was included.
By reading this article the reader will understand how to diagnose MPS IVA.
Acknowledgements
The concept of this manuscript was conceived at a summit sponsored by BioMarin Pharmaceutical Inc. (BioMarin) held June 2011 in Sausalito CA USA. BioMarin provided administrative assistance as well as editorial and critical reviews of this manuscript.
Dr. Tomatsu’s contributions were supported by the grant from International Morquio Organization (Carol Ann Foundation: www.morquio.com). S.T. was also supported by National Institutes of Health grants, 8P20GM103464-08 and 1R01HD065767-01. 1R01HD065767-01: International consortium for newborn screening and biomarkers for mucopolysaccharidoses was organized by this fund. We also appreciate for International Morquio Organization (Carol Ann Foundation) kindly providing the permission for use of International Morquio Registry data.
Abbreviations not common to the field
- CDC
Centers for Disease Control and Prevention
- CPAP
Continuous positive airway pressure
- DBS
Dried blood spots
- GALNS
N-acetylgalactosamine-6-sulfate sulfatase
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
All authors received reimbursement for participating in a BioMarin sponsored summit. The author contributions were presented in part at the summit and were not influenced by BioMarin. C.G. Gravance is an employee of BioMarin Pharmaceutical, Inc.
References
- 1.Matalon R, Arbogast B, Justice P. Morquio's syndrome: deficiency of a chondroitin sulfate N acetylhexosamine sulfate sulfatase. BiochemI.Biophys.Res.Commun. 1974;61(2):759–765. doi: 10.1016/0006-291x(74)91022-5. [DOI] [PubMed] [Google Scholar]
- 2.Morquio L. Sur une forme de dystrophie osseuse familiale. Vol. 32. Paris: Archives de medecine des infants; 1929. pp. 129–135. [Google Scholar]
- 3.Brailsford JF. Chondro-osteo-dystrophy. Roentgenographic and clinical features of a child with dislocation of vertebrae. Am.J.Sur. 1929;7:404–410. [PubMed] [Google Scholar]
- 4.Meikle P, Hopwood J, Clague AE, Carey W. Prevelence ofo lysosomal storage disorders. JAMA : the journal of the American Medical Association. 1999;281(3):249–254. doi: 10.1001/jama.281.3.249. [DOI] [PubMed] [Google Scholar]
- 5.Nelson J. Incidence of the mucopolysaccharidoses in Northern Ireland. Hum.Genet. 1997;101(3):355–358. doi: 10.1007/s004390050641. [DOI] [PubMed] [Google Scholar]
- 6.Nelson J, Crowhurst J, Carey B, Greed L. Incidence of the Mucopolysaccharidoses in Western Australia. Am.J.Med.Genet. 2003;123A(3):310–313. doi: 10.1002/ajmg.a.20314. [DOI] [PubMed] [Google Scholar]
- 7.Montano AM, Sukegawa K, Kato Z, Carrozzo R, Di NP, Christensen E, Orii KO, Orii T, Kondo N, Tomatsu S. Effect of 'attenuated' mutations in mucopolysaccharidosis IVA on molecular phenotypes of N-acetylgalactosamine-6-sulfate sulfatase. J.Inherit.Metab Dis. 2007;30(5):758–767. doi: 10.1007/s10545-007-0702-z. [DOI] [PubMed] [Google Scholar]
- 8.Kato Z, Fukuda S, Tomatsu S, Vega H, Yasunaga T, Yamagishi A, Yamada N, Valencia A, Barrera LA, Sukegawa K, et al. A novel common missense mutation G301C in the N-acetylgalactosamine-6-sulfate sulfatase gene in mucopolysaccharidosis IVA. Hum.Genet. 1997;101(1):97–101. doi: 10.1007/s004390050594. [DOI] [PubMed] [Google Scholar]
- 9.Montano AM, Kaitila I, Sukegawa K, Tomatsu S, Kato Z, Nakamura H, Fukuda S, Orii T, Kondo N. Mucopolysaccharidosis IVA: Characterization of a common mutation found in Finnish patients with attenuated phenotype. Hum.Genet. 2003;113(2):162–169. doi: 10.1007/s00439-003-0959-8. [DOI] [PubMed] [Google Scholar]
- 10.Tomatsu S, Montano AM, Nishioka T, Gutierrez MA, Pena OM, Trandafirescu GG, Lopez P, Yamaguchi S, Noguchi A, Orii T. Mutation and polymorphism spectrum of the GALNS gene in mucopolysaccharidosis IVA (Morquio A) Hum.Mutat. 2005;26(6):500–512. doi: 10.1002/humu.20257. [DOI] [PubMed] [Google Scholar]
- 11.Tomatsu S, Fukuda S, Cooper A, Wraith JE, Rezvi GMM, Yamagishi A, Yamada N, Kato Z, Isogai K, Sukegawa K, et al. Mucopolysaccharidosis IVA: Identification of a common missense mutation I113F in the N-acetylgalactosamine-6-sulfate sulfatase gene. Am.J.Hum.Genet. 1995;57(3):556–563. [PMC free article] [PubMed] [Google Scholar]
- 12.Tomatsu S, Fukuda S, Masue M, Sukegawa K, Fukao T, Yamagishi A, Hori T, Iwata H, Ogawa T, Nakashima Y, et al. Morquio disease: Isolation, characterization and expression of full-length cDNA for human N-acetylgalactosamine-6-sulfate sulfatase. Biochem.Biophys.Res.Commun. 1991;181(2):677–683. doi: 10.1016/0006-291x(91)91244-7. [DOI] [PubMed] [Google Scholar]
- 13.Dorfman A, Arbogast B, Matalon R. The enzymic defects in Morquio and Maroteaux-Lamy syndrome. Adv.Exp.Med.Biol. 1976;68:261–276. doi: 10.1007/978-1-4684-7735-1_18. [DOI] [PubMed] [Google Scholar]
- 14.Kresse H, von Figura K, Klein U, Glossl J, Paschke E, Pohlmann R. Enzymic diagnosis of the genetic mucopolysaccharide storage disorders. Meth.Enzymol. 1982;83:559–572. doi: 10.1016/0076-6879(82)83052-8. [DOI] [PubMed] [Google Scholar]
- 15.Wraith JE. The mucopolysaccharidosis: a clinical review and guide to management. Arch Dis Child. 1995;72:263–267. doi: 10.1136/adc.72.3.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Montano AM, Tomatsu S, Gottesman GS, Smith M, Orii T. International Morquio A Registry: clinical manifestation and natural course of Morquio A disease. J.Inherit.Metab Dis. 2007;30(2):165–174. doi: 10.1007/s10545-007-0529-7. [DOI] [PubMed] [Google Scholar]
- 17.Tomatsu S, Montano AM, Oikawa H, Rowan DJ, Smith M, Barrera L, Chinen Y, Thacker MM, Mackenzie WG, Suzuki Y, et al. Mucopolysaccharidosis type IVA (morquio a disease): Clinical review and current treatment: A special review. Curr.Pharm.Biotechnol. 2011;12(6):931–945. doi: 10.2174/138920111795542615. [DOI] [PubMed] [Google Scholar]
- 18.Tomatsu S, Adriana M, Montano A, Oikawa H, Giugliani R, Harmatz P, Smith M, Suzuki Y, Orii T. Impairment of Body Growth in Mucopolysaccharidoses. In: Preedy VR, editor. Handbook of growth monitoring and health and disease. London: Springer Publications; 2012. [Google Scholar]
- 19.Northover H, Cowie RA, Wraith JE. Mucopolysaccharidosis type IVA (Morquio syndrome): a clinical review. J.Inherit.Metab.Dis. 1996;19(3):357–365. doi: 10.1007/BF01799267. [DOI] [PubMed] [Google Scholar]
- 20.Nelson J, Thomas PS. Clinical findings in 12 patients with MPS IV A (Morquio's disease). Further evidence for heterogeneity. Part III: Odontoid dysplasia. Clin.Genet. 1988;33(2):126–130. doi: 10.1111/j.1399-0004.1988.tb03423.x. [DOI] [PubMed] [Google Scholar]
- 21.Thonar EJ, Pachman LM, Lenz ME, Hayford J, Lynch P, Kuettner KE. Age related changes in the concentration of serum keratan sulphate in children. J.Clin.Chem.Clin.Biochem. 1988;26(2):57–63. doi: 10.1515/cclm.1988.26.2.57. [DOI] [PubMed] [Google Scholar]
- 22.Oguma T, Tomatsu S, Okazaki O. Analytical method for determination of disaccharides derived from keratan sulfates in human serum and plasma by high-performance liquid chromatography/turbo-ionspray ionization tandem mass spectrometry. Biomed.Chromatogr. 2007;21(4):356–362. doi: 10.1002/bmc.760. [DOI] [PubMed] [Google Scholar]
- 23.Tomatsu S, Dieter T, Schwartz IV, Sarmient P, Giugliani R, Barrera LA, Guelbert N, Kremer R, Repetto GM, Gutierrez MA, et al. Identification of a common mutation in mucopolysaccharidosis IVA: Correlation among genotype, phenotype, and keratan sulfate. J.Hum.Genet. 2004;49(9):490–494. doi: 10.1007/s10038-004-0178-8. [DOI] [PubMed] [Google Scholar]
- 24.Tomatsu S, Montano AM, Oguma T, Dung VC, Oikawa H, de Carvalho TG, Gutierrez ML, Yamaguchi S, Suzuki Y, Fukushi M, et al. Validation of keratan sulfate level in mucopolysaccharidosis type IVA by liquid chromatography-tandem mass spectrometry. J.Inherit.Metab Dis. 2010 Jan 27; doi: 10.1007/s10545-009-9013-x. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 25.Tomatsu S, Fukuda S, Cooper A, Wraith JE, Yamada N, Isogai K, Kato Z, Sukegawa K, Kondo N, Suzuki Y, et al. Two new mutations, Q473X and N487S, in a Caucasian patient with mucopolysaccharidosis IVA (Morquio disease) Hum.Mutat. 1995;6(2):195–196. doi: 10.1002/humu.1380060218. [DOI] [PubMed] [Google Scholar]
- 26.Tomatsu S, Fukuda S, Cooper A, Wraith JE, Rezvi GM, Yamagishi A, Yamada N, Kato Z, Isogai K, Sukegawa K, et al. Mucopolysaccharidosis type IVA: Identification of six novel mutations among non-Japanese patients. Hum.Mol.Genet. 1995;4(4):741–743. doi: 10.1093/hmg/4.4.741. [DOI] [PubMed] [Google Scholar]
- 27.Tomatsu S, Fukuda S, Cooper A, Wraith JE, Ferreira P, Natale PD, Tortora P, Fujimoto A, Kato Z, Yamada N, et al. Fourteen novel mucopolysaccharidosis IVA producing mutations in GALNS gene. Hum.Mutat. 1997;10(5):368–375. doi: 10.1002/(SICI)1098-1004(1997)10:5<368::AID-HUMU6>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- 28.Fukuda S, Tomatsu S, Masue M, Sukegawa K, Iwata H, Ogawa T, Nakashima Y, Hori T, Yamagishi A, Hanyu Y, et al. Mucopolysaccharidosis type IVA. N-acetylgalactosamine-6-sulfate sulfatase exonic point mutations in classical Morquio and mild cases. J.Clin.Invest. 1992;90(3):1049–1053. doi: 10.1172/JCI115919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Orii T, Minami R, Chiba T, Yamaguchi M, Tsugawa S, Nakao T, Horino K, Sakuma K. Study on Morquio syndrome. Bone Metabolism (in Japanese) 1971;5(1):72–78. [Google Scholar]
- 30.Beck M, Glossl J, Grubisic A, Spranger J. Heterogeneity of Morquio disease. Clin.Genet. 1986;29(4):325–331. doi: 10.1111/j.1399-0004.1986.tb01262.x. [DOI] [PubMed] [Google Scholar]
- 31.Orii T, Kiman T, Sukegawa K, Kanemura T, Hattori S, Taga T, Hirota K. Late onset N-acetylgalactosamine-6-sulfate sulfatase deficiency in two brothers. Connect.Tissue. 1981;13(3):169–175. [Google Scholar]
- 32.Tomatsu S, Filocamo M, Orii KO, Sly WS, Gutierrez MA, Nishioka T, Serrato OP, Di Natale P, Montano AM, Yamaguchi S, et al. Mucopolysaccharidosis IVA (Morquio A): identification of novel common mutations in the N-acetylgalactosamine-6-sulfate sulfatase (GALNS) gene in Italian patients. Hum.Mutat. 2004;24(2):187–188. doi: 10.1002/humu.9265. [DOI] [PubMed] [Google Scholar]
- 33.Montano AM, Tomatsu S, Gottesman GS, Smith M, Orii T. International Morquio A Registry: Clinical manifestation and natural course of Morquio A disease. J.Inherit.Metab.Dis. 2007;30(2):165–174. doi: 10.1007/s10545-007-0529-7. [DOI] [PubMed] [Google Scholar]
- 34.Tomatsu S, Okamura K, Taketani T, Orii KO, Nishioka T, Gutierrez MA, Velez-Castrillon S, Fachel AA, Grubb JH, Cooper A, et al. Development and Testing of New Screening Method for Keratan Sulfate in Mucopolysaccharidosis IVA. Pediatr.Res. 2004;55(4):592–597. doi: 10.1203/01.PDR.0000113767.60140.E9. [DOI] [PubMed] [Google Scholar]
- 35.Whiteside JD, Cholmeley JA. Morquio's disease; a review of the literature with a description of four cases. ARCH.DIS.CHILD. 1952;27(135):487–497. doi: 10.1136/adc.27.135.487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bankart ASB. A case of achondroplasia. Proc.Roy.Soc.Med. 1913;6:155. doi: 10.1177/003591571300601874. (Sect Study Dis Child) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.McKusick VA, Howell RR, Hussels IE, Neufeld EF, Stevenson RE. Allelism, non-allelism and genetic compounds among the mucopolysaccharidoses: corrective factors in nosology, genetics and therapy. Trans.Assoc.Am.Physicians. 1972;85:151–171. [PubMed] [Google Scholar]
- 38.Osler W. Sporadic cretinism in America. Am.J.Med. Sciences. 1897;114(4):377–400. [Google Scholar]
- 39.Smith R, McCort JJ. Osteochondrodystrophy (Morquio-Brailsford type); occurrence in three siblings. Calif.Med. 1958;88(1):55–59. [PMC free article] [PubMed] [Google Scholar]
- 40.Feldman N, Davenport ME. Osteo-chondrodystrophia deformans (Morquio Brailsford disease) Arch.Dis.Child. 1951;26(128):279–288. doi: 10.1136/adc.26.128.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Langer LO, Carey LS. The roentgenographic features of the KS mucopolysaccharidosis of Morquio (Morquio-Brailsford's disease) Am.J Roentgenol.Radium.Ther.Nucl.Med. 1966;97(1):1–20. doi: 10.2214/ajr.97.1.1. [DOI] [PubMed] [Google Scholar]
- 42.Maroteaux P, Lamy M. Corneal opacities and metabolic disorders in Morquio's disease (in French) Rev.Fr.Etud.Clin.Biol. 1961;6:481–483. [PubMed] [Google Scholar]
- 43.Robins MM, Stevens HF, Linker A. Morquio's disease: An abnormality of mucopolysaccharide metabolism. J. Ped. 1963;62:881–889. doi: 10.1016/s0022-3476(63)80102-x. [DOI] [PubMed] [Google Scholar]
- 44.Medicine. Vol. 41 The Williams & Wilkins Co.; 1965. [Google Scholar]
- 45.Singh J, Di Ferrante N, Niebes P, Tavella D. N acetylgalactosamine 6 sulfate sulfatase in man. Absence of the enzyme in Morquio disease. J.Clin.Invest. 1976;57(4):1036–1040. doi: 10.1172/JCI108345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.DiFerrante N, Ginsberg LC, Donnelly PV, Di Ferrante DT, Caskey CT. Deficiencies of glucosamine-6-sulfate or galactosamine-6-sulfate sulfatases are responsible for different mucopolysaccharidoses. Science. 1978;199(4324):79–81. doi: 10.1126/science.199.4324.79. [DOI] [PubMed] [Google Scholar]
- 47.O'Brien JS, Gugler E, Giedion A, Wiessmann U, Herschkowitz N, Meier C, Leroy J. Spondyloepiphyseal dysplasia, corneal clouding, normal intelligence and acid beta-galactosidase deficiency. Clin.Genet. 1976;9(5):495–504. doi: 10.1111/j.1399-0004.1976.tb01603.x. [DOI] [PubMed] [Google Scholar]
- 48.Arbisser AI, Donnelly KA, Scott CI, Jr., DiFerrante N, Singh J, Stevenson RE, Aylesworth AS, Howell RR. Morquio-like syndrome with beta galactosidase deficiency and normal hexosamine sulfatase activity: mucopolysacchariodosis IVB. Am.J.Med.Genet. 1977;1(2):195–205. doi: 10.1002/ajmg.1320010205. [DOI] [PubMed] [Google Scholar]
- 49.Danes BS, Bearn AG. Cellular metachromasia, a genetic marker for studying the mucopolysaccharidoses. Lancet. 1967;1(7484):241–243. doi: 10.1016/s0140-6736(67)91302-5. [DOI] [PubMed] [Google Scholar]
- 50.Nelson J, Broadhead D, Mossman J. Clinical findings in 12 patients with MPS IV A (Morquio's disease). Further evidence for heterogeneity. Part I: Clinical and biochemical findings. Clin.Genet. 1988;33(2):111–120. doi: 10.1111/j.1399-0004.1988.tb03421.x. [DOI] [PubMed] [Google Scholar]
- 51.Wood TC, Harvey K, Beck M, Burin MG, Chien YH, Church HJ, D'Almeida V, van Diggelen OP, Fietz M, Giugliani R, Harmatz P, Hawley SM, Hwu WL, Ketteridge D, Lukacs Z, Miller N, Pasquali M, Schenone A, Thompson JN, Tylee K, Yu C, Hendriksz CJ. Diagnosing mucopolysaccharidosis IVA. J.Inherit. Metab.Dis. 2013;36(2):293–307. doi: 10.1007/s10545-013-9587-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sukegawa K, Orii T. Residual activity in fibroblasts from two brothers with the late-onset form of N-acetylgalactosamine-6-sulphate sulphatase deficiency. J.Inherit.Metab.Dis. 1982;5(4):231–232. doi: 10.1007/BF02179150. [DOI] [PubMed] [Google Scholar]
- 53.Montano AM, Tomatsu S, Brusius A, Smith M, Orii T. Growth charts for patients affected with Morquio A disease. Am.J.Med.Genet.A. 2008;146A(10):1286–1295. doi: 10.1002/ajmg.a.32281. 15. [DOI] [PubMed] [Google Scholar]
- 54.Mikles M, Stanton RP. A review of Morquio syndrome. Am.J.Orthop.(Belle.Mead NJ) 1997;26(8):533–540. [PubMed] [Google Scholar]
- 55.Maroteaux P, Lamy M. Studying the mucopolysaccharidoses (letter) Lancet. 1967;2:510. [Google Scholar]
- 56.Ogawa T, Tomatsu S, Fukuda S, Yamagishi A, Rezvi GM, Sukegawa K, Kondo N, Suzuki Y, Shimozawa N, Orü T. Mucopolysaccharidosis IVA: screening and identification of mutations of the N-acetylgalactosamine-6-sulfate sulfatase gene. Hum Mol Genet. 1995;4(3):341–349. doi: 10.1093/hmg/4.3.341. [DOI] [PubMed] [Google Scholar]
- 57.Dhawale AA, Thacker MM, Belthur MV, Rogers K, Bober MB, Mackenzie WG. The lower extremity in Morquio syndrome. J.Pediatr.Orthop. 2012;32(5):534–540. doi: 10.1097/BPO.0b013e318259fe57. [DOI] [PubMed] [Google Scholar]
- 58.Walker PP, Rose E, Williams JG. Upper airways abnormalities and tracheal problems in Morquio's disease. Thorax. 2003;58(5):458–459. doi: 10.1136/thorax.58.5.458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Theroux MC, Nerker T, Ditro C, Mackenzie WG. Anesthetic care and perioperative complications of children with Morquio syndrome. J.Pediatr.Anesth. 2012;22:901–907. doi: 10.1111/j.1460-9592.2012.03904.x. [DOI] [PubMed] [Google Scholar]
- 60.Summers CG, Ashworth JL. Ocular manifestations as key features for diagnosing mucopolysaccharidoses. Rheumatology (UK) 2011;50(SUPPL. 5):v34–v40. doi: 10.1093/rheumatology/ker392. [DOI] [PubMed] [Google Scholar]
- 61.Kasmann-Kellner B, Weindler J, Pfau B, Ruprecht KW. Ocular changes in mucopolysaccharidosis IV A (Morquio A syndrome) and long-term results of perforating keratoplasty. Ophthalmologica. 1999;213(3):200–205. doi: 10.1159/000027420. [DOI] [PubMed] [Google Scholar]
- 62.Wippermann CF, Beck M, Schranz D, Huth R, Michel-Behnke I, Jungst BK. Mitral and aortic regurgitation in 84 patients with mucopolysaccharidoses. Eur.J.Pediatr. 1995;154(2):98–101. doi: 10.1007/BF01991908. [DOI] [PubMed] [Google Scholar]
- 63.Nicolini F, Corradi D, Bosio S, Gherli T. Aortic valve replacement in a patient with morquio syndrome. Heart Surg.Forum. 2008;11(2):E96–E98. doi: 10.1532/HSF98.20071197. [DOI] [PubMed] [Google Scholar]
- 64.Pagel PS, Almassi GH. Perioperative implications of Morquio syndrome in a 31-year-old woman undergoing aortic valve replacement. J.Cardiothorac.Vasc.Anesth. 2009;23(6):855–857. doi: 10.1053/j.jvca.2008.12.009. [DOI] [PubMed] [Google Scholar]
- 65.Rodriguez ME, Mackenzie WG, Ditro C, Miller TL, Chidekel A, Shaffer TH. Skeletal dysplasias: evaluation with impulse oscillometry and thoracoabdominal motion analysis. Pediatr.Pulmonol. 2010;45(7):679–686. doi: 10.1002/ppul.21246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Fitzgerald J, Verveniotis SJ. Morquio's syndrome. A case report and review of clinical findings. N.Y.State Dent.J. 1998;64(8):48–50. [PubMed] [Google Scholar]
- 67.Ohashi A, Montano AM, Colon JE, Oguma T, Luisiri A, Tomatsu S. Sacral dimple: incidental incidental findings from newborn evaluation. Mucopolysaccharidosis IVA disease. Acta Paediatr. 2009;98(5):768–762. doi: 10.1111/j.1651-2227.2009.01134.x. [DOI] [PubMed] [Google Scholar]
- 68.Whitley CB, Draper KA, Dutton CM, Brown PA, Severson SL, France LA. Diagnostic test for mucopolysaccharidosis. II. Rapid quantification of glycosaminoglycan in urine samples collected on a paper matrix. Clin.Chem. 1989;35(10):2074–2081. [PubMed] [Google Scholar]
- 69.Wood T, Bodamer OA, Burin MG, D'Almeida V, Fietz M, Giugliani R, Hawley SM, Hendriksz CJ, Hwu WL, Ketteridge D, et al. Expert recommendations for the laboratory diagnosis of MPS VI. Mol.Genet.Metab. 2012;106(1):73–82. doi: 10.1016/j.ymgme.2012.02.005. [DOI] [PubMed] [Google Scholar]
- 70.Chih-Kuang C, Shuan-Pei L, Shyue-Jye L, Tuen-Jen W. MPS screening methods, the berry spot and acid turbidity tests, cause a high incidence of false-negative results in Sanfilippo and Morquio syndromes. J.Clin.Lab.Anal. 2002;16(5):253–258. doi: 10.1002/jcla.10051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Gray G, Claridge P, Jenkinson L, Green A. Quantitation of urinary glycosaminoglycans using dimethylene blue as a screening technique for the diagnosis of mucopolysaccharidoses: an evaluation. Ann.Clin.Biochem. 2007;44(Pt 4):360–363. doi: 10.1258/000456307780945688. [DOI] [PubMed] [Google Scholar]
- 72.Hata R, Nagai Y. A rapid and micro method for separation of acidic glycosaminoglycans by two-dimensional electrophoresis. Anal.Biochem. 1972;45(2):462–468. doi: 10.1016/0003-2697(72)90208-4. [DOI] [PubMed] [Google Scholar]
- 73.Hopwood JJ, Harrison JR. High-resolution electrophoresis of urinary glycosaminoglycans: An improved screening test for the mucopolysaccharidoses. Anal.Biochem. 1982;119(1):120–127. doi: 10.1016/0003-2697(82)90674-1. [DOI] [PubMed] [Google Scholar]
- 74.Humbel R, Chamoles NA. Sequential thin layer chromatography of urinary acidic glycosaminglycans. Clin. chim. acta. 1972;40(1):290–293. doi: 10.1016/0009-8981(72)90287-2. [DOI] [PubMed] [Google Scholar]
- 75.Wessler E. Analytical and preparative separation of acidic glycosaminoglycans by electrophoresis in barium acetate. Anal.Biochem. 1968;26(3):439–444. doi: 10.1016/0003-2697(68)90205-4. [DOI] [PubMed] [Google Scholar]
- 76.Pennock CA, Barnes IC. The mucopolysaccharidoses. J.Med.Genet. 1976;13(3):169–181. doi: 10.1136/jmg.13.3.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Oguma T, Tomatsu S, Montano AM, Okazaki O. Analytical method for the determination of disaccharides derived from keratan, heparan, and dermatan sulfates in human serum and plasma by high-performance liquid chromatography/turbo ionspray ionization tandem mass spectrometry. Anal.Biochem. 2007;368(1):79–86. doi: 10.1016/j.ab.2007.05.016. [DOI] [PubMed] [Google Scholar]
- 78.Auray-Blais C, Bherer P, Gagnon R, Young SP, Zhang HH, An Y, Clarke JT, Millington DS. Efficient analysis of urinary glycosaminoglycans by LC-MS/MS in mucopolysaccharidoses type I, II and VI. Mol.Genet.Metab. 2011;102(1):49–56. doi: 10.1016/j.ymgme.2010.09.003. [DOI] [PubMed] [Google Scholar]
- 79.Hintze JP, Tomatsu S, Fujii T, Montano AM, Yamaguchi S, Suzuki Y, Fukushi M, Ishimaru T, Orii T. Comparison of liquid chromatography-tandem mass spectrometry and sandwich ELISA for determination of keratan sulfate in plasma and urine. Biomark.Insights. 2011;6:69–78. doi: 10.4137/BMI.S7451. Epub; 2011;27:69–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Martell LA, Cunico RL, Ohh J, Fulkerson W, Furneaux R, Foehr ED. Validation of an LC-MS/MS assay for detecting relevant disaccharides from keratan sulfate as a biomarker for Morquio A syndrome. Bioanalysis. 2011;3(16):1855–1866. doi: 10.4155/bio.11.172. [DOI] [PubMed] [Google Scholar]
- 81.Rowan DJ, Tomatsu S, Grubb JH, Montano AM, Sly WS. Assessment of bone dysplasia by micro-CT and glycosaminoglycan levels in mouse models for mucopolysaccharidosis type I, IIIA, IVA, and VII. J.Inherit.Metab.Dis. 2010;36(2):235–246. doi: 10.1007/s10545-012-9522-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Tomatsu S, Montano AM, Oguma T, Dung VC, Oikawa H, de Carvalho TG, Gutierrez ML, Yamaguchi S, Suzuki Y, Fukushi M, et al. Dermatan sulfate and heparan sulfate as a biomarker for mucopolysaccharidosis I. J.Inherit.Metab.Dis. 2010;33(2):141–150. doi: 10.1007/s10545-009-9036-3. [DOI] [PubMed] [Google Scholar]
- 83.Tomatsu S, Montano AM, Dung VC, Ohashi A, Oikawa H, Oguma T, Orii T, Barrera L, Sly WS. Enhancement of drug delivery: enzyme-replacement therapy for murine Morquio A syndrome. Mol.Ther. 2010;18(6):1094–1102. doi: 10.1038/mt.2010.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Tomatsu S, Okamura K, Maeda H, Taketani T, Castrillon SV, Gutierrez MA, Nishioka T, Fachel AA, Orii KO, Grubb JH, et al. Keratan sulphate levels in mucopolysaccharidoses and mucolipidoses. J.Inherit.Metab.Dis. 2005;28(2):187–202. doi: 10.1007/s10545-005-5673-3. [DOI] [PubMed] [Google Scholar]
- 85.Tomatsu S, Montano AM, Oguma T, Dung VC, Oikawa H, Gutierrez ML, Yamaguchi S, Suzuki Y, Fukushi M, Barrera LA, et al. Validation of disaccharide compositions derived from dermatan sulfate and heparan sulfate in mucopolysaccharidoses and mucolipidoses II and III by tandem mass spectrometry. Mol.Genet.Metab. 2010;99(2):124–131. doi: 10.1016/j.ymgme.2009.10.001. [DOI] [PubMed] [Google Scholar]
- 86.Camelier MV, Burin MG, De MJ, Vieira TA, Marasca G, Giugliani R. Practical and reliable enzyme test for the detection of mucopolysaccharidosis IVA (Morquio Syndrome type A) in dried blood samples. Clin.Chim.Acta. 2011;412(19–20):1805–1808. doi: 10.1016/j.cca.2011.06.001. [DOI] [PubMed] [Google Scholar]
- 87.Glossl J, Kresse H. A sensitive procedure for the diagnosis of N-acetyl-galactosamine-6-sulfate sulfatase deficiency in classical Morquio's disease. Clin.Chim.Acta. 1978;88(1):111–119. doi: 10.1016/0009-8981(78)90157-2. [DOI] [PubMed] [Google Scholar]
- 88.Zhao H, Van Diggelen OP, Thoomes R, Huijmans J, Young E, Mazurczak T, Kleijer WJ. Prenatal diagnosis of morquio disease type A using a simple fluorometric enzyme assay. Prenat.Diagn. 1990;10(2):85–91. doi: 10.1002/pd.1970100204. [DOI] [PubMed] [Google Scholar]
- 89.Duffey TA, Khaliq T, Scott CR, Turecek F, Gelb MH. Design and synthesis of substrates for newborn screening of Maroteaux-Lamy and Morquio A syndromes. Bioorg.Med.Chem.Lett. 2010;20(20):5994–5996. doi: 10.1016/j.bmcl.2010.08.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Khaliq T, Sadilek M, Scott CR, Turecek F, Gelb MH. Tandem mass spectrometry for the direct assay of lysosomal enzymes in dried blood spots: Application to screening newborns for mucopolysaccharidosis IVA. Clin.Chem. 2011;57(1):128–131. doi: 10.1373/clinchem.2010.149880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Glossl J, Kresse H. Impaired degradation of keratan sulphate by Morquio A fibroblasts. Biochem.J. 1982;203(1):335–338. doi: 10.1042/bj2030335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Baker E, Guo XH, Orsborn AM, Sutherland GR, Callen DF, Hopwood JJ, Morris CP. The Morquio A syndrome (Mucopolysaccharidosis IVA) gene maps to 16q24.3. Am.J.Hum.Genet. 1993;52(1):96–98. [PMC free article] [PubMed] [Google Scholar]
- 93.Masuno M, Tomatsu S, Nakashima Y, Hori T, Fukuda S, Masue M, Sukegawa K, Orii T. Mucopolysaccharidosis IV A: Assignment of the human N-acetylgalactosamine-6-sulfate sulfatase (GALNS) gene to chromosome 16q24. Genomics. 1993;16(3):777–778. doi: 10.1006/geno.1993.1266. [DOI] [PubMed] [Google Scholar]
- 94.Masue M, Sukegawa K, Orii T, Hashimoto T. N-Acetylgalactosamine-6-sulfate sulfatase in human placenta: Purification and characteristics. J.Biochem. 1991;110(6):965–970. doi: 10.1093/oxfordjournals.jbchem.a123697. [DOI] [PubMed] [Google Scholar]
- 95.Nakashima Y, Tomatsu S, Hori T, Fukuda S, Sukegawa K, Kondo N, Suzuki Y, Shimozawa N, Orii T. Mucopolysaccharidosis IV A: Molecular cloning of the human N- acetylgalactosamine-6-sulfatase gene (GALNS) and analysis of the 5'-flanking region. Genomics. 1994;20(1):99–104. doi: 10.1006/geno.1994.1132. [DOI] [PubMed] [Google Scholar]
- 96.Cosma MP, Pepe S, Annunziata I, Newbold RF, Grompe M, Parenti G, Ballabio A. The multiple sulfatase deficiency gene encodes an essential and limiting factor for the activity of sulfatases. Cell. 2003;113(4):445–456. doi: 10.1016/s0092-8674(03)00348-9. [DOI] [PubMed] [Google Scholar]
- 97.Sukegawa K, Nakamura H, Kato Z, et al. Biochemical and structural analysis of missense mutations in N-acetylgalactosamine-6-sulfate sulfatase causing mucopolysaccharidosis IVA phenotypes. Hum.Mol.Genet. 2000;9:1283–1290. doi: 10.1093/hmg/9.9.1283. [DOI] [PubMed] [Google Scholar]
- 98.Bunge S, Kleijer WJ, Tylki-Szymanska A, Steglich C, Beck M, Tomatsu S, Fukuda S, Poorthuis BJHM, Czartoryska B, Orii T, et al. Identification of 31 novel mutations in the N-acetylgalactosamine-6-sulfatase gene reveals excessive allelic heterogeneity among patients with morquio A syndrome. Hum.Mutat. 1997;10(3):223–232. doi: 10.1002/(SICI)1098-1004(1997)10:3<223::AID-HUMU8>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 99.Fukuda S, Tomatsu S, Masuno M, Ogawa T, Yamagishi A, Maruf Rezvi G, Sukegawa K, Shimozawa N, Suzuki Y, Kondo N, et al. Mucopolysaccharidosis IVA: Submicroscopic deletion of 16q24.3 and a novel R386C mutation of N-acetylgalactosamine-6-sulfate sulfatase gene in a classical morquio disease. Hum.Mutat. 1996;7(2):123–134. doi: 10.1002/(SICI)1098-1004(1996)7:2<123::AID-HUMU6>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 100.Tomatsu S, Fukuda S, Cooper A, Wraith JE, Uchiyama A, Hori T, Nakashima Y, Yamada N, Sukegawa K, Kondo N, et al. Mucopolysaccharidosis IVA: Structural gene alterations identified by Southern blot: Analysis and identification of racial differences. Hum.Genet. 1995;95(4):376–381. doi: 10.1007/BF00208958. [DOI] [PubMed] [Google Scholar]
- 101.Yamada N, Fukuda S, Tomatsu S, Muller V, Hopwood JJ, Nelson J, Kato Z, Yamagishi A, Sukegawa K, Kondo N, et al. Molecular heterogeneity in mucopolysaccharidosis IVA in Australia and Northern Ireland: Nine novel mutations including T312S, a common allele that confers a mild phenotype. Hum.Mutat. 1998;11(3):202–208. doi: 10.1002/(SICI)1098-1004(1998)11:3<202::AID-HUMU4>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 102.Hendriksz CJ, Al-Jawad M, Berger KI, Hawley SM, Lawrence R, Mc AC, Summers CG, Wright E, Braunlin E. Clinical overview and treatment options for non-skeletal manifestations of mucopolysaccharidosis type IVA. J.Inherit.Metab Dis. 2012 Feb 23; doi: 10.1007/s10545-012-9459-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Davison JE, Kearney S, Horton J, Foster K, Peet AC, Hendriksz CJ. Intellectual and neurological functioning in Morquio syndrome (MPS IVa) J.Inherit.Metab Dis. 2012 Jan 10; doi: 10.1007/s10545-011-9430-5. [DOI] [PubMed] [Google Scholar]
- 104.Lloyd-Evans E, Platt FM. Lysosomal Ca(2+) homeostasis: role in pathogenesis of lysosomal storage diseases. Cell Calcium. 2011;50(2):200–205. doi: 10.1016/j.ceca.2011.03.010. [DOI] [PubMed] [Google Scholar]
- 105.Vitner EB, Platt FM, Futerman AH. Common and uncommon pathogenic cascades in lysosomal storage diseases. J.Biol.Chem. 2010;285(27):20423–20427. doi: 10.1074/jbc.R110.134452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Kiselyov K, Muallem S. Mitochondrial Ca2+ homeostasis in lysosomal storage diseases. Cell Calcium. 2008;44(1):103–111. doi: 10.1016/j.ceca.2007.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Kiselyov K, Jennigs JJ, Jr., Rbaibi Y, Chu CT. Autophagy, mitochondria and cell death in lysosomal storage diseases. Autophagy. 2007;3(3):259–262. doi: 10.4161/auto.3906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Lieberman AP, Puertollano R, Raben N, Slaugenhaupt S, Walkley SU, Ballabio A. Autophagy in lysosomal storage disorders. Autophagy. 2012;8(5):719–730. doi: 10.4161/auto.19469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Tessitore A, Pirozzi M, Auricchio A. Abnormal autophagy, ubiquitination, inflammation and apoptosis are dependent upon lysosomal storage and are useful biomarkers of mucopolysaccharidosis VI. Pathogenetics. 2009;2(1):4–12. doi: 10.1186/1755-8417-2-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Tomatsu S, Mackenzie WG, Theroux MC, Mason RW, Thacker MM, Shaffer TH, Montaño AM, Rowan D, Sly W, Alméciga-Díaz CJ, Barrera LA, Chinen Y, Yasuda E, Ruhnke K, Suzuki Y, Orii T. Current and emerging treatments and surgical interventions for Morquio A syndrome: a review. Res.Rep.Endo.Dis. 2012;2:65–77. doi: 10.2147/RRED.S37278. [DOI] [PMC free article] [PubMed] [Google Scholar]