Abstract
Enalapril maleate (EM) is a widely used anti-hypertensive drug which is unstable when mixed with excipients. Enalaprilate and diketopiperazine (DPK) are the main degradation products of enalapril. The in situ preparation of enalapril sodium salt (NaE) has been used to improve drug stability in dosage forms; however, gas release and product rejection ensue when the chemical reaction for obtaining the sodium salt is not completely finished before packaging. This study evaluated the effect of stearic acid (SA) on enalapril stability in microcrystalline cellulose (MCC) pellets containing EM or NaE. MCC pellets containing SA were prepared by the extrusion–spheronization technique and characterized. Enalapril stability and dissolution were then evaluated. DPK and enalaprilate formation were reduced by the addition of SA in pellets containing EM. The overall enalapril degradation in these formulations was lower when compared with pellets containing EM or even NaE prepared without SA. The immediate-release characteristic was maintained by the addition of 5% crospovidone to all the formulations tested. The incorporation of SA into NaE pellets resulted in unexpected enalapril degradation, caused by the interaction of these compounds, as suggested by a thermal analysis of the SA–NaE binary mixture. The findings presented here showed that formulations containing SA could substitute the formation of NaE, since they provide better enalapril stability in solid dosage forms. In addition, it is suggested that the stabilization effects would be observed for other N-carboxyalkyl dipeptide analogs with angiotensin converting enzyme inhibition activity, since these new entities share the same degradation pathway of enalapril.
Key words: diketopiperazine, enalapril, enalaprilate, extrusion–spheronization, stearic acid
INTRODUCTION
Enalapril maleate (EM) is a widely used anti-hypertensive drug which is unstable when mixed with excipients commonly used in the manufacture of solid dosage forms (1). Compatibility and stability studies of pure EM and EM mixtures with excipients have been reported in the literature (1–4). Enalaprilate is one of the most significant EM degradation products, which is formed by ester hydrolysis (1). Diketopiperazine (DKP) is the main thermal degradation product of EM which is formed by dehydration followed by intramolecular cyclization (1). These degradation reactions are not restricted to EM but may affect all the N-carboxyalkyl dipeptide analogs which promote the inhibition of angiotensin-converting enzyme (ACE) activity (1).
Certain reports have shown that the EM degradation rate and pathway have been influenced by environmental factors, such as temperature, humidity, and light (1–3). Excipient types and packaging material are also factors which affect the stability of EM (1,3). For instance, DKP was the main degradation product when EM was incorporated into acidic tablet matrices, while enalaprilate formation was predominant in basic matrices (1).
To improve the thermal stability of enalapril in solid dosage forms, a process for preparation of an enalapril sodium salt has been reported (5). This process consists of the reaction of EM with a sodium compound, such as sodium hydrogen carbonate (5). This reaction may occur even in solid state at room temperature (3) and promotes the formation of a more stable enalapril sodium salt (NaE), water, and CO2. This reaction is usually performed in situ during the manufacture of solid dosage forms and should be carefully monitored to guarantee that the reaction is completed before the packaging phase starts; otherwise, continuous CO2 release may cause packaging swelling and product rejection (3).
The in situ preparation of enalapril salt forms has been used in the pharmaceutical industry to improve the stability of final products containing enalapril. Nevertheless, literature reports have shown that certain enalapril solid dosage forms showed low stability and have not met the official acceptance criteria (2). In a previous study by our research group (2), in which enalapril tablets, produced by different manufactures, were submitted to an accelerated stability test it was reported that only four out of nine products were approved (drug content within ±10% of labeled amount) (2).
These results showed that a more effective and economically viable strategy to improve enalapril stability is still needed. To date, there are reports in the literature on salt formation and complexation of enalapril and β-cyclodextrin (5,6). The cyclodextrin complexation prevented enalapril interaction in a solid state from increasing drug stability (6).
EM hydrolytic conversion to enalaprilate may also be prevented by isolating the drug crystals from environmental humidity. Hydrophobic excipients in solid dosage forms may coat the drug crystals and thus improve drug stability, as reported by Kowalski and co-workers (7). These authors suggested that the hydrogenated castor oil coated the crystals of a dipeptidylpeptidase inhibitor, which protected the drug from surrounding moisture (7). In accordance with this, we believe that the stability of enalapril may be improved by isolating drug crystals from moisture and preventing drug–excipient interaction.
Stearic acid (SA) is a hydrophobic compound widely used in the manufacture of solid dosage forms as a tablet and capsule lubrificant, binder, or sustained release excipient (8). In this study, stearic acid was incorporated into microcrystalline cellulose (MCC) pellets produced by the extrusion–spheronization technique in order to improve enalapril stability without prolonging drug release. Pellets are multiparticulate solid dosage forms that offer several pharmacological and technological advantages over single-unit solid dosage forms (9). For this particular purpose, MCC was used in an attempt to accelerate EM degradation (4) and allow faster visualization of the protective effect of stearic acid. Besides, MCC is the gold standard for producing pellets via extrusion–spheronization (9). The effect of different concentrations of SA on enalaprilate and DKP formation was evaluated, and the SA incorporation was proposed as an alternative to the NaE formation technique. This approach was also suggested as a strategy for improving the stability of other ACE inhibitors.
MATERIALS AND METHODS
Materials
Stearic acid (mp 69–70°C, Chemax, Brazil), microcrystalline cellulose (Avicel PH-101, Blanver Farmoquímica, Brazil), enalapril maleate (Zhejiang Huahai Pharmaceutical, China), crospovidone (CROS) (Basf, Brazil), and sodium hydrogen carbonate were used as received. Acetonitrile and methanol (JT Baker, USA) were high-performance liquid chromatography (HPLC)-grade.
Methods
Preparation of Pellets Containing EM by Extrusion–Spheronization
Powdered mixtures of MCC, CROS, and EM were prepared in a mortar under heating (70–80°C) (Table I). Stearic acid (10% or 20%, w/w) was melted and then poured into the heated mixture and homogenized with a pestle. Next, the mixture was cooled to room temperature, and an appropriate amount of the polysorbate 80 aqueous solution (1%v/v) was added until a wetted mass was formed (Table I). Conventional pellets were prepared without SA incorporation (Table I).
Table I.
Initial Composition of Enalapril Pellets Obtained by Extrusion–Spheronization
| Formulations | EM (%,w/w) | NaHCO3 (%, w/w) | SA (%, w/w) | MCC (%, w/w) | CROS (%, w/w) | Polysorbatea (mL) |
|---|---|---|---|---|---|---|
| EMC | 5 | – | – | 90 | 5 | 106 |
| EMSA1 | 5 | – | 10 | 80 | 5 | 103 |
| EMSA2 | 5 | – | 20 | 70 | 5 | 74 |
| NaEC | 5 | 2.5 | – | 87.5 | 5 | 106 |
| NaESA | 5 | 2.5 | 10 | 77.5 | 5 | 89 |
EM enalapril maleate, NaHCO 3 sodium hydrogen carbonate, SA stearic acid, MCC microcrystalline cellulose, CROS crospovidone, EMC conventional pellets containing enalapril maleate, EMSA pellets containing EM and SA, NaEC conventional pellets containing NaE, NaESA pellets containing NaE and SA
a1% (v/v) polysorbate aqueous solution
Extrusion was performed with a Caleva Extruder 20 (Caleva, UK). The wet mass was fed into the extruder set at 25 rpm. The mass was forced through a plate containing 1 mm diameter holes. Then, what was extruded was spheronized in a CALEVA MBS spheronizer (Caleva, UK) set at 1,000 rpm for 70 s. The pellets were dried in an oven with circulating air, set at 50°C for 12 h. A schematic representation of the preparation process is shown in Fig. 1.
Fig. 1.
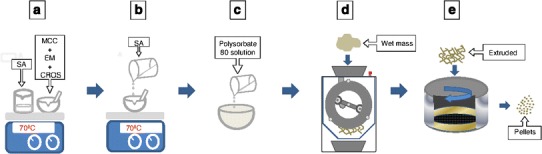
Schematic representation of preparation procedure of the enalapril pellets. a Preparation of the powder mixture and melting of stearic acid; b incorporation of stearic acid in the powder mixture; c wetting; d extrusion; e spheronization
Preparation of Pellets Containing NaE by Extrusion–Spheronization
To prepare pellets containing enalapril sodium salt (NaE), sodium hydrogen carbonate (NaHCO3) was added to the powdered mixture of MCC and EM (1:2 NaHCO3/EM weight ratio). This weight ratio is approximately equivalent to a 3:1 NaHCO3/EM molar ratio, which is needed to ensure consumption of the total amount of EM in the formulation (3). Then, distilled water was added to accelerate the salt formation reaction. Next, the wet mixture was dried by heating it in a thermostatized plate set at 70°C. This mixture was used for the preparation of pellets according to the procedure described above.
Water Content
The residual water content of the pellets was evaluated by an infrared analyzer (IV-2000, Gehaka, Brazil). Measurements were conducted at 110°C for 20 min, and the results were the mean of two measurements.
Size Analysis
The size of the pellets was evaluated on a Vibro-type sieve shaker (EML 200 Digital Plus, Haver and Boecker, USA). Mass retained (percent) in the sieves was plotted against size interval (0.063 to 1.4 mm) to build the distribution plots. The weight mean was calculated from each sample studied, according to the Eq. 1. Pellets ≥0.5 ≤ 1.4 mm in size were selected for dissolution and stability assays.
| 1 |
Where, As is the aperture size of the sieve; Mr is the percentage of mass retained on each sieve.
The aspect ratio (length/breadth) was determined by optical microscopy using a Leica DMLB microscope equipped with a Leica MPS 30 camera (Germany). Analysis was performed using Image J software (NIH, Maryland, USA).
Scanning Electron Microscopy
The pellets were coated with gold and examined in a scanning electron microscopy Jeol JSM-6610 (Thermo Scientific NSS Spectral Imaging, USA) at ×1,000 magnification.
Enalapril Stability Under Accelerated Test Conditions
Hard gelatin capsules were filled with ∼200 mg of pellets (corresponding to 10 mg of enalapril per capsule). The capsules were then packed into PVC flasks, which were sealed and placed in a 420 CLD climatic chamber (Nova Ética, Brazil) and exposed to 40°C and 75% RH for 90 days.
At each time interval, the capsules were removed and placed in 100 mL volumetric flasks containing 80 mL of sodium hydrogen phosphate buffer (pH 2.2). These mixtures were submitted to ultrasound energy for 15 min in an ultrasound cleaning bath (Ultra Cleaner 1400, Unique, Brazil). Then, the mixtures were agitated for an additional 30 min in a MA 410 shaker (Marconi, Brazil). The volume of the extraction solution was then completed to 100 mL and submitted once more to ultrasound energy for another 15 min. After that, the solution was filtered twice, and an aliquot was injected into a HPLC (ProStar 410–VARIAN) equipped with an isocratic pump, UV–vis detector (set at 215 nm) and with a ChromSpher 5 C18 (250 × 4.6 mm) 5 μm column, kept at 60°C. The mobile phase used was a mixture of sodium phosphate buffer (pH 2.2) and acetonitrile (75:25, v/v). The flow rate was 1.5 mL/min, and the injection volume was 50 μL (2). Pharmacopeial standards of enalapril maleate and enalaprilat were used for quantification. An analytical curve of diketopiperazine was prepared in accordance with United States Pharmacopeia procedures (10). Validation of the method was performed according to international guidelines (11).
Data from stability assays are given as an arithmetic mean value ± standard deviation (X ± SD). Data were analyzed using ANOVA with p < 0.05 as the minimal level of significance.
Dissolution
In vitro dissolution of enalapril was determined by filling transparent hard gelatin capsules with 200 mg of pellets (n = 6). Sinkers were wrapped around the capsules and added to cubes containing 900 mL of potassium phosphate buffer (pH 6.8) in a VK 7000 (VARIAN) dissolution apparatus equipped with USP apparatus II set at 50 rpm. The dissolution medium was kept at 37.5°C. An aliquot was withdrawn 30 min after the beginning of the test, filtered, and analyzed by HPLC.
Differential Thermal Analysis
A Shimadzu TG/DTA-60 thermobalance was used for the thermal analysis of EM, NaE, and SA. The binary physical mixtures (1:1, w/w) were prepared by grinding them together with a pestle in an agate mortar. The samples were put into standard platinum crucibles and submitted to a heating program (10°C/min) from room temperature to 400°C and under nitrogen dynamic atmosphere (50 mL min−1).
RESULTS
Extrusion–Spheronization
Preliminary studies on the preparation of enalapril pellets by extrusion–spheronization showed that the addition of SA increased the formation of agglomerates with large particle size during spheronization. Clustering was avoided when pure water was replaced by a 1% (v/v) polysorbate 80 aqueous solution, as granulating liquid (data not shown). The resulting pellets were then characterized (Table II). The yield fraction was always higher than 80%; the weight mean values, calculated from the sieve analyses, were in the range of 0.58 to 0.83 mm, and the aspect ratio was about 0.77 for all studied pellets (Table II). The moisture content of the dried pellets was in the range of 3.3% to 4.8% (Table II).
Table II.
Yield Fraction, Mean Pellet Size, Moisture Content, and Mean Aspect Ratio of Enalapril Pellets
| Abbreviation | Yield fraction (%, w/w) | Mean size (mm) | Moisture content (%, w/w) | Mean aspect ratio |
|---|---|---|---|---|
| EMC | 94.33 | 0.76 ± 0.05 | 4.75 | 0.77 ± 0.09 |
| EMSA1 | 89.53 | 0.69 ± 0.05 | 3.85 | 0.78 ± 0.08 |
| EMSA2 | 84.15 | 0.58 ± 0.02 | 4.05 | 0.77 ± 0.09 |
| NaEC | 85.93 | 0.83 ± 0.12 | 3.50 | 0.77 ± 0.07 |
| NaESA | 88.05 | 0.82 ± 0.10 | 4.85 | 0.76 ± 0.07 |
EMC conventional pellets containing enalapril maleate, EMSA pellets containing EM and SA, NaEC conventional pellets containing NaE, NaESA pellets containing NaE and SA
The morphology of the EM pellets is shown in Fig. 2. There was a pattern on the surface of the EMSA pellets (Fig. 2b, c) which was not detected on EMC pellets (Fig. 2a). A more compact surface could also be observed in the EMSA pellets. This feature was more evident in the EMSA2 formulation (Fig. 2c).
Fig. 2.
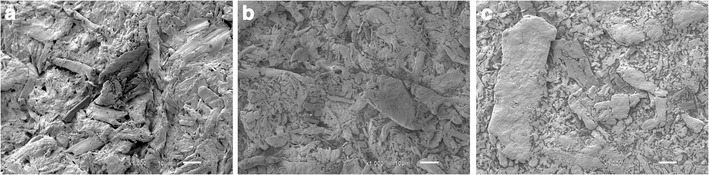
Scanning electron microscopy images of pellets containing EM. Conventional pellet at ×1,000 magnification a; pellets containing 10% SA at ×1,000 magnification b; pellets containing 20% SA at ×1,000 magnification c
Enalapril Stability
In order to determine the effect of the SA on the stability of enalapril, pellets containing different concentrations of SA and EM (EMSA1 and EMSA2, Table I) or NaE (NaESA, Table I) were submitted to accelerated stability testing and compared with conventional pellets, e.g., pellets prepared without SA (EMC and NaEC, Table I).
To perform the quantitative determination of EM, enalaprilat, and DKP in the pellets, an HPLC method (2) was validated by our research group. Linearity was determined to EM in the range of 0.14 to 0.28 mg/mL (r2 = 0.9999; y = 34,547x + 506.1), to enalaprilat in the range of 0.12 to 4.00 μg/mL (r2 = 0.9999; y = 44.74x + 7.01), and to DKP in the range 0.33 to 5.30 μg/mL (r2 = 0.9997; y = 34.36x + 0.59). The limits of quantitation and detection were 0.5 and 0.25 μg/mL, respectively, for enalapril; 0.12 and 0.06 μg/mL for enalaprilat; and 0.33 and 0.18 μg/mL for DKP. Intra- and inter-day precision and accuracy of the method showed a relative standard deviation and a relative error not greater than 5%. The HPLC method was selective, and the retention times for enalapril, enalaprilat, and DKP were 9.8, 2.8 and 30.0 min, respectively.
Figure 3 shows the degradation of EMC and NaEC pellets, as a function of time. As can be seen, NaEC pellets showed lower enalapril degradation when compared with EMC pellets (Fig. 3). The differences in the enalapril content of these formulations were statistically significant only after the first 21 days of the experiment (p < 0.05). NaEC pellets showed a higher enalaprilate formation (Fig. 4) and lower DKP formation when compared with EMC pellets (Fig. 5). The mass balance for the enalapril degradation in the pellets must consider the formation of unidentified degradation products.
Fig. 3.
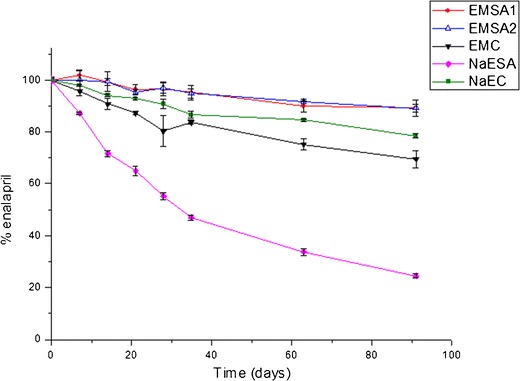
Changes in concentration of enalapril maleate (EM) and enalapril sodium salt (NaE) in conventional or pellets containing SA
Fig. 4.

Enalaprilate formation in conventional or pellets containing SA prepared with EM or NaE
Fig. 5.
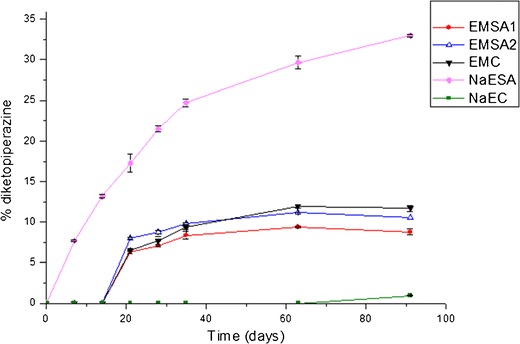
Diketopiperazine formation from conventional or pellets containing SA prepared with EM or NaE
The addition of SA resulted in contrasting effects in the enalapril pellets (Fig. 3). The incorporation of SA into pellets containing EM (EMSA1 and EMSA2, Table I) significantly increased enalapril stability. The differences in the enalapril content between EMSA and EMC pellets were statistically significant (p < 0.05) after the first 21 days of the experiment.
EMSA pellets showed a lower enalaprilate and DKP formation when compared with EMC pellets (Figs. 4 and 5). With regard to the formation of enalaprilate, EMSA1 and EMSA2 were statistically different from EMC, at all points of the curves (p < 0.05), and there were no significant differences between the EMSA1 and EMSA2 (p > 0.05) formulations. In addition, the formation of DPK was significantly lower in EMSA1 and EMSA2 than the EMC formulation, at 91 days of the experiment (p < 0.05). It is worth noting that, at this point of the curve (91 days), no statistical difference was observed between EMSA1 and EMSA2 (p > 0.05).
Of all the formulations studied here, the EMSA pellets presented the lowest decrease in enalapril concentration, while NaESA pellets showed the poorest enalapril stability (Fig. 3). A thermal study was conducted to determine the occurrence of an interaction between NaE and SA (see below). Enalaprilate and DKP formation were greatly increased in NaESA pellets (Figs. 4 and 5).
The findings presented here relating to enalapril stability in matricial pellets showed that incorporating SA into EM pellets was an efficient way of improving enalapril stability in solid dosage forms, and therefore, it could be considered an alternative to in situ salt formation during the industrial manufacture of enalapril solid dosage forms.
EM pellets containing a higher amount of SA (EMSA2, Table I) were then prepared and drug stability was evaluated. Figure 3 shows the enalapril degradation from this pellet formulation. There are no significant differences between EM content in EMSA1 and EMSA2 pellets (p < 0.05).
Thermal Analysis
To investigate the occurrence of interactions between SA and EM or NaE, binary mixtures of these constituents were submitted to DTA and TG analysis. The DTA and TG curves of the different samples are shown in Fig. 6.
Fig. 6.
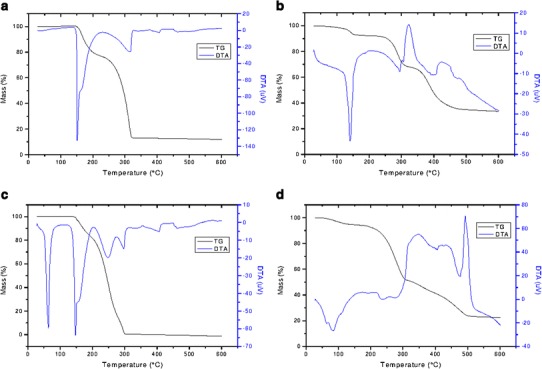
DTA and TG curves of pure EM, NaE, and EM-SA and NaE-SA binary mixtures. a EM; b NaE; c EM-SA; d NaE-SA
The DTA curve of EM (Fig. 6a) showed an endothermic event at 151.37°C, related to the melting of the drug substance (3). An event related to the formation of DKP by intramolecular cyclization overlapped the EM melting event (3). The TG curve of EM (Fig. 6a) showed a mass loss event with Tonset at 154.65°C (23.63% mass loss), which is related to water and maleic acid release during DKP formation (3).
On the other hand, the DTA curve of NaE showed an endothermic peak at 139.11°C related to a mass loss step in the TG curve (Fig. 6b). The TG curve showed that the mass loss starts at room temperature and reaches its maximum at ∼140°C (mass loss of 7.48%). This event can be ascribed to the formation of CO2 and water due to an incomplete reaction between EM and sodium hydrogen carbonate (NaHCO3) during the preparation of the pellets. This reaction is accelerated by heating (3) which could explain the occurrence of a defined event at ∼140°C, as can be observed in the DTA curve. The TG curve of NaE showed a second mass loss event starting at 268.39°C (Tpeak = 280.23°C; 23.03% of mass loss; Fig. 6b), which could be attributed to DKP formation. A comparison of the Tonset values for DKP formation in TG curves of EM and NaE showed an improvement in enalapril thermal stability brought about by salt formation.
Figure 6c shows the curve obtained from the EM/SA binary mixture (1: 1, w/w). The DTA curve showed an endothermic event probably related to the melting of the SA (Tpeak = 55.83°C) followed by a second endothermic event related to the melting peak of EM. The latter was slightly shifted to a lower temperature (Tonset = 140.60°C; Tpeak = 146.21°C) when compared with the DTA curve of pure EM. The TG curve of the EM/SA physical mixture, showed an 18.81% mass loss event starting at 148.30°C (Tpeak = 158.54°C). The Tonset of this first degradation step was slightly reduced when compared with pure EM (Fig. 6a). The shifts in the thermal events of the DTA and TG curves of the EM/SA binary mixtures were small and do not suggest the occurrence of any significant interaction between EM and SA.
On the other hand, the DTA curve of the NaE/SA physical mixture (Fig. 6d) showed the overlapping of the SA melting event with an endothermic peak probably related to the formation and release of products of the salt formation. The second mass loss step related to DKP formation started at 246.61°C (Tpeak = 269.47°C; 39.45% mass loss), which was ∼22°C lower than that presented by pure NaE. This difference indicates the occurrence of an interaction between these substances, which could explain the unexpected degradation behavior of NaESA pellets.
Dissolution
The United States Pharmacopeia established that enalapril dissolution from solid dosage forms should be ≥80% in 30 min (10). Conventional EM pellets prepared with 2% (w/w) crospovidone showed an enalapril release of 69.75% (±7.67%) in 30 min. This formulation was excluded from further characterization. Then, EMC pellets were prepared with 5% crospovidone (Table I), which resulted in an acceptable release of EM (94.54 ± 1.04% in 30 min). EMSA pellets were unable to release more than 80% of enalapril in 30 min (∼52.19%). However, an addition of 5% crospovidone in the EMSA1 formulation resulted in 89.51 ± 3.90% of enalapril release, which met the acceptance criteria of the United States Pharmacopoeia (10).
DISCUSSION
The incorporation of a lipophilic binder in granules containing a moisture sensitive drug was undertaken by Kowalski and co-workers (7). They showed an increase in drug stability that was dependent on the binder concentration in the formulation. It was also observed that the lipophilic binder did not affect the immediate-release characteristics of the drug product (7).
A similar strategy was proposed in this study with the aim of improving the stability of enalapril in solid dosage forms. MCC was selected as the main constituent of the enalapril pellets to force drug degradation, since MCC has already been recognized as an excipient which is capable of accelerating enalapril degradation (4).
Other approaches to improving enalapril stability in solid dosage forms have already been evaluated (5,6). Of these, the preparation of enalapril sodium salt (NaE) is a well known method for the thermal stabilization of this drug. However, the reaction of salt formation should be carefully controlled, since an incomplete reaction may cause packaging swelling and product rejection (3).
The incorporation of SA into pellets containing enalapril was studied here as an alternative to the salt formation procedure. It was discovered that the EMSA pellets presented superior stability when compared with EMC or even NaEC pellets. As expected, SA strongly decreased the hydrolytic conversion to enalaprilate in the pellets. It seems likely that this decrease is due to the coating of the drug crystals, which protected them from moisture, as previously reported in the literature (7). The enalaprilate formation was about seven times more pronounced in NaEC pellets when compared with the EMSA1 formulation. In addition, SA also decreased DKP formation in EMSA pellets when compared with EMC. It seems likely that the coating of EM crystals by SA prevents the MCC-EM interaction (4), thereby increasing enalapril stability. However, NaEC pellets still showed the lowest DKP formation, which is in agreement with previously reported data (3,5).
As observed for enalapril, other N-carboxyalkyl dipeptide analogs with ACE inhibition activity, such as moexipril, may undergo hydrolysis and intramolecular cyclization during peptide synthesis or long-term storage (1,12). It can therefore be assumed that SA incorporation in solid dosage forms can also improve the stability of these substances.
The discussion above shows that SA provided the best protection against enalaprilate formation while NaE was responsible for the lowest DKP formation. Thus, it seems likely that the association of SA and NaE presents the best stabilizing effect for enalapril in solid dosage forms. However, a destabilizing effect was observed in NaESA pellets. Both enalaprilate and DKP were formed in higher amounts when compared with EMC or EMSA formulations, and consequently, the overall enalapril degradation was far superior for this formulation. NaE is a non-crystalline substance which is probably in closer contact with MCC fibres, preventing SA coating and stabilization. However, the solid state properties of NaE cannot fully explain the destabilizing effect observed in NaESA pellets, so a thermal investigation was performed to elucidate the occurrence of an interaction between SA and NaE.
The resulting TG and DTA curves showed that pure NaE was more thermostable than pure EM, a fact which is in harmony with previous reports in the literature (3,5). The TG curves of NaE/SA binary mixtures showed a shift of Tonset value for the first step of enalapril degradation, which would indicate the occurrence of an interaction between these substances, which, in turn, could explain the fast intense enalapril degradation in NaESA pellets. This difference could not be observed in the binary mixtures of EM/SA.
Conventional formulations prepared with 2% crospovidone did not provide a high enough enalapril release to meet the official acceptance criteria for immediate-drug release (10). The incorporation of SA into EM pellets caused a slight decrease in enalapril release. The acceptance criterion was reached only by incorporating 5% crospovidone in conventional and pellets containing SA.
CONCLUSION
The stability of enalapril in MCC pellets was improved by incorporating SA (10% or 20%) into the formulation. SA could reduce the hydrolytic instability of the enalapril as well as decrease the formation of DKP, the main product of enalapril thermal degradation. Pellets containing SA showed higher enalapril stability when compared with conventional pellets prepared with EM or NaE. Thus, the incorporation of SA can be considered an alternative to the in situ salt formation, and it can be assumed that the incorporation of SA into pellets prepared without MCC may result in a better stabilization effect. In conclusion, incorporating SA into the formulation is a simpler and more economically viable way to improve the chemical stability of enalapril and has potential to stabilize other N-carboxyalkyl dipeptide analogs with ACE inhibition activity.
Acknowledgments
Financial support from INCT_if and CNPQ is gratefully acknowledged.
References
- 1.Al-Omari MM, Abdelah MK, Badwan AA, Jaber AMY. Effect of the drug-matrix on the stability of enalapril maleate in tablet formulations. J Pharm Bio Anal. 2001;25:893–02. doi: 10.1016/S0731-7085(01)00399-5. [DOI] [PubMed] [Google Scholar]
- 2.Lima DM, Santos LD, Lima EM. Stability and in vitro release profile of enalapril maleate from different commercially available tablets: possible therapeutic implications. J Pharm Bio Anal. 2008;47:934–7. doi: 10.1016/j.jpba.2008.02.030. [DOI] [PubMed] [Google Scholar]
- 3.Resende RLO, Santoro MIRM, Matos JR. Stability and compatibility study on enalapril maleate using thermoanalytical techniques. J Ther Anal Cal. 2008;93:881–6. doi: 10.1007/s10973-007-8187-4. [DOI] [Google Scholar]
- 4.Cotton ML, Wu DW, Vadas EB. Drug-excipient interaction study of enalapril maleate using thermal analysis and scanning electron microscopy. Int J Pharm. 1987;40:129–42. doi: 10.1016/0378-5173(87)90058-5. [DOI] [Google Scholar]
- 5.Merslavic SM, Jozica Razen NM, Ales Rotar L. Stable formulation of enalapril salt, a process for the preparation thereof and the use thereof. WO Patent. 1994.
- 6.Zoppi A, Garnero C, Linck YG, Chattah AK, Monti GA, Longhi MR. Enalapril: β-CD complex: stability enhancement in solid state. Carbohydr Polym. 2011;86:716–721. doi: 10.1016/j.carbpol.2011.05.008. [DOI] [Google Scholar]
- 7.Kowalski J, Kalb O, Joshi YM, Serajuddin ATM. Application of melt granulation technology to enhance stability of a moisture sensitive immediate-release drug product. Int J Pharm. 2009;381:56–61. doi: 10.1016/j.ijpharm.2009.05.043. [DOI] [PubMed] [Google Scholar]
- 8.Rowe RC, Sheskey PJ, Quinn ME. Handbook of pharmaceutical excipients. 6 rd ed. London: Pharmaceutical Press; 2009. [Google Scholar]
- 9.Dukic-Ott A, Thommes M, Remon JP, Kleinebudd P, Vervaet C. Production of pellets via extrusion- spheronization without the incorporation of microcrystalline cellulose: a critical review. Eur J Pharm Biopharm. 2009;71:38–46. doi: 10.1016/j.ejpb.2008.08.005. [DOI] [PubMed] [Google Scholar]
- 10.United States Pharmacopeia 31, National Formulary, United States Pharmacopeia Convention, Rockville, 2008.
- 11.International Conference of Harmonization, Q2 (R1) Validation of analytical procedures: text and methodology, 2005, ICH, Geneva, Switzerland.
- 12.Gu L, Strickley RG. A profound solvent effect on the diketopiperazine formation of the new dipeptide angiotensin-converting enzyme inhibitor, moexipril. Int J Pharm. 1990;60:99–107. doi: 10.1016/0378-5173(90)90295-F. [DOI] [Google Scholar]


