Abstract
In transdermal drug delivery systems, it is always a challenge to achieve stable and prolonged high permeation rates across the skin since the concentrations of the drug dissolved in the matrix have to be high in order to maintain zero order release kinetics. Several attempts have been reported to improve the permeability of poorly soluble drug compounds using supersaturated systems. However, due to thermodynamic challenges, there was a high tendency for the drug to nucleate immediately after formulating or even during storage. The present study focuses on the efficiency of nanoparticles and influence of different concentrations of solubilizer such as vitamin E TPGS (d-a-tocopheryl polyethylene glycol 1000 succinate) to improve the permeation rate through the skin. Effects of several formulation factors were studied on the nanosuspension systems using ibuprofen as a model drug. The overall permeation enhancement process through the skin was influenced mostly by the solubilizer and also by the size of nanoparticles. The gel formulation developed with vitamin E TPGS + HPMC nanosuspension, consequently represent a promising approach aiming to improve the permeability performance of a poorly water soluble drug candidate.
KEY WORDS: dermal drug delivery, human skin, nanosuspension, permeation rate, porcine skin, vitamin E TPGS
INTRODUCTION
Oral drug delivery has many disadvantages and this becomes even more apparent when this route is used for localized therapy in the skin. For example, the anti-inflammatory (non-steroidal anti-inflammatory drugs (NSAID)) used for the treatment of acute and chronic arthritic conditions can cause gastric mucosal damage which may result in ulceration and/or bleeding. Therefore, topical delivery of these classes of drugs overcomes many side effects such as gastric complications (1).
Despite extensive research and development efforts, only a limited number of drugs can be administered by the topical route due to various limitations. One of the reasons is the limitation in permeation of effective concentrations of drugs through the skin barrier for desired therapeutic action. The permeation challenge becomes more pronounced in case of poorly soluble drug molecules (2). Even though these molecules should possess enhanced permeation rates due to their higher lipophilicity, the rate of release of the drug becomes rate limiting for these compounds.
Several studies have been reported in the literature using topical gels for enhanced drug delivery through the skin (3–5). In order to improve the permeability of the drug through the skin, penetration enhancers were incorporated into the gel. This approach has succeeded in many cases in overcoming the skin barrier, however, is restricted by the skin irritation that may be caused by some of these compounds.
Another approach to achieve drug enhancement involves the use of supersaturated systems with co-solvents and solubilizers (6–9). Linear relationships have been shown to exist between drug content in the transdermal matrix and drug release, resulting in an increased drug flux due to higher thermodynamic activity. However, these systems are often thermodynamically unstable due to the crystallization of drug molecules immediately after formulation or even during storage.
Recently, nanotechnology has been extensively explored for transdermal drug delivery enhancement. The crucial factors that need to be considered for formulation design include drug loading, stability of drug compound, scale-up ability and most importantly, the permeability factor. The mechanism responsible for skin permeation of nano- and micro-particles depends on the particle size. Recent studies conducted using smaller sized particles provided new insights concerning the correlation between particle size and skin permeation route. It was shown that 40-nm nanoparticles penetrated the skin via the follicular route; however, limited permeation was observed for larger-sized particles due to the tight network of epidermal Langerhan’s cells (10). In another study, it was also shown that when the particle size was higher than 5 μm, almost no permeation was observed through the stratum corneum, however particles with a diameter of about 750 nm demonstrated better permeation into the hair follicle of the human skin (11).
One of the approaches studied recently, was to reduce the size of crystals by the wet media milling approach for poorly soluble drug compounds (12,13). This helped to improve the rate of release of drug substance by increasing the surface area of the crystals during the milling process. Once the particle size was decreased, probably to the nano range, the saturation solubility increased. This increase promoted the enhancement of the permeation rate through the skin due to an increased concentration gradient. One of the challenges during this approach is the abrasion of grinding media. The risk of contamination of bead to the product has been minimized by process optimization (14).
In this current study, an evaluation of a nanosuspension was performed to enhance the permeability of a poorly soluble drug through the skin. d-a-Tocopheryl polyethylene glycol 1000 succinate (known as Vitamin E TPGS or TPGS) and hydroxylpropyl methylcellulose—lower viscosity grade (HPMC K4) were used as the basic components in this formulation. In a separate study, the authors evaluated the significance of these compounds in the topical formulation (15). Vitamin E TPGS was used in to enhance the permeability of the poorly soluble drug. Also, it was used to stabilize the system by hydrophobic interactions. HPMC K4 was used as a steric stabilizer to inhibit crystal growth of the drug in the formulation.
This manuscript is divided into three main sections. The first section deals with the design and assessment of gel formulation. Gel forming polymers such as hydroxypropyl methylcellulose—higher viscosity grade (HPMC K100), sodium carboxy methyl cellulose (Na-CMC) and polaxamer (Pluronic F127) were used in order to optimize the final variant, which was tested for permeability study using porcine skin. In the second section of the research, a factorial design study was conducted to evaluate the individual effects from the three critical components (particle size of drug crystals, concentration of Vitamin E TPGS and concentration of gel forming polymer) on the permeation rate of drug through porcine skin. In the third or final part of the study, the optimal formulation was tested on human skin to confirm the permeability assessment.
Ibuprofen was used as the model drug for this study. It is a potent NSAID often used for the treatment of acute and chronic arthritic conditions. Although topical delivery is the preferred approach to overcome the challenges of gastric complications occurring with oral delivery of this drug (16,17), the drug molecule exhibits poor aqueous solubility and also has a high tendency of crystal growth in the high energized system.
MATERIALS AND METHODS
Materials
Ibuprofen, an anti-inflammatory drug from Doctors Organic Chemical Limited (Tanaku, AP, India), was used as a model drug in this study. The free base form of this drug is poorly water soluble with an equilibrium water solubility of 0.02 mg/ml and molecular weight of 206.28 g/mol. The excipients used in this study include, vitamin E TPGS from Eastman Chemical. Co. (Kingsport, TN, USA), Pluronic F-127 from BASF (Florham Park, NJ, USA), HPMC K4 and HPMC K100 from Dow Chemical Company (Midland, MI, USA), Sodium-carboxymethylcellulose/Na-CMC from Hercules (Wilmington, DE, USA). Deionized water was used as dispersion media.
Preparation of Gel Formulations from Nanosuspension
During this process, Vitamin E TPGS was dissolved in water at 70–80°C to produce a 1% w/v solution. The stabilizer (HPMC K4) was dissolved in the solution (2% w/v). The drug substance (5% w/v) was dispersed into the system and the resulting suspension was wet milled with the grinding media (0.2 mm diameter) using a conventional planetary mill, Model PM400, Retsch GmbH, Germany, equipped with beaker with a chamber volume of 50 ml. The agitation rate was maintained at 400 rpm. High shear force generated during collision of the milling media with the solid drug provided the energy to fracture drug crystals into smaller particles. Due to the collision of the drug crystals with the beads and with the wall of the grinding chamber, smaller crystals at sub-micron or nano size range were produced. Once the desired nanosuspension was formed, gel forming polymers were dispersed into the solution using high-speed homogenizer. Three different polymers were used at varying concentrations. The compositions of the different formulations used are outlined in Table I.
Table I.
Compositions of Ibuprofen Nanosuspension Formulations
| Code | Vit. E TPGS % (w/v) | HPMC K4 % (w/v) | Drug % (w/v) | HPMC K100 % (w/v) | Na-CMC % (w/v) | Pluronic F127 % (w/v) | Assay (%) | Mean PS (nm) | PDI |
|---|---|---|---|---|---|---|---|---|---|
| N1 | 1 | 2 | 5 | 1 | 96.5 | 396.8 | 0.212 | ||
| N2 | 1 | 2 | 5 | 2.5 | 98.1 | 320.7 | 0.236 | ||
| N3 | 1 | 2 | 5 | 5 | 99.1 | 344.6 | 0.245 | ||
| N4 | 1 | 2 | 5 | 1 | 95.8 | 582.3 | 0.299 | ||
| N5 | 1 | 2 | 5 | 2.5 | 98.1 | 480.6 | 0.312 | ||
| N6 | 1 | 2 | 5 | 5 | 96.5 | 466.2 | 0.221 | ||
| N7 | 1 | 2 | 5 | 10 | 98.6 | 756.3 | 0.300 | ||
| N8 | 1 | 2 | 5 | 20 | 96.3 | 652.3 | 0.302 | ||
| N9 | 1 | 2 | 5 | 25 | 98.9 | 854.6 | 0.502 | ||
| N10 | 1 | 2 | 5 | 96.5 | 284.5 | 0.211 |
Preparation of Gel Formulations as a Control for Human Skin Permeation Study
Initially Vitamin E TPGS was dissolved in water at 70–80◦ C to produce a final concentration of 5% (w/v). Excess drug was added into this system and the suspension was stirred for 48 h at 37◦C using an insulated shaker (Innova 4000, New Brunswick Scientific, Edison, NJ, USA). The suspension was then centrifuged using a centrifuge (CT422, Jouan Inc., Winchester, VA, USA) at 3000 rpm and the supernatant clear solution was collected. This aliquot was mixed with 2% w/v of HPMC K4 as steric stabilizer. After forming the micellar solution, gel forming polymer (HPMC K100; 3% w/w) was dispersed into the solution using high-speed homogenizer and the formulation was kept overnight in order to achieve complete hydration.
Evaluation of Drug Content in the Gel Formulation
The gels were evaluated for drug content. The drug content of the gels was determined by dissolving about 100 mg of gel in acetonitrile–water mixture (1:1), which was diluted with PBS solution (pH 7.4) to make a volume of 100 ml. The drug content was estimated using HPLC method that was discussed in later section.
Short-Term Stability Study
The stability study was conducted to identify the appropriate conditions for inhibiting the crystal growth in the formulations. The gel formulations were kept on short-term stability and the crystal growth/increase of particle size was monitored. The formulations were stored at 2–8°C and samples were collected at different time points from 0 to 6 weeks for particle size analysis.
Particle Size Analysis
The growth of crystals in the nanosuspension gel system was detected by Photon Correlation Spectroscopy. Photon Correlation Spectroscopy determines velocity distribution of particle movement, by measuring dynamic fluctuations of intensity of scattered light. The suspensions were characterized by intensity-weighted particle size using photon correlation spectroscopy (PCS) particle size analyzer (Beckman Coulter, Jersey City, NJ, USA). Once the required intensity reached, analysis was performed to obtain the mean particle size and polydispersity index (PI). Analysis was performed in triplicate (angle—90°, diluent—water, temp.—25°C, run time—200 s.).
Solubility Study
Solubility studies were performed by mixing excess amount of drug into water containing various concentrations of vitamin E TPGS and kept at 37°C with constant shaking until 72 h. After that, the solution was centrifuged (CT422, Jouan Inc., Winchester, VA, USA) at 3,000 rpm; the supernatant liquid was collected and the concentration of drug dissolved was analyzed using HPLC (as described below).
Permeation Study
Permeation rates were determined using porcine and human skin. Dermatomed (∼500 μm) porcine (pig) skin was obtained from the abdominal regions of young Yorkshire pigs (26.5–28 kg, UMDNJ, Newark, NJ). Human skin was obtained from NY firefighters (New York, NY) and was also dermatomed to 500 μm. The human skin was collected from right posterior leg of a 40-year-old female Hispanic donor.
The skin was stored at −80°C. Prior to each permeation experiment; the skins were allowed to thaw at room temperature. After washing and equilibration with PBS, the skin was mounted on static vertical Franz Diffusion cells—Permegear Inc., Bethlehem, PA (receptor volume 5.1 ml, donor area 0.64 cm2) by clamping them between the donor and receptor compartments. The receptor compartment was filled with PBS (pH 7.4) and maintained at 37 ± 0.5°C with constant stirring at 600 RPM. Formulations were added to the donor compartment as an infinite dose to completely cover the membrane surface. Receptor samples were collected at predetermined time points and then replaced with equivalent amount of buffer. The drug content in the samples was analyzed by HPLC.
Statistical Analysis
A statistical analysis was performed with nanosuspension gel formulations in order to evaluate the effect of individual components and the interaction between these parameters. A 23 factorial design with three critical parameters at two different levels (high and low) was executed. Three replicates were used for each formulation during permeation study. The concentration of HPMC K4 and drug substance was kept constant during this study. The overall study design is shown in Table II. The Pareto chart (from factorial design analysis) was used as a statistical tool to analyze the effect and magnitude of the above parameters. The objective for this statistical analysis was to investigate the change of permeability profile from each of the following components:
Size of drug crystals
Vitamin E TPGS concentration
Concentration of gel forming polymer
Table II.
Characterization of Ibuprofen Nanosuspension Formulations
| B.N. | X1 | X2 | X3 | Drug content (SD) | Flux μg/cm2/h (SD) | Cum. amount of drug permeated (μg) | ||
|---|---|---|---|---|---|---|---|---|
| PS | Vit. E TPGS level | HPMC K100 level | 24 h (SD) | 48 h (SD) | 72 h (SD) | |||
| F1 | +1 (300) | +1 (2.0) | +1 (1.0) | 96.4 (0.7) | 26.0 (3.6) | 298.0 (57.8) | 536.5 (63.4) | 1142.4 (107.5) |
| F2 | +1 (300) | +1 (2.0) | −1 (3.0) | 96.0 (1.2) | 29.7 (4.1) | 155.9 (11.4) | 580.2 (58.6) | 1178.9 (144.2) |
| F3 | −1 (900) | +1 (2.0) | −1 (3.0) | 98.5 (0.8) | 19.3 (2.4) | 140.2 (35.9) | 543.5 (112.5) | 759.6 (94.4) |
| F4 | +1 (300) | −1 (0.1) | −1 (3.0) | 96.3 (1.5) | 19.3 (3.3) | 132.3 (65.7) | 464.8 (18.2) | 734.4 (195.2) |
| F5 | −1 (900) | −1 (0.1) | −1 (3.0) | 100.1 (2.5) | 14.1 (2.2) | 312.3 (53.1) | 542.5 (65.1) | 799.5 (133.3) |
| F6 | −1 (900) | +1 (2.0) | +1 (1.0) | 98.3 (2.3) | 19.8 (0.9) | 148.8 (28.6) | 551.2 (123.0) | 808.0 (128.9) |
| F7 | −1 (900) | −1 (0.1) | +1 (1.0) | 96.2 (2.3) | 12.5 (2.2) | 123.9 (24.6) | 260.1 (81.5) | 387.9 (146.1) |
| F8 | +1 (300) | −1 (0.1) | +1 (1.0) | 96.4 (1.6) | 17.7 (2.4) | 132.3 (51.6) | 464.8 (110.8) | 734.4 (88.8) |
MVDA Modeling of Nanosuspension Gel Formulations
Besides the Factorial design analysis using Pareto chart approach, multi variant data analysis (MVDA) modeling was performed to study the influence of different components of nanosuspension gel formulations on the permeation rate of the drug through pig skin. SIMCA P + (version 11.5) software was used for this analysis. MVDA is an important modeling tool for very large data set. In research, this model helps to identify the influential formulation parameters and to establish the correlation pattern among these parameters. This model is established based on the equation: y = f(x) + e, where, f(x) = the part explained by the model and e = noise (the remaining unexplained part of the data).
HPLC Analysis
The assay was determined by using a gradient HPLC (Waters 2695 HPLC system) equipped with UV–Vis detector (Waters 2487, Dual I Absorbance Detector) and a C18 column detection (X Terra column, Waters, Ireland, analytical C18 column, 5 μm particle size, 4.6 × 150 mm). The mobile phase consists of a mixture of acetonitrile and phosphate buffer (pH 3.5) with a ratio of 60/40 (v/v). The detection wavelength used was 230 nm with a flow rate of 1.2 ml/min and run time of 6 min. The precision and linearity was established for concentration ranged from 5–500 μg/ml. All the test samples were within these ranges.
RESULTS AND DISCUSSIONS
Formulation Design of Gel System Produced from Nanosuspension
As shown in Table I, several formulations were evaluated using nanosuspensions. The nanosuspensions were prepared by the wet milling process using 5% drug, 1% vitamin E TPGS, and 2% HPMC K4 polymer. The advantages of using the top down media milling approach for the formation of nanosuspensions included high drug loading capacity, elimination of organic solvent and easy scale-up. In a separate study, a detailed evaluation was conducted in order to optimize the vitamin E TPGS and HPMC K4 polymer concentrations based upon the success of producing nanocrystals during the milling process. Since we intend to develop these formulations for dermal application, therefore gel forming polymers were added for better application and adherence on the skin. We used different types of gel forming polymers at three different concentrations (Table I). Mean particle size and assay values were recorded for the different formulations.
Particle Size Analysis of Nanosuspension Gel System
One of the most important characterization studies of nanosuspension was the particle sizing of the drug crystals. The three popular analytical techniques used for size measurement of nanoparticles are PCS by dynamic light scattering (DLS), laser diffraction (LD) by static light scattering technique and microscopy (18). Among these techniques microscopic study is a visible method which gave qualitative information of the particles. However, this method does not provide any statistical data analysis. On the other hand, PCS produces accurate results of particles size measurement between 50 nm to about 2 μm and sometimes even up to 5 μm. Also, this technique requires a small amount of sample (a few milliliters) with less dilution and thus minimizes the probability of dissolution of the smaller particles. However, it is not possible to detect larger particles (more than 6 μm) by using the PCS technique (19). The LD technique is a very robust technique for measuring the size of particles; however, it can only analyze larger particles (more than 400 nm). Based on the above facts, during the preliminary phase of this research, we used both, PCS and LD techniques to detect the size of drug crystals formed during the process and also during storage. However, crystals were not detected accurately by LD method due to lower intensity of particles in the sample. Thus, PCS technique was selected as the suitable technique for particle size analysis. Also during the analytical method development, in order to study the effect of any possible dissolution on drug crystals during particle size analysis, a study was performed by diluting the nanosuspension with unsaturated, partially saturated and completely saturated dispersion media. No difference in the results was observed between these experiments.
An increase of particle size during (a) gel formation process and (b) during storage was identified as the most important quality attribute during the initial screening experiments. As shown in Table I, the size of drug crystals increased during the preparation of the gel from the nanosuspension. The size of drug crystals increased significantly in presence of Pluronic F127. However, the increase in size of drug crystal was comparatively lower for HPMC K100 and Na-CMC. One of the reasons that can be stated to explain this kind of observation is that, unlike Pluronic F127, the other two polymers did not change the solubility of the drug. Based on these data, Pluronic F127 was ruled out for further screening experiments. For HPMC K100, almost no particle size increase was observed. The reason for the low particle size increase for HPMC K100 can be explained by the fact that HPMC possessed a hydrophobic interaction with the drug crystals, resulted to inhibition of crystal growth during the gel forming process. HPMC K100 probably was adsorbed onto drug crystals due to the interaction of the hydrophobic (methoxyl) and hydrophilic (hydroxypropyl) groups with the drug and provided steric stabilization as reported in the published literature (20,21).
During the stability study, significant increase of drug particle size was observed (Table III) when polymer level of HPMC K100 and Na-CMC was lower (1%). However, at 2.5 and 5% polymer concentration, rate of increase of particle size was reduced significantly. Overall stability of the gel formulation was better for HPMC K100 gel as compared to Na-CMC. Both HPMC K100 and Na-CMC based nanosuspension gel formulations at 2.5% polymer concentration were selected for the permeation study.
Table III.
Stability Study of Ibuprofen Nanosuspension Formulations
| Formulation | Weeks | Particle size (nm) | ||||
|---|---|---|---|---|---|---|
| Mean | PDI | d10 | d50 | d90 | ||
| N1 (HPMC K100 gel: 1% polymer) | 0 | 396.8 | 0.212 | 302.2 | 412.3 | 599.6 |
| 3 | 698.3 | 0.289 | 369.9 | 723.3 | 1021.3 | |
| 6 | 896.3 | 0.496 | 563.3 | 921.3 | 1369.3 | |
| N2 (HPMC K100 gel: 2.5% polymer) | 0 | 320.7 | 0.236 | 256.0 | 369.8 | 532.3 |
| 3 | 493.6 | 0.255 | 279.1 | 502.3 | 652.3 | |
| 6 | 550.3 | 0.241 | 320.1 | 553.2 | 744.2 | |
| N3 (HPMC K100 gel: 5% polymer) | 0 | 344.6 | 0.245 | 233.6 | 349.6 | 589.3 |
| 3 | 487.9 | 0.266 | 255.3 | 496.3 | 623.1 | |
| 6 | 569.7 | 0.231 | 287.3 | 589.3 | 711.9 | |
| N4 (Na-CMC gel: 1% polymer) | 0 | 582.3 | 0.299 | 361.2 | 599.6 | 856.3 |
| 3 | 978.6 | 0.356 | 689.3 | 1023.3 | 1690.3 | |
| 6 | 1789.3 | 0.496 | 1023.3 | 1799.8 | 2130.0 | |
| N5 (Na-CMC gel: 2.5% polymer) | 0 | 480.6 | 0.312 | 171.1 | 489.3 | 789.6 |
| 3 | 785.6 | 0.298 | 296.3 | 796.3 | 963.5 | |
| 6 | 986.3 | 0.357 | 369.8 | 998.6 | 1463.3 | |
| N6 (Na-CMC gel: 5% polymer) | 0 | 466.2 | 0.221 | 236.9 | 489.6 | 796.3 |
| 3 | 788.9 | 0.269 | 300.0 | 789.6 | 902.1 | |
| 6 | 897.3 | 0.329 | 355.8 | 921.3 | 1398.7 | |
| N10 (Nanosuspension with no gel forming polymer) | 0 | 284.5 | 0.211 | 184.6 | 302.3 | 489.6 |
| 3 | 356.3 | 0.189 | 203.3 | 377.3 | 596.3 | |
| 6 | 460.5 | 0.241 | 236.9 | 462.3 | 689.6 | |
In Vitro Permeation Study Using Porcine Skin
The permeation rate for the gel formulations was determined using Fick’s law
| 1 |
where, J is the flux per unit area, P is the permeability coefficient, and Cd and Cr are the concentrations of drug in the donor and receptor solutions, respectively. In the case where the sink conditions are maintained on the receptor side, (Cd − Cr) is replaced by Cd and the Eq. 1 can be simplified as:
| 2 |
The rate of permeation can be thus defined as:
| 3 |
The rate of permeation/flux (Js) was actually measured from the slope of the Q vs. t plots3. The control samples were used for respective formulations using un-micronized drug substance.
In the nanosuspension formulations, the increase of total area (A) of the particles due to size reduction, decrease in the thickness (h) of the diffusion layer surrounding the particles and fast replacement of diffused molecules due to continuous dissolution of nanocrystals are all responsible for the higher flux. There can be a further increase of saturation solubility (Cs) by decreasing the particle size to nano range (<500 nm), which can be explained due to the increase of particle curvature as interpreted by the Ostwald Freundlich equation (explained in the later section) (22,23).
Effect of Polymers on the Permeation of Drug from Nanosuspension Gel System
While evaluating the effect of polymer type on the permeation rate, the highest permeation was observed for HPMC K100 followed by Na-CMC (Fig. 1). The flux observed for HPMC K100 was 15.2 μg/cm2/h (SD-0.8; n = 3) compared to 12.0 μg/cm2/h (SD-0.8; n = 3) for Na-CMC polymer. The differences at early time points were observed due to difference in the swelling properties of the gel forming polymers which impacted the lag time (mainly for HPMC K100).
Fig. 1.
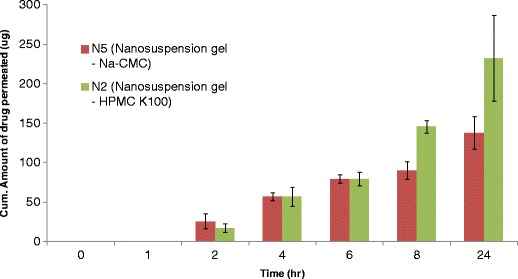
Cumulative amount of ibuprofen permeated through pig skin from nanosuspension formulations in presence of different polymers (n = 3; ±SD)
Statistical Design of Formulation Parameters for Nanosuspension Gel System
The influence of the three critical components – size of drug particles in nanosuspension range, level of Vitamin E TPGS in the suspension and the concentration of gel forming polymer (HPMC K100) were studied on the skin permeability of ibuprofen. These parameters were adjusted in a factorial design analysis in order to evaluate their significance in determining the flux. The different particle size of ibuprofen crystals were obtained by optimizing the milling time.
The permeation study was conducted using dermatomed porcine skin. The Franz cell receptor samples were collected at predetermined time points (4, 8, 12, 24, 36, 48, 72 h) and analyzed using HPLC. During the DOE analysis the study was extended until 72 h in order to rule out any significant precipitation/crystal growth of drug in the nanosuspension gel system during its contact with skin surface which is rough or gritty in nature. However, during the permeation study, we did not observe significant decrease in the rate of drug release even up to 72 h. This may be explained by the shorter duration of permeation experiments to initiate significant crystal growth. From the permeation experiment, no change of the permeation rate was observed for 72 h, which confirmed that supersaturation was maintained during the study. The plot showing the drug permeated for different formulations is presented in Fig. 2.
Fig. 2.

Cumulative amount of ibuprofen permeated through pig skin during design of experiments (n = 3; ±SD)
The cumulative amount of drug permeated through the skin after 24, 48, and 72 h is shown in Table II. The initial drug content in the gel formulations used for the DOE study was incorporated in Table II. The drug content for all the formulations were 96–100%. If we correlate the drug content with the cumulative amount of drug permeated after 72 h no change of conclusion was confirmed. Also the saturation solubility of ibuprofen at pH 7.4 PBS (+37°C) was determined to be 1.6 mg/mL (1,600 μg/ml). Therefore, the sink condition was very well maintained. The flux was determined for each formulation in order to identify the significance of the variables. The following sections summarized the results of these studies and the significance of the findings.
Flux values were determined for each permeation study (Table II) and these values were used as response factors in the factorial design. The absolute values of effects were used for rank ordering between the factors and the p value (from un-paired Student’s t test) was used to determine the significance of different parameters. The results from the permeation studies demonstrated a rank order in correlation between the formulation parameters and drug permeability through the skin. From the Puerto chart (Fig. 3a), concentration of vitamin E TPGS seemed to be the most significant parameter (p value < 0.005). The magnitude of effect was positive (+) which indicated that increase in vitamin E TPGS concentration influenced the drug solubility and also the permeability of the drug through the skin. Additionally, we had performed some separate studies in order to co-relate the solubility of the drug in the formulation and the permeability rate. It was observed that the solubility of the drug in 0.5, 1, and 2.5% TPGS solution was 258.66, 262.26, and 423.3 μg/ml, respectively. If we relate the solubility trend with the data obtained from the factorial design analysis, it was observed that the flux of the drug through the skin was significantly higher for formulation F2 (flux—29.7 μg/cm2/h.) as compared to F4 (flux −19.3 μg/cm2/h.) due to higher amount of TPGS used in the formulation. A similar result was also obtained for formulations F1 and F8. Although, the polymers used in this research did not produce any significant influence on the solubility of the drug; however, they probably improved the stability of the systems, which was explained in the later section.
Fig. 3.

a Pareto chart model (from Factorial Design) to analyze the influence of the critical formulation parameters from ibuprofen nanosuspension formulations. b Summary of influence of formulation variables (from Multi Variant Data Analysis using SIMCA P+ software). c Observed vs. Predicted Plot: shows the agreement between the actual results of our runs vs. the calculated/predicted values by the PLS (partial least square) model
The particle size of drug crystals was the second most significant parameter (p value < 0.005). The magnitude of effect was negative (−). The decrease of crystal size influenced the drug dissolution rate and hence the drug fluxes through the skin. The influence of particle size on the permeability can be explained by the larger surface area and potentially higher dissolution velocity of the nanosuspension system. The phenomenon can be explained by the following Ostwald–Freundlich’s equation:
| 4 |
where S is the solubility, S0 is the solubility of a flat sheet (r = ∞), M is the molecular weight of the solid, γ is the interfacial tension, R is the gas constant, T is the absolute temperature, r is the radius and ρ is the density of the solid. As can be seen from this equation, the solubility of a certain material is inversely correlated to the particle size. Therefore, the gel system that contained larger drug particles (∼900 nm) resulted in a concentration gradient between the differently sized particles. At the vicinity of the skin surface, the smaller particles easily diffused from the high concentration to the low concentration and precipitated on the surface of the large particles. Basically, the higher solubility of smaller sized nanoparticles and lower solubility of larger-sized nanoparticles was responsible for creating a concentration gradient in contact with the skin, which resulted in Ostwald ripening. A similar observation was reported in the past (24).
In addition, the increase in particle curvature during particle size reduction increases the dissolution pressure that consequently increases the saturation solubility. However, this effect is still questionable since Grant and Brittain suggested that an increase in solubility due to increased particle curvature might only become significant for particles having a radius of less than about 100 nm (25).
Finally, the concentration of HPMC K100 polymer seemed to be the non-significant parameter (p value > 0.005). However, the analysis of HPMC K100 parameter revealed interesting insights. The magnitude of effect was positive (+), that contradicted the prediction, which assumed that the level of HPMC K100 concentration should have a negative (−) impact on the permeability of drug through the skin. The increase in concentration of HPMC K100 should have reduced permeability rate by increasing the viscosity of the gel system. However, an additional effect needed to be considered. The increase of HPMC K100 level inhibited the formation of crystal growth of the drug and thus improved the permeation rate to certain extent. At the same time, by increasing the amount of HPMC K100, the viscosity of the suspension also increased. This provided additional resistance to the Ostwald ripening process. Thus, the performance of these stabilizers can be explained by the combination of steric hindrance of polymer and diffusion resistance due to higher viscosity of the system. Finally, the use of HPMC K100 along with vitamin E TPGS could have a synergistic effect in stabilizing the highly energized crystals. Similar observation from Vitamin E TPGS was reported in the past (26,27).
The above result suggests that factorial design is a useful tool for identifying the impact of individual formulation parameters on the drug permeability profiles through the skin. It becomes apparent that presence of surfactant (vitamin E TPGS) and size of nanosuspension crystals have a significant impact on the permeability profile. The nanosuspension gel system with optimized formulation was prepared and used for the subsequent permeation study through human skin.
Additionally, by using the MVDA model, similar results were obtained as compared to the Pareto chart approach. Figure 3b has shown the importance and magnitude of four different variables (particle size of drug crystals, vitamin E TPGS concentration, concentration of gel forming polymer and viscosity of gel formulations) on the two response factors (cumulative amount permeated after 72 h and flux).
In this article, the authors studied the influence of formulation and process variables on the most important critical quality attributes such as (a) stability of the gel (with regards to nucleation and crystal growth) during process and storage and (b) permeation rate through the skin. Once the final formulation was selected, factorial design analysis using Pareto chart approach and MVDA modeling was performed to study the influence of different components of nanosuspension gel formulations on the permeation rate of the drug through pig skin. These statistical analyses find relationships between variables measured on the process (X) and corresponding values of “result variables” (Y). The MVDA modeling study produced R2 value of about 0.8 between observed vs. predicted plot. It demonstrated a strong agreement between the actual results of these runs vs. the calculated/predicted values (Fig. 3c). Finally, the optimized formulation (F2) from the statistical analysis was subjected to permeation study though the human skin.
Permeation Study of Optimized Gel Formulation through the Human Skin
The in vitro permeation study through the human skin was conducted with nanosuspension gel formulation. The flux observed for nanosuspension gel formulation was 23.1 μg/cm2/h (SD-0.7; n = 3) compared to 14.1 μg/cm2/h (SD-0.6; n = 3) for control formulation (Fig. 4).
Fig. 4.
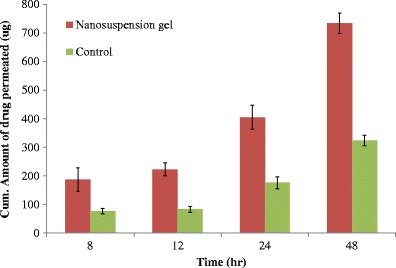
Cummulative amount of ibuprofen permeated through human skin from the optimized nanosuspension formulation (n = 3; ±SD)
The major contribution to the enhanced permeation of a poorly soluble drug like ibuprofen can be explained by Noyes–Whitney Eq. 1.
| 1 |
where dC/dt is the rate of dissolution of the drug particles, D is the diffusion coefficient of the drug in the formulation matrix, h is the thickness of the diffusion layer around each drug particle, Cs is the saturation solubility of the drug in solution in the diffusion layer, and C is the concentration of the drug in the gel. This equation was described to explain the effect of particle size reduction of drug crystals on dissolution of the drug in the gel matrix.
In summary, several hypotheses may be generated to explain the experimental findings during the research.
Increase of total area (A) of the particles due to size reduction;
Decrease in the thickness (h) of the diffusion layer surrounding the particles;
In the present study, the increase of drug solubility occurred due to the addition of surface active agent (Vitamin E TPGS in this case). In a separate study, the unique property of vitamin E TPGS has been evaluated on the permeability enhancement of the drug through the skin. TGPS may contribute the level of lipophilicity in the skin. More detailed investigations on the mechanism of skin permeation of ibuprofen at the nanosuspension state and the influence of Vitamin E TPGS will possibly shed some light on the observed effects.
The control gel formulation (as stated in the “MATERIALS AND METHODS” section) was selected from our previous study (15). This system had shown promising permeation results through the skin. The nanosuspension gel formulation had actually demonstrated superior result as compared to our control. This can be explained due to the fact that the control system used in this study is a meta stable system, where the concentration of the drug was probably above the equilibrium solubility which leads to the thermodynamic instability of the system as a function of time. Although the polymer used in this system helped to inhibit the nucleation process and also the rate of crystal growth, however might not be sufficient enough to sustain the stability during the permeation study through the skin (8,9,28). On the other hand, the superior permeation results obtained from the gel system produced from nanosuspension can be explained due to the influence from the solubilizers as well the particle size of the drug crystals. These factors resulted in higher drug release due to the formation of supersaturated solution around the crystals and thus a high concentration gradient was produced between the drug crystals and skin surface (29,30). The depletion of the dissolved drug due to any precipitation was immediately replaced by the dissolution of nanoparticles (due to the presence of higher surface area of drug particles) and thus equilibrium state was maintained in the system. Therefore, fast replacement of diffused molecules occurred due to fast and continuous dissolution from the new crystal surface (12,31) and thus drug release became continuous or zero order.
CONCLUSIONS
In conclusion, this study demonstrated a clear correlation between the vitamin E TPGS and particle size of nanocrystals with the permeation rate (flux) of ibuprofen through the skin. The explanation for the high permeation rate through the skin was mainly because of high surface area created in the formulation system that resulted in a high and continual drug release from the formulation to the external phase as a result of a constant driving force. In addition, the components used in the system also influenced the drug delivery potential from the formulation that improved the wettability of the poorly soluble drug and thus affected the mobility parameters through the skin. The formulation developed with vitamin E TPGS and HPMC 3cps provided hydrophobic interactions that resulted in the stabilization of nanocrystals. In conclusion, a number of factors including the particle size of the drug crystals, surface properties of the carrier, interaction of drug molecule with the stabilizer needed to be considered while designing a suitable dermal formulation for the poorly soluble compound. In summary, for BCS II compounds like ibuprofen, nanosuspension gel formulations seem to be an attractive approach for improving the drug permeability through the skin.
Acknowledgments
The authors would like to thank Theresa Bello, Ami Shah, Hakim Robinson (Ernest Mario School of Pharmacy) and Joshua Wang (Biomedical Engineering) for their support with the permeation experiments and HPLC studies. The author would also like to thank Victoria Kai (Novartis Pharmaceuticals) for her support with the MVDA studies.
References
- 1.Choi JS, Shin SC. Preparation and evaluation of pranoprofen gel for percutaneous administration. Drug Dev Ind Pharm. 2007;33:19–26. doi: 10.1080/03639040600975071. [DOI] [PubMed] [Google Scholar]
- 2.Davis AF, Hadgraft J. Supersaturated solutions as topical drug delivery systems. Pharmaceutical Skin Penetration Enhancement. Marcel Dekker. 1993; 243–67.
- 3.Choi CW, Choi JS, Shin SC. Development of the ambroxol gels for enhanced transdermal delivery ambroxol gels for enhanced transdermal delivery. Drug Dev Ind Pharm. 2008;34:330–5. doi: 10.1080/03639040701662644. [DOI] [PubMed] [Google Scholar]
- 4.Song JH, Shin SC. Development of the loratadine gel for enhanced transdermal delivery. Drug Dev Ind Pharm. 2009;5:897–903. doi: 10.1080/03639040802680289. [DOI] [PubMed] [Google Scholar]
- 5.Baboota S, Shakeel F, Kohli K. Formulation and evaluation of once-a-day transdermal gels of diclofenac diethylamine. Methods Find Exp Clin Pharmacol. 2006;28:109–14. doi: 10.1358/mf.2006.28.2.977842. [DOI] [PubMed] [Google Scholar]
- 6.Iervolino M, Raghavan SL, Hadgraft J. Membrane penetration enhancement of ibuprofen using supersaturation. Int J Pharm. 2000;198:229–38. doi: 10.1016/S0378-5173(00)00346-X. [DOI] [PubMed] [Google Scholar]
- 7.Hadgraft J. Passive enhancement strategies in topical and transdermal drug delivery. Int J Pharm. 1999;184:1–6. doi: 10.1016/S0378-5173(99)00095-2. [DOI] [PubMed] [Google Scholar]
- 8.Pellett MA, Davis AF, Hadgraft J. Effect of supersaturation on membrane transport: 2. Piroxicam. Int J Pharm. 1994;111:1–6. doi: 10.1016/0378-5173(94)90395-6. [DOI] [Google Scholar]
- 9.Davis AF, Hadgraft J. Effect of supersaturation on membrane transport: 1. Hydrocortisone acetate. Int J Pharm. 1991;76:1–8. doi: 10.1016/0378-5173(91)90337-N. [DOI] [PubMed] [Google Scholar]
- 10.Vogt A, Combadiere B, Hadam S, Stieler KM, Lademann J, Schaefer H, Autran B, Sterry W, Blume-Peytavi U. 40 nm, but not 750 or 1,500 nm, nanoparticles enter epidermal CD1aþ cells after transcutaneous application on human skin. J Investig Dermatol. 2006;26:1316–22. doi: 10.1038/sj.jid.5700226. [DOI] [PubMed] [Google Scholar]
- 11.Lademann J, Richter H, Teichmann A, Otberg N, Blume-Peytavi U, Luengo J, Weiß B, Schaefer U, Lehr CM, Wepf R, Sterry W. Nanoparticles—an efficient carrier for drug delivery into the hair follicles. Eur J Pharm Biopharm. 2007;66:159–64. doi: 10.1016/j.ejpb.2006.10.019. [DOI] [PubMed] [Google Scholar]
- 12.Mishra PR, Shaal LA, Müller RH, Keck CM. Production and characterization of esperetin nanosuspensions dermal delivery. Int J Pharm. 2009;371:182–9. doi: 10.1016/j.ijpharm.2008.12.030. [DOI] [PubMed] [Google Scholar]
- 13.Kobierski S, Ofori-Kwakye K, Müller RH, Keck CM. Resveratrol nanosuspensions for dermal application–production, characterization and physical stability. Pharmazie. 2009;64:741–7. [PubMed] [Google Scholar]
- 14.Juhnke M, Märtin D, John E. Generation of wear during the production of drug nanosuspensions by wet media milling. Eur J Pharm Biopharm. 2012;81:214–22. doi: 10.1016/j.ejpb.2012.01.005. [DOI] [PubMed] [Google Scholar]
- 15.Ghosh I, Michniak-Kohn B. A comparative study of Vitamin E TPGS/HPMC supersaturated system and other solubilizer/polymer combinations to enhance the permeability of a poorly soluble drug through the skin. Drug Dev Ind Pharm. 2012; 1–9, Early Online. [DOI] [PubMed]
- 16.Garcia MTJ, Silva CHTP, Oliveira DCR, Braga ECA, Thomazini JA, Bentley MVLB. Transdermal delivery of ketoprofen: the influence of drug–dioleylphosphatidylcholine interactions. Pharm Res. 2006;8:1776–85. doi: 10.1007/s11095-006-9040-3. [DOI] [PubMed] [Google Scholar]
- 17.Berba J, Goranson S, Langle J, Banakar UV. In vitro release of selected non-steroidal anti-inflammatory analgesic from reservoir type transdermal formulations. Drug Dev Ind Pharm. 1991;146:255–62. [Google Scholar]
- 18.Ghosh I, Bose S, Vippagunta R, Harmon F. Nanosuspension for improving the bioavailability of a poorly soluble drug and screening of stabilizing agents to inhibit crystal growth. Int J Pharm. 2011;409:260–8. doi: 10.1016/j.ijpharm.2011.02.051. [DOI] [PubMed] [Google Scholar]
- 19.Keck CM. Particle size analysis of nanoparticles: improved analysis method. Int J Pharm. 2012;390:3–12. doi: 10.1016/j.ijpharm.2009.08.042. [DOI] [PubMed] [Google Scholar]
- 20.Raghavan SL, Trividic A, Davis AF, Hadgraft J. Crystallization of hydrocortisone acetate: influence of polymers. Int J Pharm. 1999;93:231–7. doi: 10.1016/s0378-5173(00)00610-4. [DOI] [PubMed] [Google Scholar]
- 21.George M, Ghosh I. Identifying the correlation between drug/stabilizer properties and critical quality attributes (CQAs) of nanosuspension formulation prepared by wet media milling technology. Eur J Pharm Sci. 2013;48:142–52. doi: 10.1016/j.ejps.2012.10.004. [DOI] [PubMed] [Google Scholar]
- 22.Van Eerdenburgh B, Van den Mooter G, Augustijns P. Top-down production of drug nanocrystals: nanosuspension stabilization, miniaturization and transformation into solid products. Int J Pharm. 2008;364:64–75. doi: 10.1016/j.ijpharm.2008.07.023. [DOI] [PubMed] [Google Scholar]
- 23.Kesisoglou F, Panmai S, Wu Y. Nanosizing—oral formulation development and biopharmaceutical evaluation. Adv Drug Deliv Rev. 2007;59:631–44. doi: 10.1016/j.addr.2007.05.003. [DOI] [PubMed] [Google Scholar]
- 24.Gao G, Li Z, Sun M, Li H, Guo C, Cui J, Li A, Cao F, Xi Y, Lou H, Zhai G. Preparation, characterization, pharmacokinetics, and tissue distribution of curcumin nanosuspension with TPGS as stabilizer. Drug Dev Ind Pharm. 2010;36:1225–34. doi: 10.3109/03639041003695139. [DOI] [PubMed] [Google Scholar]
- 25.Grant DJW, Brittain HG. Solubility of pharmaceutical solids. In: Brittain HG, editor Physical Characterization of Pharmaceutical Solids. Marcel Dekker. 1995; 321–86.
- 26.Li S, Pollock-Dove C, Dong LC, Chen J, Creasey AA, Dai WG. Enhanced bioavailability of a poorly water-soluble weakly basic compound using a combination approach of solubilization agents and precipitation inhibitors: a case study. Mol Pharm. 2012;9:1100–8. doi: 10.1021/mp200352q. [DOI] [PubMed] [Google Scholar]
- 27.Ghosh I, Schenck D, Bose S, Liu F, and Motto M. Identification of critical process parameters and its interplay with nanosuspension formulation prepared by top down media milling technology—A QbD perspective. Pharm Dev Technol. 2012; Early Online: 1–11. [DOI] [PubMed]
- 28.Raghavan SL, Trividic A, Davis AF, Hadgraft J. Effect of cellulose polymers on supersaturation and in vitro membrane transport of hydrocortisone acetate. Int J Pharm. 2000;193:231–7. doi: 10.1016/S0378-5173(99)00345-2. [DOI] [PubMed] [Google Scholar]
- 29.Mitria K, Shegokara R, Gohlac S, Anselmib C, Müllera RH. Lutein nanocrystals as antioxidant formulation for oral and dermal delivery. Int J Pharm. 2011;420:141–6. doi: 10.1016/j.ijpharm.2011.08.026. [DOI] [PubMed] [Google Scholar]
- 30.Müller RH, Shegokar RGS, Keck CM. Nanocrystals: production, cellular drug delivery, current and future products. Fundam Biomed Technol. 2011;5:411–32. doi: 10.1007/978-94-007-1248-5_15. [DOI] [Google Scholar]
- 31.Ghosh I, Michniak-Kohn B. Design and characterization of submicron formulation for a poorly soluble drug: the effect of vitamin E TPGS and other solubilizers on skin permeability enhancement. Int J Pharm. 2012;434:90–8. [DOI] [PubMed]


