Abstract
Gallic acid (GA) is well known for its antioxidant and hepatoprotective activity, though its effectiveness is restricted due to rapid metabolism and elimination. To overcome these problems, gallic acid–phospholipid complex was prepared and the effect of phospholipid complexation was investigated on carbon tetrachloride (CCl4)-induced oxidative damage in rat liver. The complex significantly reduced the hepatic marker enzymes in rat serum and restored the antioxidant enzyme levels with respect to CCl4-induced group (P < 0.05 and P < 0.01). Also, the complex improved the pharmacokinetics of GA by increasing the relative bioavailability and elimination half-life. The study therefore suggests that phospholipid complexation has enhanced the therapeutic efficacy of GA which may be due to its improved absorption and increased bioavailability in rat serum.
Electronic supplementary material
The online version of this article (doi:10.1208/s12249-013-9991-8) contains supplementary material, which is available to authorized users.
KEY WORDS: bioavailability, gallic acid, hepatoprotective activity, pharmacokinetic, phospholipid complex
INTRODUCTION
Gallic acid (GA) is a naturally occurring polyphenol present in tea leaves, strawberries, pineapples, bananas, amla, red and white wines, gallnuts, sumac, witch hazel, oak bark, and apple peels—some of the natural products which are rich in GA (1). There are several reports of this beneficial phytochemical for its potential antioxidant activity studied in animal models (2–7). Other biological activities of GA studied in animal models are anticancer (1), antihyperglycemic (5), hepatoprotective (6), and antiviral (7) activity.
Carbon tetrachloride (CCl4) causes hepatocellular degeneration and centrilobular necrosis (8,9) and impairs different enzymatic systems (10). It is metabolized in the liver by cytochrome P-450 to produce trichloromethyl radicals. These radicals initiate a cascade of free radical reaction causing an increase of lipid peroxidation and reduction of antioxidant enzyme activity (11). GA is a potent antioxidant which is able to scavenge these free radicals and provide hepatoprotective effect by reducing the levels of serum liver marker enzymes and lipid peroxidation in rat liver as well as improve the antioxidant marker enzymes in rat liver homogenate (2,3).
Despite these health benefits, the usage of GA is restricted due to its poor absorption, low bioavailability, and rapid elimination from the body studied both in human and animal (12,13). GA metabolizes rapidly to its major metabolite 4-O-methylgallic acid and pyrogallol which are converted to pyrogallol-1-O-β-d-glucuronide, 4-O-methylgallic acid-3-O-sulfate, 2-O-methylpyrogallol-1-O-β-d-glucuronide, 2-O-methylpyrogallol, and 4-O-methylgallic acid (14–17). It is quite evident that rapid metabolism is a factor for low bioavailability and faster elimination of GA. Also, these metabolites possess inferior antioxidant potential compared to the parent molecule (17). Therefore, to get desirable therapeutic activity, it is required to improve the bioavailability and reduce the elimination of GA. Complexation with hydrogenated soy phosphatidylcholine (HSPC), which is an important carrier of drug molecule, can improve the bioavailability and elimination-related problems. The recent studies in our laboratory with andrographolide, ellagic acid, and naringenin demonstrated that complexation with phospholipid improves the bioavailability and enhances the bioactivity of these phytochemicals (18–20) which is may be due to sustained-release delivery of the complex (21). So, the aim of this work was to develop GA-HSPC complex, to evaluate the effect of complexation on hepatoprotective and in vivo antioxidant activity of the complex in CCl4-intoxicated rats, and to study how the level of GA in rat plasma is improved along with the main pharmacokinetic parameters due to complex formation.
MATERIALS AND METHODS
Chemicals
HSPC was purchased from Lipoid, Germany. GA (CAS number 149-91-7), glutathione reductase, and alpha alumina powder were purchased from Sigma Chemical, St. Louis, MO. Ethylenediaminetetraacetic acid (EDTA), thiobarbituric acid, trichloroacetic acid, sodium dodecyl sulfate, n-hexane, dichloromethane, and other chemicals were obtained from S.D. Fine Chem., Biosar, India. Glutathione, nitro blue tetrazolium, 5,5′-dithiobis(2-nitrobenzoic acid), phenazine methosulfate, 1-chloro-2,4-dinitrobenzene (CDNB), nicotinamide adenine dinucleotide phosphate in reduced form (NADPH), and nicotinamide adenine dinucleotide in reduced form (NADH) were purchased from SRL Chemicals, Mumbai, India. Acetonitrile and orthophosphoric acid of high-performance liquid chromatography (HPLC) grade and potassium bromide (KBr) spectroscopy grade were procured from Merck (Mumbai, India).
Preparation of Gallic Acid–Phospholipid Complex
Complex of GA with phospholipids was prepared by a method based on an earlier reported method (22). In short, 1 mol of gallic acid was refluxed with 1 mol of HSPC in 20 mL of dichloromethane till all the gallic acid was dissolved. The volume of the resulting solution was reduced to 2–3 mL and 10 mL of n-hexane was added to the above solution with continuous stirring. As a result, GA-HSPC complex gets precipitated. The complex was then filtered and dried under vacuum to remove traces of solvent. Resultant GA-HSPC complex (yield of the complex 87.5% w/w) was kept in an amber-colored glass bottle flushed with nitrogen and stored at room temperature (20–25°C).
HPLC Analysis of Gallic Acid
The HPLC technique developed and validated by Santagati et al. is adopted for the analysis of gallic of GA (23). The HPLC system (Shimadzu, Japan) was used for the analysis, consisting of binary HPLC pump, a photodiode array detector, and a Rheodyne 7725i injector equipped with a 20-μL loop. Separation was achieved using Luna C18(2) 100A, 250 × 4.6 mm, filled with 5 μm particles (Phenomenex, Torrance).
A reference stock solution of gallic acid was prepared at a concentration of 1.0 mg/mL by transferring 10 mg of gallic acid to a 10-mL volumetric flask and dissolving in mobile phase. Calibration standard solutions with different concentrations were prepared by appropriate dilutions from the stock using mobile phase as diluent.
HPLC assay was performed using isocratic conditions by external standard method. The mobile phase was acetonitrile and 1% orthophosphoric acid in deionized water (10:90, v/v). All the samples were filtered through a 0.45-μm nylon membrane prior to injection and ultrasonically degassed prior to use. Twenty microliters of the sample was injected with the flow rate of 1 mL/min. The detection was performed at a wavelength of 254 nm.
Entrapment Efficiency and Drug Content
The entrapment efficiency (EE) of the complex was performed by the HPLC technique explained above. The EE and drug content of GA-HSPC were calculated according to the following equations:
| 1 |
| 2 |
Characterization of the Complex
Infrared Spectroscopy
The infrared absorption spectra of the samples were taken with a Bruker Alpha FT-infrared (IR) spectrometer (Bruker, Germany). The spectra were recorded in the region of 4,000 to 400 cm−1. KBr pellets were prepared by mixing 10 mg of sample with 1 g KBr using a glass pestle and mortar.
Scanning Electron Microscopy
The sample was sprinkled on a double-sided carbon tape and placed on a brass stub. The surface was coated with a thin layer of palladium (about 30 μm) in an auto fine coater (Jeol JFC1600, Japan). Then, it is placed in the sample chamber of a scanning electron microscope (Jeol JSM 5200, Japan) and the morphology of the complex was observed.
Differential Thermal Analysis
Thermograms of GA, HSPC, GA-HSPC complex, and physical mixture of GA and HSPC were recorded using a differential scanning calorimeter (Pyris Diamond TG/DTA, PerkinElmer, Singapore). The thermal behavior was studied by heating 2.0 ± 0.2 mg of each individual sample in a covered sample pan under nitrogen gas flow (150 mL/min). The investigations were carried out over the temperature range of 25–350°C with a heating rate of 10°C min−1. Alpha alumina powder was used as reference.
Evaluation of Gallic Acid–Phospholipid Complex
In Vivo Antioxidant Activity
Animals
Male albino rats (Wistar strain) weighing 180–220 g, age about 2–3 months, were used for this study. Animals were housed in groups of six in colony cages at an ambient temperature of 20–25°C and 45–55% relative humidity with 12 h light/dark cycles. They had free access to pellet chow (Brook Bond, Lipton India) and water ad libitum. The experiment was performed with the ethical guidelines as provided by the Committee for the Purpose of Control and Supervision of Experiments on Animals.
Dosing
The adult male Wistar rats were divided into six groups of six animals each. The animals of control group and CCl4 control group received only distilled water with Tween 20 (1% v/v) p.o. for 7 days via gastric intubation. GA50 group was treated with GA in distilled water with Tween 20 (1% v/v) at a dose level of 50 mg/kg body weight, per day p.o., for 7 days. GA-HSPC50 group was treated with GA-HSPC complex suspension in Tween 20 (1% v/v) at doses of 50 mg/kg equivalent to pure GA, per day p.o., for 7 days. GA+HSPC50 group received physical mixture of GA and HSPC in 1:1 molar ratio suspended in Tween 20 (1% v/v) at doses of 50 mg/kg equivalent to pure GA, per day p.o., for 7 days. HSPC250 group received pure HSPC in Tween 20 (1% v/v) at doses of 250 mg/kg/day p.o. (since 50 mg/kg dose of GA-HSPC is equivalent to 250 mg/kg dose of pure HSPC approximately). On the seventh day, a single dose of equal mixture of carbon tetrachloride and olive oil was given (50% v/v, 1 mL/kg i.p.) to all animals except the control group.
On the eighth day, exactly after 24 h of CCl4 injection, all the animals were sacrificed by cervical dislocation under diethyl ether-induced anesthesia. The blood was aspirated from the left ventricle and centrifuged to collect the plasma. The rat liver was dissected out, washed with ice-cold saline, and the homogenate was prepared in 0.1 M phosphate-buffered saline (pH 7.4).
Liver Marker Enzyme Estimation
Serum glutamate oxaloacetate transaminase (SGOT) and serum glutamate pyruvate transaminase (SGPT), serum alkaline phosphatase (ALP), and total bilirubin were determined.
SGOT and SGPT were determined by the method of Reitman and Frankel (24). Each substrate (0.5 mL) [α-l-alanine (200 mM) for SGOT or l-aspartate (200 mM) with 2 mM α-ketoglutarate for SGPT] was incubated for 5 min at 37°C. Serum (0.1 mL) was added and the volume was adjusted to 1 mL with sodium phosphate buffer (pH 7.4; 0.1 M). The reaction mixture was incubated for 30 and 60 min for SGPT and SGOT, respectively. 2,4-Dinitrophenyl hydrazine (0.5 mL; 1 mM) was added to the reaction mixture and left for 30 min at room temperature. Finally, the color as developed by the addition of 5 mL sodium hydroxide (NaOH) (0.4 N) and the product formed was read at 505 nm.
ALP was determined by the method of Kind and King (25). One milliliter of the substrate was incubated in 1 mL of sodium bicarbonate buffer for 3 min at 37°C. One hundred microliters of serum was added, vortexed well, and incubated again for 15 min at 37°C. After incubation, 0.8 mL of 0.5 N NaOH, 1.2 mL of sodium bicarbonate (NaHCO3) (0.5 N), 1 mL aminoantipyrine (0.6%), and 1 mL potassium ferricyanide were added and mixed well, and the absorbance was measured at 520 nm.
Total bilirubin in plasma was determined by the method of Malloy and Evelyn (26). Briefly, 5 mL of sulfanilic acid solution (4 mmol/L) mixed with 0.1 mL of sodium nitrite solution (144 mmol/L) and 0.25 mL serum was added to this mixture. The mixture was incubated for 10 min at 37°C. The absorbance was recorded at 670 nm.
In Vivo Antioxidant Marker Enzyme Estimation
The rat liver homogenate was centrifuged and the supernatant was used for the assay of oxidative stress biomarkers which is explained below.
Reduced glutathione (GSH) in rat liver homogenate was estimated using the method of Ellman (27). Briefly, equal quantity of homogenate and 10% trichloroacetic acid was mixed and centrifuged to separate the proteins. To 0.01 mL of this supernatant, 2 mL of phosphate buffer (pH 8.4), 0.5 mL of 5,5′-dithiobis(2-nitrobenzoic acid), and 0.4 mL double-distilled water were added. Mixture was vortexed and the absorbance read at 412 nm within 15 min. The concentration of reduced glutathione was expressed as micrograms per milligram of protein.
Glutathione peroxidase (GPx) in rat liver homogenate was estimated using the method of Paglia and Valentine (28). The reaction mixture consisted of 400 μL 0.25 M potassium phosphate buffer (pH 7.0), 200 μL supernatant, 100 μL reduced GSH (10 mM), 100 μL NADPH (2.5 mM), and 100 μL glutathione reductase (6 U/mL). Reaction was started by adding 100 μL hydrogen peroxide (12 mM) and absorbance was measured at 340 nm at 1 min intervals for 5 min. Data were expressed as units per milligram of protein.
Glutathione S-transferase (GST) in rat liver homogenate was estimated using the method of Habig and Pubst (29). The reaction mixture consisted of 2.75 mL of sodium phosphate buffer (0.1 M; pH 7.4), 0.1 mL reduced glutathione (l mM), and 0.1 mL supernatant in a total volume of 3.0 mL. Reaction was started by adding 0.1 mL CDNB (100 mM) and absorbance measured at 340 nm at 1 min intervals for 5 min. Data were expressed as units per milligram of protein.
Glutathione reductase (GRD) in rat liver homogenate was estimated using the method of Dubler and Anderson (30). The assay system consisted of 1.65 mL sodium phosphate buffer (0.1 M; pH 7.4), 0.1 mL EDTA (0.5 mM), 0.05 mL oxidized glutathione (1 mM), 0.1 mL NADPH (0.1 mM), and 0.05 mL supernatant in a total mixture of 2 mL. The enzyme activity was quantified by measuring the disappearance of NADPH at 340 nm at 30 s intervals for 3 min. The activity was expressed as units per milligram protein.
Superoxide dismutase (SOD) in rat liver homogenate was estimated using the method of Kakkar et al. (31). The assay mixture contained 0.1 mL of supernatant, 1.2 mL of sodium pyrophosphate buffer (pH 8.3; 0.052 M), 0.1 mL of phenazine methosulfate (186 μm), 0.3 mL of nitro blue tetrazolium (300 μM) and 0.2 mL of NADH (750 μM). Reaction was started by addition of NADH. After incubation at 30°C for 90 s, the reaction was stopped by the addition of 0.1 mL of glacial acetic acid. Reaction mixture was stirred vigorously with 4.0 mL of n-butanol. Color intensity of the chromogen in the butanol layer was measured spectrophotometrically at 560 nm.
Catalase (CAT) in rat liver homogenate was estimated using the method of Beers and Seizer (32). In brief, 0.1 mL of supernatant was added to cuvette containing 1.9 mL of 50 mM phosphate buffer (pH 7.0). Reaction was started by addition of 1.0 mL of freshly prepared 30 mM H2O2. The rate of decomposition of H2O2 was measured spectrophotometrically at 240 nm.
Thiobarbituric acid reactive substances (TBARS) in rat liver homogenate were estimated using the method of Ohkawa et al. (33). In brief, 1.5 mL of acetic acid (20%; pH 3.5), 1.5 mL of thiobarbituric acid (0.8%), and 0.2 mL of sodium dodecyl sulfate (8.1%) were added to 0.1 mL of supernatant and heated at 100°C for 60 min. Mixture was cooled and 5 mL of n-butanol–pyridine (15:1) mixture and 1 mL of distilled water were added and vortexed vigorously. After centrifugation at 1,200×g for 10 min, the organic layer was separated and absorbance was measured at 532 nm using a spectrophotometer.
Total protein in rat liver homogenate was estimated using the method of Lowry et al. (34). Tissue homogenate (0.01 mL) (2.5%) was diluted to 1.2 mL and mixed with 6 mL of solution A (1 mL copper sulfate (1%) + l mL sodium potassium tartrate (2%) + 98 mL 2% sodium carbonate in 0.l N sodium hydroxide). The mixture was incubated at room temperature for 10 min and 0.3 mL of solution B (phosphomolybdate–phosphotungstate reagent) was added, mixed immediately, and kept at room temperature for 30 min. Absorbance was taken at 750 nm.
HPLC Method for Determination of GA in Rat Plasma
Apparatus and Chromatography
Apparatus and chromatography were the same as described previously.
Preparation of Stock Solution and Calibration Curves
Stock solutions of standard gallic acid were prepared at a concentration of 1.0 mg/mL of methanol and stored at 4°C till analysis. The stock solutions at different concentrations were spiked into blank plasma to obtain final concentrations in the range of 0.5–50 μg/mL. Quality control (QC) samples were prepared at low, medium, and high concentration levels of 0.5, 5, and 50 μg/mL.
Extraction of Gallic Acid from Plasma and Preparation of Sample
The different aliquots containing 50 μL of plasma and 200 μL of acetonitrile were mixed and vortexed for 1 min and centrifuged at 800×g for 10 min. One hundred microliters of the supernatant was transferred to a second tube, evaporated, and reconstituted with 100 μL of mobile phase. After mixing, a 20-μL aliquot was injected into the HPLC system for analysis.
Validation of the Extraction and Quantification Method
Validation of the HPLC method was done based on the guideline of the recommended International Conference on Harmonization. Calibration curves were obtained after determining the peak areas of standard plasma spiked with various concentrations of gallic acid and plotting the peak areas against corresponding concentration of gallic acid. Intra-day accuracy and precision were evaluated from replicate analysis (n = 6) of QC samples at different concentrations on the same day. Inter-day accuracy and precision were also assessed from the analysis of the same QC samples on three consecutive days in replicates (n = 6). QC samples were analyzed against the calibration curve. The standard deviation and relative standard deviation were calculated from the QC samples and used to estimate the intra- and inter-day precision. Accuracy was assessed by comparison of the calculated mean concentrations with the known concentrations. The extraction recoveries of GA were tested at three QC levels by comparing the peak areas from extracted tissues samples with those found by direct injection of standard solutions at the same concentration.
The QC samples stored at −20°C for 24 h and percent loss of the analyte after three cycles were determined by comparing the concentrations with those obtained before freezing. They were also analyzed for short-term (24 h, 25°C) and long-term stability (14 days, −20°C).
Study of Pharmacokinetic Parameters
Male albino Wistar rats were divided into two main groups. First group was administered a single dose of GA in distilled water with Tween 20 (1% v/v) at a dose of 150 mg/kg p.o. via gastric intubation and the other group GA-HSPC in distilled water with Tween 20 (1% v/v) at a dose equivalent to 150 mg/kg of GA p.o. The animals of each group were subdivided in subgroups (n = 6) for each different time points (0.5, 1.0, 1.5, 2.0, 2.5, 3.0, 3.5, 4.0, 6.0, 8.0, 10.0, 12.0 h). Blood samples (0.5 mL) were collected from the retro-orbital plexus of rats into microcentrifuge tubes containing EDTA at each time points. Blood was centrifuged at 800×g for 10 min and plasma was separated and kept at −20°C prior to analysis.
The main pharmacokinetic parameters of GA-HSPC complex were obtained with the help of a computer-designed program “WINNONLIN-4.1” and the parameters were compared to that of free gallic acid. Maximum concentration (Cmax) and time to reach maximum concentration (Tmax) are the values obtained directly from concentration–time curve. Area under the concentration–time curve (AUC0 − tn and AUC0 – tα), elimination half-life (t1/2el), elimination rate constant (Kel), clearance, and volume of distribution were determined. Relative bioavailability (F) was calculated as a ratio of the plasma AUC (0–infinity) of the pure gallic acid and its complex.
Statistical Analysis
All data were expressed as data were expressed as mean ± standard error of the means (SEM) except entrapment efficiency, drug content, and pharmacokinetic parameters where data were expressed as mean ± standard deviation (SD). For antioxidant activity, the statistical analysis was carried out by one-way analysis of variance followed by Tukey’s post hoc test using GraphPad Prism software 4.01 (San Diego, CA). For serum concentration study, data were analyzed by Student’s “t” test. The differences between means were considered to be significant when the P value was <0.05.
RESULT AND DISCUSSION
EE and Drug Content
The EE of the formulation was calculated as 91.95 ± 2.1% w/w. The GA content in the GA-HSPC complex was found to be 16.35 ± 1.3% w/w.
Characterization of the Complex
Infrared Spectroscopy
The formation of the complex can be confirmed by the FTIR spectroscopy comparing the spectrum of the complex with pure GA. The FTIR spectrum of gallic acid showed broad aromatic and carboxylic O–H stretching at 3,285.89 and 3,386.32 cm−1; also, it showed the occurrence of characteristic intense band of C=O ketonic double bond stretching at 1,706.05 cm−1 and C–O stretching at 1,244.49 and 1,339.69 cm−1. The C=C aromatic double bond stretching can be observed at 1,618.74, 1,540.69, and 1,460.30 cm−1. The out of the plane aromatic bending can be observed at 1,025.14, 865.91, and 700.14 cm−1. But in the case of the FTIR spectrum of GA-phospholipid complex, disappearance or shifting of these bands was observed. The aromatic O–H stretching disappeared due to the formation of the HSPC complex.
Differential Thermal Analysis
Differential thermal analysis (DTA) is a fast and reliable method to identify drug–excipient interaction. An interaction is concluded by elimination of endothermic peak(s), appearance of new peak(s), change in peak shape and its onset, peak temperature/melting point, and relative area or enthalpy (35). Figure 1 shows DTA thermograms of pure GA (a), HSPC (b), GA-HSPC complex (c), and physical mixture of GA and HSPC (d). HSPC (Fig. 1b) showed two major peaks at 100.07°C and 177.74°C and two minor peaks at 226.86°C and 264.84°C. The first one (100.07°C) may have appeared due to the hot movement of the phospholipid polar head group. The second peak (177.74°C) may be appeared because of phase transition from gel to liquid crystalline state. The physical mixture of GA and HSPC showed similar thermogram (Fig. 1d) as HSPC having two major peaks at 108.25.07°C and 177.19°C and one minor peak at 229.76°C. The pure GA (Fig. 1a) showed a sharp endothermic peak at 249.21°C and one small peak at 87.99°C. On the other hand, GA-HSPC complex (Fig. 1c) showed two small peaks at 51.62°C and 62.66°C and a sharp peak at 192.76°C which appeared due to phase transition. So, it is evident that the original peaks of the GA and HSPC disappeared from the thermogram of the complex and the phase transition temperature of the complex has shifted to higher temperature than that of HSPC thus confirming the formation of the complex.
Fig. 1.
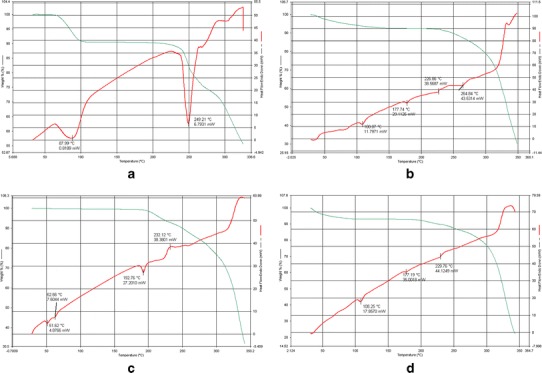
DTA thermogram of a pure GA, b HSPC, and c GA-HSPC complex, d physical mixture of GA and HSPC
Scanning Electron Microscopy
The scanning electron microscopic view (Fig. 2) indicated the presence of spheroid structures of the complex.
Fig. 2.

The scanning electron microscope image of GA-HSPC complex
Estimation of Liver Marker Enzymes
CCl4-induced hepatic damage caused a significant rise in marker enzymes like SGOT, SGPT, and ALP as well as in serum bilirubin level. Pretreatment with GA at a dose 50 mg/kg as well as GA-HSPC complex (equivalent to 50 mg/kg of pure GA) and physical mixture of GA and HSPC (equivalent to 50 mg/kg of pure GA) gave a significant reduction (*P < 0.05 and **P < 0.01) in the serum liver marker enzyme levels when compared with CCl4 group. But pure HSPC at a dose of 250 mg/kg failed to produce a significant change in the serum marker enzyme levels when compared with the CCl4 group. It was also observed that GA-HSPC complex group produced significant reduction of liver marker enzyme levels when compared to GA50 group (#P < 0.05 and ##P < 0.01), but the GA+HSPC physical mixture group could not produce any significant changes in comparison to the GA50 group. The results have been shown in Table I.
Table I.
Effect of GA and Its HSPC Complex on SGOT, SGPT, ALP, Total Bilirubin, and Total Protein Levels in CCl4-Induced Toxicity in Rats
| Parameters | Control | CCl4-control (CCl4 1 mL/kg) | GA50 (GA 50 mg/kg) | GA-HSPC50 (GA-HSPC complex ≈ 50 mg/kg GA) | GA+HSPC50 (physical mixture of GA and HSPC ≈ 50 mg/kg GA) | HSPC250 (HSPC 250 mg/kg) |
|---|---|---|---|---|---|---|
| SGOT (IU/L) | 76.43 ± 1.2**, ## | 136.40 ± 7.8## | 108.35 ± 3.5** | 88.31 ± 1.5**, # | 111.96 ± 2.8* | 132.64 ± 6.7## |
| SGPT (IU/L) | 53.22 ± 2.84**, ## | 98.21 ± 1.29## | 79.25 ± 3.76** | 63.37 ± 3.34**, # | 81.59 ± 2.98** | 95.21 ± 1.29## |
| ALP (units/L) | 124.03 ± 4.81**, ## | 210.7 ± 7.37## | 164.33 ± 3.06** | 136.23 ± 3.56**,## | 159.33 ± 4.56** | 205.7 ± 6.47## |
| Total bilirubin (mg/dL) | 0.53 ± 0.02**,## | 0.94 ± 0.03## | 0.70 ± 0.01** | 0.59 ± 0.01**,## | 0.65 ± 0.01** | 0.87 ± 0.03## |
Values are mean ± SEM of six animals
*P <0.05, **P < 0.01 significant with respect to CCl4-treated group; # P < 0.05, ## P < 0.01 significant with respect to GA50 group
In Vivo Antioxidant Activity
CCl4-intoxicated rats showed significantly (**P < 0.01) reduced level of GSH, GPx, GST, and GRD in the rat liver homogenate with respect to control group. The groups pretreated with pure GA, GA-HSPC complex, and GA+HSPC physical mixture showed significant (*P < 0.05, **P < 0.01) improvement in the levels of these enzymes, but pure HSPC at a dose of 250 mg/kg failed to produce significant improvement with respect to CCl4-intoxicated group. Also, GA-HSPC complex group showed significant enhancement of GSH and GPx levels (##P < 0.05) with respect to pure GA group, whereas GA+HSPC physical mixture group failed to produce significant changes (Table II).
Table II.
Effect of GA and Its HSPC Complex on the Enzymes of Liver Glutathione System of CCl4-Induced Rats
| Parameters | Control | CCl4− control (CCl4 1 mL/kg) | GA50 (GA 50 mg/kg) | GA-HSPC50 (GA-HSPC complex ≈ 50 mg/kg GA) | GA+HSPC50 (physical mixture of GA and HSPC ≈ 50 mg/kg GA) | HSPC250 (HSPC 250 mg/kg) |
|---|---|---|---|---|---|---|
| GSH (μg/mg) of protein | 5.17 ± 0.43**,## | 1.910 ± 0.27## | 3.54 ± 0.32** | 4.810 ± 0.21**,# | 3.590 ± 0.29** | 2.12 ± 0.18# |
| GPx (units/mg) of protein | 13.35 ± 1.04**,## | 3.72 ± 0.42## | 8.37 ± 0.76** | 11.76 ± 0.74**,# | 9.10 ± 0.93** | 4.52 ± 0.37# |
| GST (units/mg) of protein | 97.24 ± 5.31**,## | 44.59 ± 2.37## | 73.71 ± 3.06** | 88.13 ± 5.46** | 78.20 ± 3.2** | 47.91 ± 2.71## |
| GRD (units/mg) of protein | 19.21 ± 1.11** | 7.82 ± 0.73# | 13.61 ± 0.95* | 17.78 ± 1.01** | 14.82 ± 1.88** | 8.72 ± 1.12# |
Values are mean ± SEM of six animals
*P < 0.05, **P < 0.01 significant with respect to CCl4-treated group; # P < 0.05, ## P < 0.01 significant with respect to GA50 group
CCl4 intoxication increases TBARS level in rat liver. Pretreatment with pure GA (50 mg/kg), GA-HSPC complex (equivalent to 50 mg/kg of pure GA), as well as GA+HSPC physical mixture (equivalent to 50 mg/kg of pure GA) showed significant (*P < 0.05 and **P < 0.01) decrease in TBARS levels in liver homogenate, whereas the HSPC group could not produce any significant change in TBARS levels when compared to CCl4-induced rats (Fig. 3).
Fig. 3.
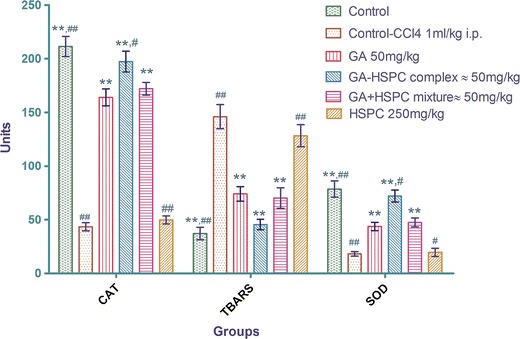
Effect of pure GA and GA-HSPC complex on catalase (units per milligram protein), TBARS (10−1 nM/mg protein), and SOD (10−1 units/mg protein). Values are mean ± SEM of six animals. * P < 0.05, ** P < 0.01 significant with respect to CCl4-treated group. # P < 0.05, ## P < 0.01 significant with respect to GA50 group
A significant reduction of SOD and CAT level occurred in CCl4-induced animals as compared to normal (**P < 0.01). Pretreatment with GA (50 mg/kg) as well as GA-HSPC complex (equivalent to 50 mg/kg of pure GA) as well as GA+HSPC physical mixture (equivalent to 50 mg/kg of pure GA) showed significant (**P < 0.01) increase in SOD and CAT levels, but group pretreated with HSPC (250 mg/kg) failed to produce any significant improvement with respect to CCl4-induced group. Also, GA-HSPC complex group showed significant enhancement of SOD and CAT levels (##P < 0.05), whereas GA+HSPC physical mixture group failed to produce significant changes when compared with pure GA group (Fig. 3).
HPLC Method Validation
A good linear precision relationship between the concentrations (0.5–50 μg/mL) and peak areas was obtained with the correlation coefficient (r) of 0.9986. The limit of detection and limit of quantification were estimated to be 53 and 185 ng/mL, respectively. The intra- and inter-day precisions of GA were within 4.0%. The intra-and inter-day accuracy ranged from 97.12% to 102.73%. The result showed good extraction efficiency. Mean absolute recoveries of GA from rat plasma were in the range of 93.1–94.3% at three QC levels. GA was found to be stable after three cycles of freeze and thaw and the variation of GA content was within 7%. GA is also found stable during short-term (24 h) and long-term (14 days) stability experiments and the variation of analyte content was found within 7% and 15%, respectively.
Pharmacokinetic Parameters
Figure 4 shows the level of GA in rat serum when applied in pure and complex form. The main pharmacokinetic parameters of GA (pure and complex form) are depicted in Table III. Cmax was increased from 2.5 to 5.57 μg/mL in case of the complex, so did the Tmax. The elimination half-life of GA was increased when it was in the complex form with phospholipids and eventually the clearance of the molecule in complex form was also lowered. The AUC 0 to infinity for the GA-HSPC complex was 13.33 units, whereas that of pure GA was 3.09 units. Thus, the GA-HSPC complex has a relative bioavailability of 4.31 compared to that of pure GA. The results indicate that the relative bioavailability of the GA-HSPC complex is 4.31-fold compared to the normal GA.
Fig. 4.
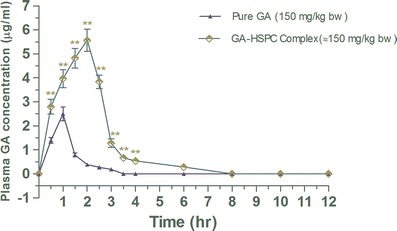
Effect of complexation on serum concentration of gallic acid in rats. Values are mean ± SEM (n = 6/group/time point). ** P < 0.001 (significant with respect to pure gallic acid-treated group)
Table III.
Main Pharmacokinetic Parameters of Pure GA (150 mg/kg, p.o.) and GA-HSPC Complex (equivalent to 150 mg/kg of pure GA, p.o.) in Rats (n = 6)
| Pharmacokinetic parameters | Pure GA | GA-HSPC complex |
|---|---|---|
| C max (μg mL−1) | 2.5 ± 0.18 | 5.57 ± 0.48 |
| T max (h) | 1.08 ± 0.2 | 2.08 ± 0.2 |
| Area under concentration–time curve (AUC0 – tn) (μg mL−1 h) | 2.82 ± 0.2 | 12.45 ± 0.93 |
| Area under concentration–time curve (AUC0 – tα) (μg mL−1 h) | 3.09 ± 0.21 | 13.33 ± 0.87 |
| Elimination half-life (t 1/2el) (h) | 0.98 ± 0.07 | 2.12 ± 0.18 |
| Elimination rate constant (K el) (h−1) | 0.71 ± 0.03 | 0.33 ± 0.01 |
| Clearance (cl) (L h−1) | 32.39 ± 2.8 | 7.5 ± 0.69 |
| Volume of distribution (V d) (L) | 45.55 ± 5.7 | 22.93 ± 0.31 |
Values are means ± SD
Gallic acid, a naturally occurring plant phenol, was found to be hepatoprotective and antioxidant in CCl4-induced toxicity in rats (2–4). Our previous works on hesperitin (21), quercetin (22), and curcumin (35) show that the hepatoprotective activity of these phytomolecules was improved by the phospholipid complexation in a dose-dependent manner. Silipide (a silybin–phospholipid complex) was found to improve the hepatoprotection of silybin both in human and animal models (36,37). In the present study, GA-HSPC complex showed better efficacy in comparison with pure molecule-treated group as well as group receiving physical mixture of GA and HSPC. There is no significant difference in the activity of GA-treated group and GA+HSPC physical mixture-treated group. Moreover, pure HSPC-treated group failed to show significant improvement when compared to the CCl4 control group. This phenomenon denotes that HSPC itself did not contribute in hepatoprotective action against CCl4 intoxication, but improves the activity of GA when complexed with it. The previous works by other researchers showed that the in vitro DPPH free radical scavenging activity of phospholipid complexes of quercetin, catechin, and gallic acid showed quite similar results like their parent molecules. But when investigated in vivo, the phospholipid complexes of phytomolecules show improvements in antioxidant activity (38–40). So, it is important to find out the exact mechanism by which phospholipid complexes show improved antioxidant activity when tested in vivo.
In general, the bioavailability of GA is very low. In one experiment conducted in healthy human volunteers, the maximum concentration of GA in plasma was 1.83 ± 0.16 μmol/L after intake of 50 mg GA (12). In another experiment, after oral administration of 50, 100, and 150 mg/kg grape seed extract containing 91 mg GA/g of extract in male Sprague–Dawley rats, the Cmax of GA was 309.8 ± 102.6, 239.6 ± 39.2, and 323.4 ± 76.9 ng/mL (13). In the present study, the Cmax of GA was 2.5 ± 0.18 μg/mL after 150 mg/kg oral dose of pure GA, which is quite comparable to previous works. But, the Cmax of GA for administration of GA-HSPC complex in same dose was found to be 5.57 ± 0.48 μg/mL. Not only that the complex increased the bioavailability, but also reduced the rate of elimination of GA. The improved pharmacokinetics may be the reason for enhancement of in vivo antioxidant activity of GA imparted by the complex. These findings are well supported by the previous works in our laboratory on androgapholide (18), ellagic acid (19), hesperetin (21), and curcumin (35) as well as the works of other researchers [with silybin (41) and ginkgolides A and B (42)] where similar effects of phospholipid complexation on pharmacokinetics of those phytomolecules were observed. The improvement of the pharmacokinetics may be attributed due to sustained-release action of the complex which maintained the minimum effective concentration of the drug for a longer period of time. The phospholipid complexes of hesperetin (21), catechin (39) and gallic acid (43) had shown sustained-release properties when tested in vitro.
The sustained-release formulations without a carrier system like phospholipid are seldom designed for improving the oral bioavailability of drugs. Some sustained-release formulations of gallic acid like gallic acid-loaded cellulose acetate electrospun nanofibers (44) and gallic acid-loaded electrospun poly(l-lactic acid) fiber (45) were developed and investigated for their in vitro release properties, but sufficient data on the effect of pharmacokinetic parameters are not available. Due to the nontoxic nature of phospholipids, the phospholipid delivery systems are favored than other delivery systems which utilize polymer. Moreover, the easier method of preparation is an advantage over other phospholipid formulations like liposomes. Additionally, the ability of phospholipids to increase absorption of phytochemicals could make the complexes popular nutritional supplements.
CONCLUSION
It is obvious from the present study that the GA-HSPC complex can be utilized as effective dietary supplement which improves the bioavailability of gallic acid by enhancing its absorption and reducing its clearance, prolonging its duration of action. The improved pharmacokinetics of the complex may be the reason of enhanced hepatoprotective and in vivo antioxidant efficacy of gallic acid in CCl4-intoxicated rats.
Electronic supplementary material
Below is the link to the electronic supplementary material.
(DOC 177 kb)
ACKNOWLEDGMENTS
The authors express their gratitude to the State Government Fellowship Scheme, Government of West Bengal, India for providing financial assistance.
REFERENCES
- 1.Kaur M, Velmurugan B, Rajamanickam S, Agarwal R, Agarwal C. Gallic acid, an active constituent of grape seed extract, exhibits anti-proliferative, pro-apoptotic and anti-tumorigenic effects against prostate carcinoma xenograft growth in nude mice. Pharm Res. 2009;26:2133–40. doi: 10.1007/s11095-009-9926-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tung YT, Wu JH, Huang CC, Peng HC, Chen YL, Yang SC, et al. Protective effect of Acacia confusa bark extract and its active compound gallic acid against carbon tetrachloride-induced chronic liver injury in rats. Food Chem Toxicol. 2009;47:1385–92. doi: 10.1016/j.fct.2009.03.021. [DOI] [PubMed] [Google Scholar]
- 3.Jadon A, Bhadauria M, Shukla S. Protective effect of Terminalia belerica Roxb. and gallic acid against carbon tetrachloride induced damage in albino rats. J Ethnopharmacol. 2007;109:214–8. doi: 10.1016/j.jep.2006.07.033. [DOI] [PubMed] [Google Scholar]
- 4.Kanai S, Okano H. Mechanism of the protective effects of sumac gall extract and gallic acid on the progression of CCl4-induced acute liver injury in rats. Am J Chin Med. 1998;26:333–41. doi: 10.1142/S0192415X98000373. [DOI] [PubMed] [Google Scholar]
- 5.Punithavathi VR, Prince PS, Kumar R, Selvakumari J. Antihyperglycaemic, antilipid peroxidative and antioxidant effects of gallic acid on streptozotocin induced diabetic Wistar rats. Eur J Pharmacol. 2011;650:465–71. doi: 10.1016/j.ejphar.2010.08.059. [DOI] [PubMed] [Google Scholar]
- 6.Rasool MK, Sabina EP, Ramya SR, Preety P, Patel S, Mandal N, et al. Hepatoprotective and antioxidant effects of gallic acid in paracetamol-induced liver damage in mice. J Pharm Pharmacol. 2010;62:638–43. doi: 10.1211/jpp.62.05.0012. [DOI] [PubMed] [Google Scholar]
- 7.Choi HJ, Song JH, Bhatt LR, Baek SH. Anti-human rhinovirus activity of gallic acid possessing antioxidant capacity. Phytother Res. 2010;24:1292–6. doi: 10.1002/ptr.3101. [DOI] [PubMed] [Google Scholar]
- 8.Kim HJ, Bruckner JV, Dallas CE, Gallo GM. Effects of dosing vehicles on the pharmacokinetics of orally administered carbon tetrachlorides in rats. Toxicol Appl Pharmacol. 1990;102:50–60. doi: 10.1016/0041-008X(90)90082-6. [DOI] [PubMed] [Google Scholar]
- 9.Valles EG, De Castro CR, De Castro JA. N-Acetylcysteine is an early but also a late preventive agent against carbon tetrachloride-induced liver necrosis. Toxicol Lett. 1994;71:87–95. doi: 10.1016/0378-4274(94)90202-X. [DOI] [PubMed] [Google Scholar]
- 10.Glende EA, Hruszewycz AM, Recknagel RO. Critical role of lipid peroxidation in carbon tetrachloride-induced loss of aminopyrine demethylase, cytochrome P-450 and glucose-6-phosphatase. Biochem Pharmacol. 1976;25:2163–70. doi: 10.1016/0006-2952(76)90128-3. [DOI] [PubMed] [Google Scholar]
- 11.Recknagel RO, Glende EA, Dolak JA, Waller RL. Mechanisms of carbon tetrachloride toxicity. Pharm Ther. 1989;43:139–54. doi: 10.1016/0163-7258(89)90050-8. [DOI] [PubMed] [Google Scholar]
- 12.Shahrzad S, Aoyagi K, Winter A, Koyama A, Bitsch I. Pharmacokinetics of gallic acid and its relative bioavailability from tea in healthy humans. J Nutr. 2001;131:1207–10. doi: 10.1093/jn/131.4.1207. [DOI] [PubMed] [Google Scholar]
- 13.Ferruzzi MG, Lobo JK, Janle EM, Cooper B, Simon JE, Wu QL, et al. Bioavailability of gallic acid and catechins from grape seed polyphenol extract is improved by repeated dosing in rats: implications for treatment in Alzheimer’s disease. J Alzheimers Dis. 2009;18:113–24. doi: 10.3233/JAD-2009-1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zong L, Inoue M, Nose M, Kojima K, Sakaguchi N, Isuzugawa K, et al. Metabolic fate of gallic acid orally administered to rats. Biol Pharm Bull. 1999;22:326–9. doi: 10.1248/bpb.22.326. [DOI] [PubMed] [Google Scholar]
- 15.Watanabe A, Oshima Y. Metabolism of gallic acid and tea catechin by rabbit. Agric Biol Chem. 1965;29:90–3. doi: 10.1271/bbb1961.29.90. [DOI] [Google Scholar]
- 16.Booth AN, Masri MS, Robbins DJ, Emerson OH, Jones FT, Deeds F. The metabolic fate of gallic acid and related compounds. J Biol Chem. 1959;234:3014–6. [PubMed] [Google Scholar]
- 17.Yasuda T, Inaba A, Ohmori M, Endo T, Kubo S, Ohsawa K. Urinary metabolites of gallic acid in rats and their radical-scavenging effects on 1,1-diphenyl-2-picrylhydrazyl radical. J Nat Prod. 2000;63:1444–6. doi: 10.1021/np0000421. [DOI] [PubMed] [Google Scholar]
- 18.Maiti K, Mukherjee K, Murugan V, Saha BP, Mukherjee PK. Enhancing bioavailability and hepatoprotective activity of andrographolide from Andrographis paniculata, a well-known medicinal food, through its herbosome. J Sci Food Agric. 2010;90:43–51. doi: 10.1002/jsfa.3777. [DOI] [PubMed] [Google Scholar]
- 19.Murugan V, Mukherjee K, Maiti K, Mukherjee PK. Enhanced oral bioavailability and antioxidant profile of ellagic acid by phospholipids. J Agric Food Chem. 2009;57:4559–65. doi: 10.1021/jf8037105. [DOI] [PubMed] [Google Scholar]
- 20.Maiti K, Mukherjee K, Gantait A, Saha BP, Mukherjee PK. Enhanced therapeutic potential of naringenin-phospholipid complex in rats. J Pharm Pharmacol. 2006;58:1227–33. doi: 10.1211/jpp.58.9.0009. [DOI] [PubMed] [Google Scholar]
- 21.Maiti K, Mukherjee K, Muruga V, Saha BP, Mukherjee PK. Exploring the effect of hesperetin–HSPC complex—a novel drug delivery system on the in vitro release, therapeutic efficacy and pharmacokinetics. AAPS PharmSciTech. 2009;10:943–50. doi: 10.1208/s12249-009-9282-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Maiti K, Mukherjee K, Gantait A, Ahamed KFH, Saha BP, Mukherjee PK. Enhanced therapeutic benefit of quercetin–phospholipid complex in carbon tetrachloride-induced acute liver injury in rats: a comparative study. Iran J Pharmacol Ther. 2005;4:84–90. [Google Scholar]
- 23.Santagati NA, Salerno L, Attaguile G, Savoca F, Ronsisvalle G. Simultaneous determination of catechins, rutin, and gallic acid in Cistus species extracts by HPLC with diode array detection. J Chromatogr Sci. 2008;46:150–6. doi: 10.1093/chromsci/46.2.150. [DOI] [PubMed] [Google Scholar]
- 24.Reitman S, Frankel S. A colourimetric method for the determination of serum oxaloacetatic and glutamic pyruvic transaminases. Am J Clin Pathol. 1957;28:56–63. doi: 10.1093/ajcp/28.1.56. [DOI] [PubMed] [Google Scholar]
- 25.Kind PRN, King EJ. Estimation of plasma phosphatase by determination of hydrolysed phenol with amino-antipyrine. J Clin Pathol. 1954;7:322–6. doi: 10.1136/jcp.7.4.322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Malloy HT, Evelyn KA. The determination of bilirubin with the photoelectric colorimeter. J Biol Chem. 1973;119:481–90. [Google Scholar]
- 27.Ellman GL. Tissue sulfahydryl groups. Arch Biochem Biophys. 1959;82:70–7. doi: 10.1016/0003-9861(59)90090-6. [DOI] [PubMed] [Google Scholar]
- 28.Paglia DE, Valentine WN. Studies on the quantitative and qualitative characterisation of erythrocyte glutathione peroxidase. J Lab Clin Med. 1967;70:158–69. [PubMed] [Google Scholar]
- 29.Habig WH, Pabst M. The first enzymatic step in mercapturic acid formation. J Biol Chem. 1974;249:7130–9. [PubMed] [Google Scholar]
- 30.Dubler RE, Anderson BM. Simultaneous inactivation of the catalytic activities of yeast glutathione reductase by N-alkylmeleimides. Biochim Biophys Acta. 1981;659:70–85. doi: 10.1016/0005-2744(81)90272-2. [DOI] [PubMed] [Google Scholar]
- 31.Kakkar B, Das PN, Viswanathan A. Modified spectrophotometer assay of SOD. Ind J Biochem Biophys. 1984;21:130–2. [PubMed] [Google Scholar]
- 32.Beers RF, Jr, Seizer IW. A spectrophotometric method for measuring breakdown of hydrogen peroxide by catalase. J Biol Chem. 1952;115:130–40. [PubMed] [Google Scholar]
- 33.Ohkawa H, Hash N, Yagi K. Assay for lipid peroxide for animal tissue by thiobarbituric acid reaction. Ann Biochem. 1979;95:351–8. doi: 10.1016/0003-2697(79)90738-3. [DOI] [PubMed] [Google Scholar]
- 34.Lowry OH, Rosebrough NJ, Forr AL, Ramdall RJ. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193:265–75. [PubMed] [Google Scholar]
- 35.Maiti K, Mukherjee K, Gantait A, Saha BP, Mukherjee PK. Curcumin-phospholipid complex: preparation, therapeutic evaluation and pharmacokinetic study in rats. Int J Pharm. 2007;330:155–63. doi: 10.1016/j.ijpharm.2006.09.025. [DOI] [PubMed] [Google Scholar]
- 36.Marena C, Lampertico M. Preliminary clinical development of silipide: a new complex of silybin in toxic liver disorders. Planta Med. 1991;57:A124–5. doi: 10.1055/s-2006-960420. [DOI] [Google Scholar]
- 37.Conti M, Malandrino S, Magistretti MJ. Protective activity of silipide on liver damage in rodents. Jpn J Pharmacol. 1992;60:315–21. doi: 10.1254/jjp.60.315. [DOI] [PubMed] [Google Scholar]
- 38.Singh D, Rawat MS, Semalty A, Semalty M. Quercetin-phospholipid complex: an amorphous pharmaceutical system in herbal drug delivery. Curr Drug Discov Technol. 2012;9:17–24. doi: 10.2174/157016312799304507. [DOI] [PubMed] [Google Scholar]
- 39.Semalty A, Semalty M, Singh D, Rawat MSM. Phyto-phospholipid complex of catechin in value added herbal drug delivery. J Incl Phenom Macrocycl Chem. 2012;73:377–86. doi: 10.1007/s10847-011-0074-8. [DOI] [Google Scholar]
- 40.Kumawat RS, Mruthunjaya K, Gupta MK. Preparation, characterization and antioxidant activities of gallic acid phospholipids complex. Int J Res Pharm Sci. 2012;2:138–48. [Google Scholar]
- 41.Gatti G, Perucca E. Plasma concentrations of free and conjugated silybin after oral intake of a silybin-phosphatidylcholine complex (silipide) in healthy volunteers. Int J Clin Pharmacol Ther. 1994;32:614–7. [PubMed] [Google Scholar]
- 42.Mauri P, Simonetti P, Gardana C, Minoggio M, Morazzoni P, Bombardelli E, et al. Liquid chromatography/atmospheric pressure chemical ionization mass spectrometry of terpene lactones in plasma of volunteers dosed with Ginkgo biloba L. extracts. Rapid Commun Mass Spectrom. 2001;15:929–34. doi: 10.1002/rcm.316. [DOI] [PubMed] [Google Scholar]
- 43.Singh D, Singh M, Rawat M, Semalty A, Semalty M. Gallic acid-phospholipid complex: drug incorporation and physicochemical characterization. Lett Drug Des Discov. 2011;8:284–91. doi: 10.2174/157018011794578240. [DOI] [Google Scholar]
- 44.Phiriyawirut M, Thawatchai P. Gallic acid-loaded cellulose acetate electrospun nanofibers: thermal properties, mechanical properties, and drug release behavior. Open J Polym Chem. 2012;2:21–9. doi: 10.4236/ojpchem.2012.21004. [DOI] [Google Scholar]
- 45.Chuysinuan P, Chimnoi N, Techasakul S, Supaphol P. Gallic acid-loaded electrospun poly(l-lactic acid) fiber mats and their release characteristic. Macromol Chem Phys. 2009;210:814–22. doi: 10.1002/macp.200800614. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOC 177 kb)


