Abstract
Lornoxicam is a potent oxicam class of non steroidal anti-inflammatory agent, prescribed for mild to moderate pain and inflammation. Niosomal gel of lornoxicam was developed for topical application. Lornoxicam niosomes (Lor-Nio) were fabricated by thin film hydration technique. Bilayer composition of niosomal vesicles was optimized. Lor-Nio dispersion was characterized by DSC, XRD, and FT-IR. Morphological evaluation was performed by scanning electron microscopy (SEM). Lor-Nio dispersion was incorporated into a gel using 2% w/w Carbopol 980 NF. Rheological and texture properties of Lor-Nio gel formulation showed suitability of the gel for topical application. The developed formulation was evaluated for in vitro skin permeation and skin deposition studies, occlusivity test and skin irritation studies. Pharmacodynamic activity of the Lor-Nio gel was performed by carragenan-induced rat paw model. Optimized Lor-Nio comprised of Span 60 and cholesterol in a molar ratio of 3:1 with 30 μM dicetyl palmitate as a stabilizer. It had particle size of 1.125 ± 0.212 μm (d90), with entrapment efficiency of 52.38 ± 2.1%. DSC, XRD, and IR studies showed inclusion of Lor into niosomal vesicles. SEM studies showed spherical closed vesicular structure with particles in nanometer range. The in vitro skin permeation studies showed significant improvement in skin permeation and skin deposition for Lor-Nio gel (31.41 ± 2.24 μg/cm2, 30.079 ± 1.2 μg/cm2) over plain lornoxicam gel (7.37 ± 1.27 μg/cm2, 6.6 ± 2.52 μg/cm2). The Lor-Nio gel formulation showed enhanced anti-inflammatory activity by exhibiting mean edema inhibition (87.69 ± 1.43%) which was significantly more than the plain lornoxicam gel (53.84 ± 2.21%).
KEY WORDS: anti-inflammatory activity, lornoxicam, niosomes, rheology, texture analysis
INTRODUCTION
Lornoxicam (Lor) belongs to an oxicam class of nonsteroidal anti-inflammatory drug. It inhibits cyclooxygenase enzyme and thereby interferes with prostaglandins synthesis. This leads to desensitization of peripheral nociceptors which further reduces inflammation (1,2). It is prescribed for mild to moderate pain and inflammation in osteoarthritis and rheumatoid arthritis (3). It is available as an oral immediate release formulation and parenteral formulations for intravenous and intramuscular administration (4 mg/ml). The total recommended dose by oral route is 8–16 mg/day and the daily should not exceed by 16 mg/day (4–6). There is no topical dosage form of the drug available commercially.
Oral administration requires repeated administration of Lor. It has a very poor solubility in acidic pH of the stomach and hence remains in the stomach wall for a long period, aggravating its potential side effects like peptic ulcers and gastric irritation. Parenteral administration, on the other hand, is not applicable to chronic conditions (7). Topical delivery of Lor with high therapeutic efficacy will avoid its systemic side effects by targeting the drug to the skin.
Various nanotechnology based formulations are available for topical delivery of drugs and cosmeceuticals. Among them, vesicular systems like liposomes and niosomes represent an attractive carrier system. Niosomes offer advantages over liposomes in terms of improved physical and chemical stability (8), low cost, availability of range of surfactants (9), etc. Niosomes is the submicron vesicular system which is comprised of nonionic surfactants. These nonionic amphiphiles self assemble themselves in the aqueous media, to form bilayer structures (10,11). The nonionic amphiphiles consist of hydrophilic head groups and hydrophobic tails. When they are dispersed in water, the molecules arrange the structure so that the hydrophobic part is shielded from the aqueous medium by the hydrophilic head groups (12,13). Being a bilayer structure, it can incorporate both hydrophilic and hydrophobic drugs into the structure (14). Niosomes are reported to improve the residence time of the drug into the skin, thereby increasing its skin deposition (15). They are also reported to modulate permeability of the horny layer of the skin by reducing transepidermal water loss thereby increasing its smoothness by replenishing the lipid composition of the skin (16,17). Thus, niosome-based gel of lornoxicam can be a suitable alternative for efficient topical delivery and improved anti-inflammatory activity.
MATERIALS AND METHODS
Materials
Lornoxicam was obtained from Sun Pharma Ltd, India. Cholesterol was purchased from Thomas Baker, India. Dicetyl phosphate was purchased from Sigma Chemical Co., USA. Tween 20, Tween 80, Span 20, Span 60, Span 80 were obtained from Merck India Ltd. Carbopol 974 NF was obtained from Lubrizol Corporation. All the other chemicals and reagents were of analytical grade.
Methods
Fabrication of Lornoxicam-Loaded Niosomes
Lornoxicam niosomes (Lor-Nio) were prepared by thin film hydration technique using rotary evaporator (Buchi Laboratory Equipment, Switzerland). Different surfactants were screened for preparation of Lor-Nio dispersion. Surfactants and cholesterol were dissolved in a mixture (5 ml) of chloroform and methanol (2:1 v/v) in various molar ratios (1:1, 2:1, 3:1, 4:1). Dicetyl phosphate (DCP) (15 μM) and lornoxicam (1% w/w) were dissolved in a 5 ml mixture of chloroform and methanol (2:1 v/v). This solution was added to the surfactant–cholesterol solution. Organic solvent was slowly removed at 60°C under reduced pressure using rotary evaporator at 50 rpm. A thin lipid film was formed on the walls of the round bottom flask. It was kept in a vacuum desiccator for removal of residual solvents. The dry film was then hydrated using 10 ml of double distilled water at 55°C, which is above the phase transition temperature of the surfactants. The niosomal dispersion so formed was subjected to sonication (Dakshin Ultrasonics, India) for particle size reduction. It was kept overnight to form stable vesicles.
Effect of Bilayer Composition on Vesicular Size and Drug Entrapment
Various surfactants like Span 20, Span 60, Span 80, Tween 20, and Tween 80 were screened for preparation of lornoxicam niosomes. Lornoxicam niosomes were fabricated using 1:1 molar ratio of these surfactant and cholesterol (Table I). Effect of surfactant on particle size, entrapment efficiency (% EE) and stability of the niosomal vesicles was evaluated and the composition with stable niosomes was selected for further evaluation.
Table I.
Optimization of Components of Lor-Nio Dispersion
| Formulation variablea | Formulation | Surfactant | Surfactant: cholesterol (μM) | DCP (μM) |
|---|---|---|---|---|
| Surfactant type | Lor-Nio 1 | Tween 80 | 1:1 | 15 |
| Lor-Nio2 | Tween 20 | 1:1 | 15 | |
| Lor-Nio3 | Span 80 | 1:1 | 15 | |
| Lor-Nio4 | Span 60 | 1:1 | 15 | |
| Lor-Nio5 | Span 20 | 1:1 | 15 | |
| Surfactant concentration | Lor-Nio6 | Span 60 | 1.5:1 | 15 |
| Lor-Nio7 | Span 60 | 2:1 | 15 | |
| Lor-Nio8 | Span 60 | 3:1 | 15 | |
| Cholesterol concentration | Lor-Nio9 | Span 60 | 1:1.5 | 15 |
| Lor-Nio10 | Span 60 | 1:2 | 15 | |
| Lor-Nio11 | Span 60 | 1:3 | 15 | |
| Stabilizer concentration | Lor-Nio 12 | Span 60 | 3:1 | 15 |
| Lor-Nio 13 | Span 60 | 3:1 | 30 | |
| Lor-Nio 14 | Span 60 | 3:1 | 45 |
(aConcentration of lornoxicam was kept as 1% w/w)
Cholesterol forms an integral part of the vesicular structures, as it influences various physical properties and stability of the vesicular system. Niosomes were fabricated using various molar ratios (1:1, 1.5:1, 2:1, and 3:1) of surfactant and cholesterol (Table I). Effect of cholesterol concentration on particle size, entrapment efficiency and colloidal stability of the Lor-Nio dispersion was evaluated (Table I).
Dicetyl palmitate (DCP) is incorporated as a stabilizer in a vesicular system. It has a net negative charge and a long fatty chain (C16) which provides electrostatic and steric stabilization to the niosomal dispersion (10). DCP concentration in the niosomal formulation was varied from 15 to 45 μM (Table I) and its optimum concentration was determined.
Optimization of Process Parameters
Optimization of Hydration Time and Sonication Time
Lipid film was hydrated with double distilled water using rotary evaporator. Hydration time influences particle size and entrapment efficiency of the vesicles. Hydration time was varied from 15 to 60 min. Niosomes so formed, were further subjected to size reduction using probe sonicator. Sonication time was varied from 1 min to 6 min and Lor-Nio dispersion was further evaluated for its physicochemical properties.
Characterization of Lor-Nio
Particle Size
Particle size of the Lor-Nio dispersion was evaluated by dynamic light scattering using Malvern Mastersizer Hydro 2000, UK. Lor-Nio dispersion was added to approximately 800 ml of double distilled water to get a laser obstruction in the range 1–5. Samples were analyzed in triplicate and the average particle size was adopted.
Entrapment Efficiency
Entrapment of the drug into the niosomal vesicles was determined. Niosomes were separated by ultracentrifugation at 35,000 rpm at 20°C for 60 min (Thermo Sorwall WX Ultra, USA). Niosomes were settled in the form of pellets. The supernatant containing unentrapped drug was separated carefully and analyzed by UV–Visible spectroscopy (Jasco, Japan) at 380 nm. The entrapment efficiency was determined using the following formula:
Colloidal Stability
Colloidal stability of the niosomes was determined by evaluating its particle size, entrapment efficiency, and physical stability at room temperature and 4°C after 30 days.
Characterization of Optimized Lor-Nio Dispersion
Morphological Evaluation
Morphological features of the Lor-Nio dispersion were evaluated using Scanning Electron Microscope (SEM) (JEOL JSM 840 SEM HITACHI Japan). Lor-Nio dispersion was diluted 1:1,000 times with double distilled water. The dispersion was fixed on an adhesive carbon tape and dried at room temperature. The tape was further coated with platinum under vacuum and its microscopic examination was performed.
Differential Scanning Calorimetry
Thermal characteristics of the Lor niosomes were evaluated using differential scanning calorimetry using M Perkin Elmer DSC: Pyris 6 Instrument. Empty standard aluminum pans were used as reference. DSC thermographs of bulk lornoxicam, Lor niosomes and blank niosomes were recorded at a heating rate of 10°C/min in a temperature range of 30–250°C.
X-ray Diffraction
Solid-state characteristics of Lor niosomes were evaluated by X-ray diffraction technique (Rigaku Dmax 2500, Rigaku, Tokyo, Japan). X-ray diffraction spectra of the bulk lornoxicam, Lor niosomes and blank niosomes were recorded using a Phillips X-ray diffractometer equipped with an X-ray generator. Nickel-filtered copper target operating at a 40 kV voltage and 20 mA current was used at a scanning speed of 2°/min.
Infra Red (10) Spectroscopy
Interaction and entrapment of the drug into the niosomal vesicles was studied using IR spectroscopy. Bulk lornoxicam, Lor niosomes and the blank niosomes were characterized by IR spectroscopy (Perkin Elmer system 2000 FT-IR Spectrophotometer, Germany) in the region of 4,000 cm−1 to 400 cm−1 by KBr disc method. IR spectrums were compared to investigate the interaction of niosomes with the drug (18).
Preparation and Evaluation of Lor-Nio Based Topical Gel
The optimized lornoxicam niosomes were incorporated in to a topical gel using Carbopol 974 NF. Weighed quantity of Carbopol 974 NF was dispersed in water (2% w/w). The dispersion was stirred for 2–3 h. Lor niosomes (equivalent to 1% w/w of lornoxicam) were incorporated into the gel base. It was stirred for 1 h. pH was adjusted to 6.0 ± 0.05, using sodium hydroxide solution (1 N).
Rheological Behavior of the Lor-Nio Gel
The rheological studies of the Lor-Nio gel were performed using Anton Paar mcr 101 rheometer (Anton Paar Gmbh Grazh, Austria), having cone and plate measuring system. Sample holder situated between the gap of the cone and plate was filled with the Lor-Nio gel and it was subsequently closed. Dynamic shear strain ranging from 0.1 to 100 S−1 was applied to the sample and the shear stress and the viscosity expended by the sample by imposed shear strain was measured. The measurements were performed at isothermal condition of 37°C (19–21).
Spreadability
Uniform spreading of the topical formulation ensures even application of the gel and delivery of a standard dose of the drug. The optimum spreadability of such formulations helps to ensure delivery of suitable dose at the targeted site. Spreadability is the rheological property which determines the ease of the topical application. Thus, spreadability is a critical parameter affecting the delivery of the drug to the targeted site (22). Spreadability of the plain Lor gel and Lor-Nio gel was determined by Texture Analyzer (Brookfield Engineering Laboratories, USA). The standard procedure dictated by Brookfield Engineering Laboratories, USA was used for determining spreadability of the gel. Spreadability assembly was fixed with the female cone on the base holder. The sample was placed in the female cone. Male cone was attached to the load cell. Male cone was aligned to coincide with the female cone. Compression type of test was performed. Male cone was lowered at a pretest speed of 1 mm/s. The test speed and the post test speed was set to 2 mm/s. Spreadability of the plain Lor gel and the Lor-Nio gel was compared in terms of work done to deform the samples at specified distance (22,23).
Skin Permeation and Skin Deposition Study
In vitro skin permeation and deposition of the Lor-Nio gel and the plain Lor gel was evaluated on Wistar Rat skin using vertical modified Keshary Chien type of diffusion cell. The epidermal layer of the rat skin was separated carefully. It was fixed on the donor compartment of the diffusion cell. Permeation medium (phosphate buffer pH 7.4, 10 ml) was placed in the diffusion cell. Gels were applied to the donor area. Temperature was maintained at 37°C. Aliquots (2 ml) were withdrawn at different time intervals and replaced by fresh medium to maintain sink condition. The drug concentration was analyzed using a validated HPLC method. HPLC unit consisted of a Jasco Intelligent pump (Japan) with an ODS HYPERSIL C-18 (II) 250 × 4.6 mm (5 μ) columns. Mobile phase was composed of methanol: water (0.25 M Na acetate) (90: 10) mixture with a flow rate 1.0 ml/min. Jasco MD 2015 plus (Photodiode array detector) was used as a detector and the eluate was monitored at 284 nm. Skin was removed at the end of the permeation study and was washed with distilled water. It was cut into fine pieces and homogenized with methanol for complete extraction of the drug. The sample was analyzed by HPLC after appropriate dilution.
Primary Skin Irritation Study
Skin irritation potential of the Lor-Nio gel was evaluated by acute skin irritation test as per OECD Guidelines. Protocol for the study was approved by Animal Ethical Committee (ICT/IAEC/2011/P 29). Healthy male New Zealand rabbits weighing 2.5–3 kg were used for the study (n = 3). Animals were divided into four groups. The groups were categorized into positive control (1% formaldehyde solution), blank niosomal gel, Lor-Nio gel and negative control (without any treatment). An area of rabbits back (0.4 cm2) was shaved carefully and applied with 0.5 g of the test substances individually. It was covered with an adhesive tape. The skin was evaluated and scored for erythema and edema which serves as an indicator of irritation potential of samples (23,24).
In Vitro Occlusivity Study
Niosomes are the lipid-based vesicular system with particle size in nanometer range. Niosome-based semisolid formulations provide an excellent occlusivity to the skin on topical application. The lipidic and colloidal nature of the vesicles provides an extensive surface area, which efficiently reduces the transepidermal water loss from the skin surfaces. This further increases the hydration status of the skin resulting in enhanced deposition of the drug into the skin (25).
The method adopted by Vringer et al. was used for determining the occlusivity of the formulations. Occlusivity factor of the plain Lor gel and Lor-Nio gel was evaluated. Distilled water (25 g) was placed in beakers. Each beaker was covered with Whatman glass microfiber filter (9.0 cm). Test formulations were then applied on its surface. The beaker covered with filter in which no test formulation was applied served as a control for water loss. These beakers were then placed at 30 ± 2°C/60 ± 5% RH for a period of 48 h. All the formulations were tested in triplicate keeping all the condition constant. Occlusive factor (F) was calculated in reference to the control using following formula (26)
Where F is the occlusivity factor, A is the % water loss without sample (reference), and B is the water loss with sample.
Pharmacodynamic Evaluation
Protocol for the pharmacodynamic study was approved by Institutional Animal Ethical Committee (IAEC) (ICT.IAEC/2011/P12). Anti-inflammatory activity of the Lor-Nio gel was evaluated using carragenan-induced rat paw edema model. Wistar rats of either sex weighing 200–250 g were used for the study. A 1% w/v solution of carragenan in saline was injected through 26 gauge needle in sub-plantar tissue of the right hind paw of each rat. The paw thickness was measured immediately before and after administration of the carragenan solution. Plain Lor gel and Lor-Nio gel was applied to the right hind paw and it was occluded with 3 M surgical tape and gauge to prevent licking of the gel (n = 3). The group of animals administered with carragenan, without application of anti-inflammatory agent, served as positive control (26,27).
The paw volume was measured by a mercury displacement technique using plethysmometer after an hour’s interval. The percent edema volume inhibition was calculated using following formula:
Where, Vtreated is the volume of the paw after the topical treatment of gel and Vuntreated is the volume of the paw without treatment of topical gel.
Statistical Analysis
All the results were statistically evaluated using one-way ANOVA test at p < 0.05.
RESULTS
Fabrication of Lor-Nio Dispersion
Effect of Bilayer Composition on Vesicular Size and Drug Entrapment
Components of bilayer structure of niosomes like surfactants, cholesterol, and stabilizers affect their vesicular properties. Niosomes were prepared using 1:1 molar ratio of various surfactants and cholesterol. The stabilizer (DCP) concentration was fixed to 15 μM. There was increase in entrapment efficiency with various surfactants in the order of Tween 20 < Span 80 < Tween 80 < Span 20 < Span 60. Particle size was reduced in the order of Tween 80 < Span 80 < Tween 20 < Span 20 < Span 60. Colloidal stability of the Lor-Nio dispersion was evaluated for a month period. Lor-Nio dispersion prepared with Span 60 had the least particle size (d90) of 1.125 ± 0.212 μ with the highest entrapment efficiency of 32.35 ± % 1.06 (Fig. 1a). Span 60-loaded niosomes further remained stable for a minimum 1 month period with no significant difference (p < 0.05) in particle size and entrapment efficiency.
Fig. 1.
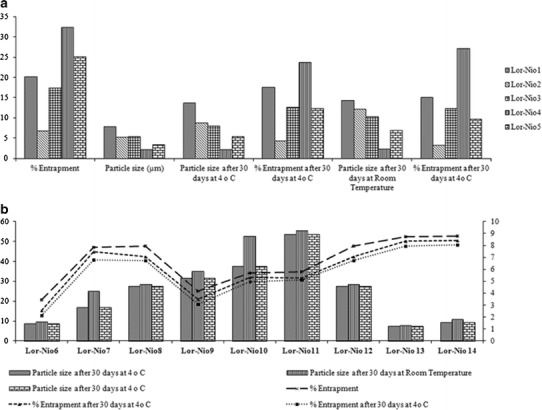
Optimization of bilayer components of Lor-Nio dispersion. a Screening of surfactants. b Optimization of components
Cholesterol forms an integral part of the vesicular delivery system. Its composition with respect to surfactant (1:1, 1.5:1, 2:1, and 3:1) was optimized. Entrapment efficiency was increased up to a molar ratio of 2:1, followed by a significant reduction from 47.15 ± 0.95% to 37.03 ± 0.94% after that (Fig. 1b).
Stabilizer concentration was optimized. Niosomes were prepared with varied DCP concentration from 15 μM to 45 μM. Niosomes with DCP concentration of 30 μM were highly stable and did not show significant increase (p < 0.05) in particle size for 1 month (Fig. 1b).
Optimization of Process Parameters
Hydration time and sonication time are the important process parameters during thin film hydration technique. Lipid film was hydrated using a rotary evaporator. Hydration time of the surfactant lipid film was varied from 15 min to 60 min. There was no significant difference (p < 0.05) in entrapment of the drug into the bilayer vesicles (Fig. 2a). Hydration time was optimized to 30 min. The bilayered vesicles so formed were subjected to ultrasonication for size reduction. Sonication time was increased from 1 min to 6 min. Particle size of niosomes was reduced from 4.032 μm to 523 nm with subsequent decrease in the drug entrapment from 54.55% to 24.21% (Fig. 2b). Ultrasonication disrupts the vesicular structure of the niosomes and causes leakage of the drug. Thus, sonication time of 3 min was optimized.
Fig. 2.
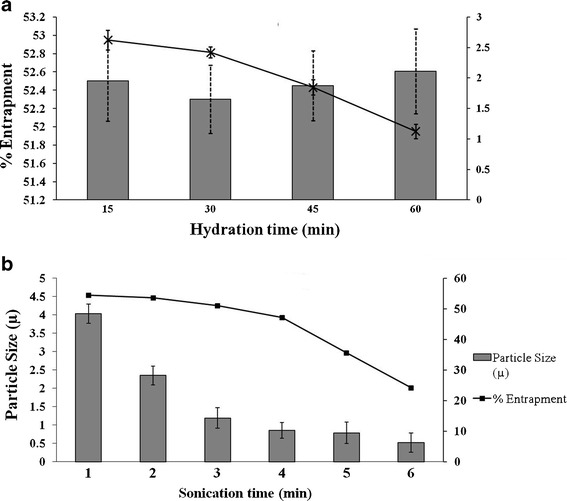
Process parameters optimization. a Optimization of hydration time. b Optimization of time of sonication
Characterization of Optimized Lor-Nio Dispersion
Solid-State Characterization
SEM study showed spherical and closed vesicular structure in a nanometer size range (Fig. 3a). DSC studies of the Lor-Nio dispersion was carried out. Bulk Lor shows a sharp melting point at 225°C, depicting a typical crystalline nature of the drug. When incorporated into niosomes, the characteristic endotherm of Lor disappeared. This suggests complete amorphization of Lor when incorporated into the niosomes. Blank niosomes showed a sharp characteristic endotherm at 55°C. Its crystallinity was reduced when incorporated into niosomes (Fig. 4a). XRD study was in agreement with the results of DSC study. Lornoxicam is a crystalline substance with characteristic peaks at 7.8°, 10.2°, 12.2°, 14.5°, 18.2°, 22.2°, and 24.5° (Fig. 4b). The characteristic peaks of lornoxicam were absent in niosomes. Blank niosomes were used to eliminate the interference of the other constituents of the niosomes (28). FT-IR study showed characteristic peaks of Lor as –NH stretching (3,090 cm−1), –C═O stretching (Primary amide at 1,642 cm−1), N-H bending (secondary amine at 1,537 cm−1) and –O═S═O stretching (1150, 1378 and 1328.9 cm−1). All these characteristic peaks of lornoxicam were completely masked in the Lor niosomes, suggesting entrapment of the drug into the niosomal vesicles.
Fig. 3.
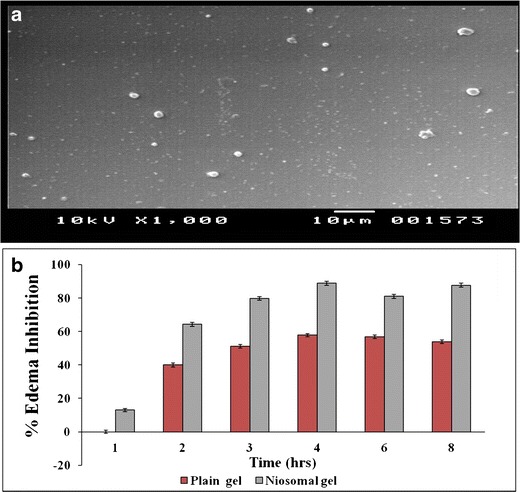
a Morphological evaluation of Lor-Nio by SEM. b In vivo anti-inflammatory activity of Lor-Nio gel
Fig. 4.
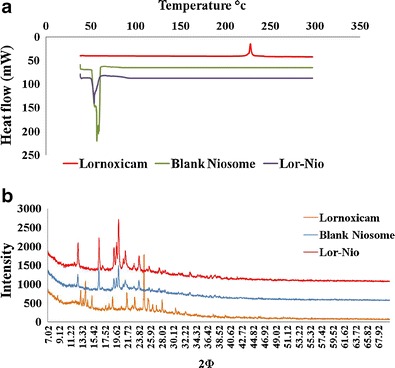
Characterization of optimized Lor-Nio dispersion. a DSC Study of Lor-Nio dispersion. b XRD study of Lor-Nio dispersion
Preparation and Evaluation Lor-Nio Gel
Rheology of the Lor-Nio Gel
Rheological Behavior
Rheological properties of the semisolid gel were evaluated using a rheometer. Lor-Nio gel and plain Lor gel were studied for its rheological behavior. The rheogram showed a typical concave curve (Fig. 5a) inclined towards the shear rate axis specific to the shear thinning system with pseudo plastic flow.
Fig. 5.
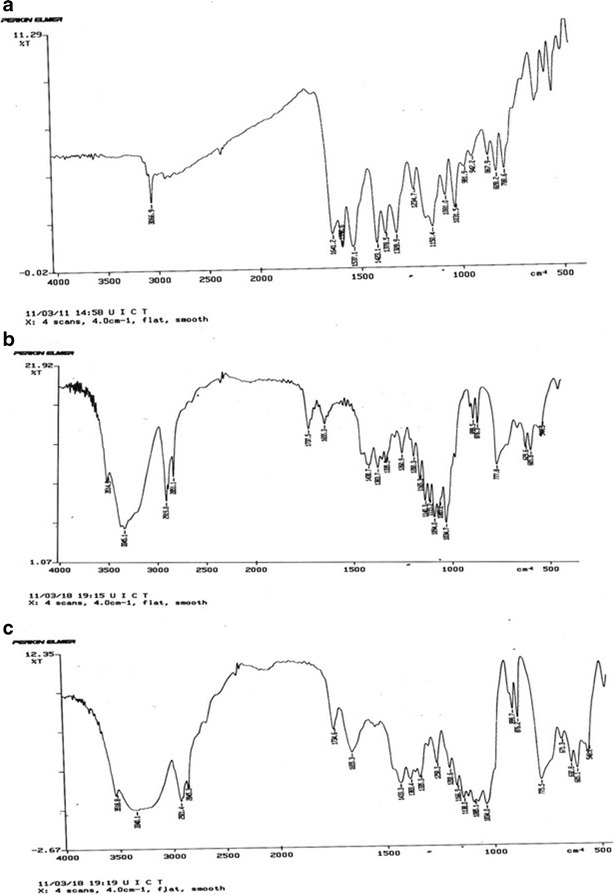
IR Spectrum of a Lornoxicam. b Plain niosomes. c Lor-Nio
Spreadability
The graph of work done against time was plotted to determine spreadability of the Lor-Nio gel. Maximum value on the ordinate axis of the spreadability curve indicates the firmness of the semisolid formulation at specified depth. The area under the positive curve measures the force required to deform the sample to the defined distance. Spreadability of Lor-Nio gel was compared with plain Carbopol gel. Lor-Nio gel showed lower value of peak load with less area under the curve, indicating better spreadability (Fig. 5b).
Skin Permeation and Skin Deposition Study
Skin permeation of the lornoxicam in the form of Lor-Nio gel and plain Carbopol gel was evaluated using modified Keshary–Chien diffusion cell. The results showed statistically significant increase in the permeation of lornoxicam when incorporated into niosomes (31.41 ± 2.24 μg/cm2) as compared to plain Lor gel (7.37 ± 1.27 μg/cm2) at p < 0.05. There was a fivefold increase in deposition of Lor when encapsulated into the niosomes-based gel (30.079 ± 1.2 μg/cm2) compared to plain Lor gel (6.6 ± 2.52 μg/cm2) (Fig. 6a).
Fig. 6.
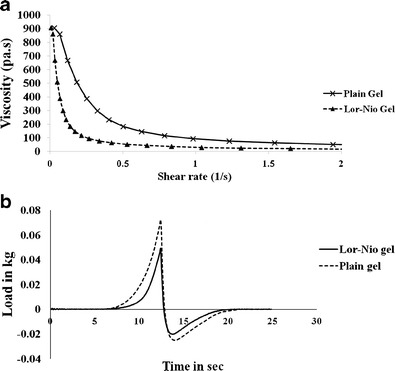
Rheology and texture analysis of Lor-Nio gel. a Rheological behavior of Lor-Nio gel. b Spreadability of Lor-Nio gel
Primary Skin Irritation Test
The Lor-Nio gel was free of any irritation when applied to the skin. There were no signs of erythema and edema (Fig. 7a).
Fig. 7.
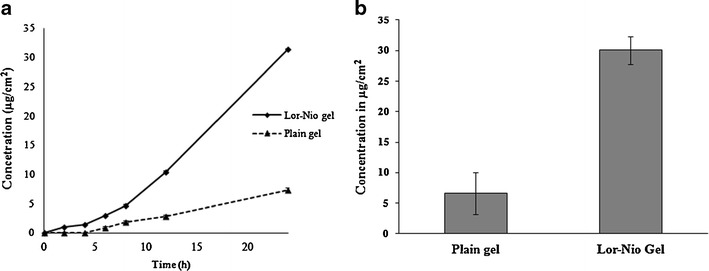
Permeation profile of Lor-Nio gel. a Skin permeation of Lor-Nio gel. b Skin deposition of Lor-Nio gel
In Vitro Occlusivity Test
The ability of niosomes to reduce the transepidermal loss of water was evaluated by in vitro occlusivity test. The occlusivity factor of the plain Lor gel and the Lor-Nio gel was calculated. Lor-Nio gel and Lor gel had an occlusivity factor of 65.88 ± 1.23 and 32.65 ± 1.43 respectively. Lor-Nio gel thus had significantly higher occlusivity properties over plain Lor gel at p < 0.05 (Fig. 8).
Fig. 8.
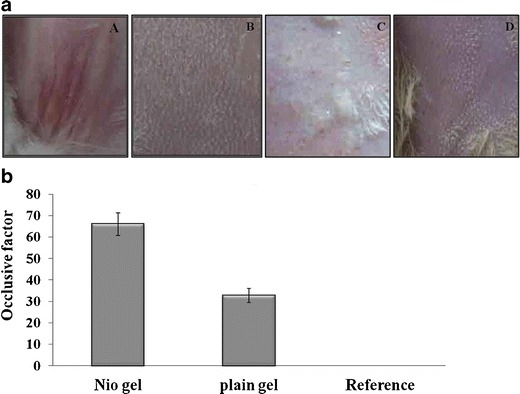
Evaluation of Lor-Nio gel. a Skin irritation study of Lor-Nio gel. b Occlusivity of Lor-Nio gel
Pharmacodynamic Evaluation
In vivo performance of the Lor-Nio gel was evaluated by measuring anti-inflammatory activity of the Lor-Nio gel in rats. Carragenan-induced rat paw edema model was used for the studies. The change in the paw volume in the groups was determined. The results of the paw edema test were analyzed using one-way ANOVA at p < 0.05. Niosomal gel showed 87.69% inhibition of the rat paw edema which was significantly higher than the plain Lor gel (53.84%) at p < 0.05 (Fig. 3b). This suggests improved anti-inflammatory activity of Lor when incorporated into a niosomal system.
DISCUSSION
Span 60 (sorbitan monosterate) is a hydrophobic surfactant with long alkyl chain length (C18), which accommodates high concentration of drug into its hydrophobic region. Span 60 also has the highest transition temperature (50°C) of all the surfactants, which further increases entrapment efficiency of the drug (Fig. 1). Span 20(sorbitan monolaurate) has small alkyl chain (C12) and thus has less capacity to incorporate hydrophobic molecules. Tween 80 is polyoxyethylene sorbitan monooleate (HLB 15) with a monounsaturated alkyl chain (C18) and Tween 20 is polyoxyethylene sorbitan monolaurate (C12) with HLB of 16.7. The long hydrophobic fatty acid chain of Tween 80 facilitates incorporation of hydrophobic molecules (29,30). Therefore, Span 60 and Tween 80 had high entrapment amongst their respective class.
Incorporation of cholesterol in the niosomes bilayer increases the entrapment efficiency of the drug in the vesicles. Cholesterol increases viscosity of niosomal dispersion and imparts rigidity to the flexible bilayer, which results in the formation of highly ordered structures of surfactants with cholesterol embedded in the bilayer. This further facilitates partitioning of the drug in the bilayer and increases the entrapment of the drug in niosomal vesicles. Increased hydrophobicity and rigidity of the bilayered vesicles imparts stability and reduces permeability of the niosomes thereby increasing the entrapment efficiency. Increasing cholesterol concentration beyond 2 μM showed decrease in entrapment of the drug. Higher amounts of cholesterol compete with the drug for packing space within the bilayer and effluxes the drug when the amphiphiles assemble into the vesicles. It also disrupts the regular bilayer structure and causes leakage of the drug (29,31).
DCP was added as a stabilizer. DCP stabilizes the niosomal vesicles by inducing negative charge in the bilayer, which avoids aggregation and fusion of the niosomes and thus maintains the integrity and stability of the niosomal vesicles (32).
Process parameters being hydration time and sonication time were optimized. Increasing hydration time increases the wetting and thus swelling of the lipid film which further enhances density of vesicle formation (29). Ultrasound waves generate the cavitation bubbles in liquids which oscillate nonlinearly and collapse eventually. This results in local rise in the temperature and pressure of the system which further decreases particle size of the vesicles. Sonication time was optimized to 3 min. Sonication of the niosomal dispersion beyond 3 min showed no significant reduction in particle size and instead led to disruption of the vesicular structure of the niosomes, resulting in leakage of the drug.
Solid-state characterization of the Lor-Nio dispersion was performed by FT-IR, DSC and XRD studies. FT-IR studies showed inclusion of the drug into the bilayer vesicles. DSC and XRD studies showed loss of characteristic peaks of lornoxicam when incorporated into niosomes. This suggests complete amorphization of the drug when incorporated into niosomes. Amorphous drug shows high permeation and deposition over its crystalline form, which further aids in efficient delivery of lornoxicam on topical application.
Lor niosomes were further incorporated into a topical gel using Carbopol 974 NF as a gelling agent. Incorporation of niosomes in semisolid gel forms a colloidal network that aligns itself in the direction of applied shear showing pseudoplastic behavior (a shear thinning system showing decrease in viscosity on application of shear stress) which facilitates its topical application (19–21). Lor-Nio gel had better spreadability over plain Carbopol gel.
Skin permeation and deposition studies were performed on Wistar rat skin. Lor-Nio gel showed increased deposition into the skin over plain Lor gel. Plain Lor gel is a Carbopol based gel of lornoxicam representing a simple, aqueous system with lornoxicam dispersed in the matrix. Permeation of Lor from plain gel is thus determined by permeation properties of the drug. Improved skin permeation and deposition of the drug from Lor-Nio gel can be justified by several mechanisms. Nonionic surfactants in the formulation act as permeation enhancer. The surfactants in vesicular form decrease the crystallinity of the intracellular lipid bilayers of the skin and thus enhance drug deposition. Increased solubility of the Lor enhances its skin permeation. Vesicular nature of niosomes enhances the permeation and deposition of the drug in the skin. Lipid-based vesicles in nanometer size range showed superior occlusivity over plain aqueous gel which improved hydration of the skin and hence drug deposition (Fig. 6b) (29,30).
In vitro occlusivity studies showed superior occlusivity of Lor-Nio gel. Plain Lor gel is a hydrophilic gel system, without a lipid or oil component, while Lor-Nio gel is comprised of cholesterol and hydrophobic surfactants with vesicular size in nanometer size range. This offers extensive surface area which forms a superior occlusive film on the skin surface and thus prevents the transepidermal water loss. It further improves hydration status of the skin and enhances skin permeation and deposition of the drug.
Decreased particle size, decreased degradation, sustained release and amorphization of the drug, improved hydration status of the skin cumulatively enhance the deposition of the drug and its pharmacodynamic activity on topical application (31–33).
CONCLUSION
Lornoxicam-loaded niosomes were successfully formulated by thin film hydration technique. Morphological studies showed spherical, closed vesicles of lornoxicam niosomes. Span 60 formed stable niosomes with d90 value of 1.125 ± 0.212 μ and entrapment efficiency of 52.38 ± 2.1%. DSC and XRD studies showed complete amorphization of lornoxicam when incorporated into niosomes. Niosomes were further incorporated into a semisolid gel formulation. Niosomal gel showed good rheological and texture properties which was favorable for its topical application. Lornoxicam showed significantly enhanced skin permeation and deposition in the form of niosomal gel over conventional plain gel. Niosomal gel of lornoxicam showed enhanced anti-inflammatory activity in rats over plain lornoxicam gel. Thus, a novel niosome-based topical gel formulation can be an efficient alternative to the commercially existing oral and intravenous formulations of lornoxicam.
Acknowledgments
The authors wish to acknowledge University Grant Commission (UGC), Government of India for providing fellowship. Authors would like to thank AICTE-NAFETIC for providing the laboratory facilities.
Declaration of Interest
The authors report no conflict of interests.
References
- 1.Zhang Y, Zhong D, Si D, Guo Y, Chen X, Zhou H. Lornoxicam pharmacokinetics in relation to cytochrome P450 2C9 genotype. Br J Clin Pharmacol. 2005;59(1):14–17. doi: 10.1111/j.1365-2125.2005.02223.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Norholt SE, Sindet-Pedersen S, Larsen U, Bang U, Ingerslev J, Nielsen O, et al. Pain control after dental surgery: a double-blind, randomised trial of lornoxicam versus morphine. Pain. 1996;67(2):335–343. doi: 10.1016/0304-3959(96)03126-0. [DOI] [PubMed] [Google Scholar]
- 3.Staunstrup H, Ovesen J, Larsen UT, Elbaek K, Larsen U, Kroner K. Efficacy and tolerability of lornoxicam versus tramadol in postoperative pain. J Clin Pharmacol. 1999;39(8):834–841. doi: 10.1177/00912709922008362. [DOI] [PubMed] [Google Scholar]
- 4.Hamza YES, Aburahma MH. Innovation of novel sustained release compression-coated tablets for lornoxicam: formulation and in vitro investigations. Drug Dev Ind Pharm. 2010;36(3):337–349. doi: 10.3109/03639040903170768. [DOI] [PubMed] [Google Scholar]
- 5.Radhofer-Welte S, Dittrich P. Determination of the novel non-steroidal anti-inflammatory drug lornoxicam and its main metabolite in plasma and synovial fluid. J Chromatogr B Biomed Sci Appl. 1998;707(1):151–159. doi: 10.1016/S0378-4347(97)00597-5. [DOI] [PubMed] [Google Scholar]
- 6.Skjodt NM, Davies NM. Clinical pharmacokinetics of lornoxicam: a short half-life oxicam. Clin Pharmacokinet. 1998;34(6):421–428. doi: 10.2165/00003088-199834060-00001. [DOI] [PubMed] [Google Scholar]
- 7.Lin SZ, Wouessidjewe D, Poelman MC, Duchene D. In vivo evaluation of indomethacin/cyclodextrin complexes gastrointestinal tolerance and dermal anti-inflammatory activity. Int J Pharm. 1994;106(1):63–67. doi: 10.1016/0378-5173(94)90276-3. [DOI] [Google Scholar]
- 8.Vora B, Khopade AJ, Jain N. Proniosome based transdermal delivery of levonorgestrel for effective contraception. J Control Release. 1998;54(2):149–165. doi: 10.1016/S0168-3659(97)00100-4. [DOI] [PubMed] [Google Scholar]
- 9.Manconi M, Sinico C, Valenti D, Lai F, Fadda AM. Niosomes as carriers for tretinoin: III. A study into the in vitro cutaneous delivery of vesicle-incorporated tretinoin. Int J Pharm. 2006;311(1):11–19. doi: 10.1016/j.ijpharm.2005.11.045. [DOI] [PubMed] [Google Scholar]
- 10.Baillie A, Florence A, Hume L, Muirhead G, Rogerson A. The preparation and properties of niosomes—non‐ionic surfactant vesicles. J Pharm Pharmacol. 1985;37(12):863–868. doi: 10.1111/j.2042-7158.1985.tb04990.x. [DOI] [PubMed] [Google Scholar]
- 11.Azmin MN, Florence AT, Handjani-Vila RM, Stuart JFB, Vanlerberghe G, Whittaker JS. The effect of non-ionic surfactant vesicle (niosome) entrapment on the absorption and distribution of methotrexate in mice. J Pharm Pharmacol. 1985;37(4):237–242. doi: 10.1111/j.2042-7158.1985.tb05051.x. [DOI] [PubMed] [Google Scholar]
- 12.Handjani-Vila RM, Ribier A, Rondot B, Vanlerberghie G. Dispersions of lamellar phases of non-ionic lipids in cosmetic products. Int J Cosmet Sci. 1979;1(5):303–314. doi: 10.1111/j.1467-2494.1979.tb00224.x. [DOI] [PubMed] [Google Scholar]
- 13.Toshimitsu Y, Florence AT. Vesicle (niosome)-in-water-in-oil (v/w/o) emulsions: an in vitro study. Int J Pharm. 1994;108(2):117–123. doi: 10.1016/0378-5173(94)90322-0. [DOI] [Google Scholar]
- 14.Junginger HE, Hofland HEJ, Bouwstra JA. Liposomes and niosomes: interactions with human skin. Cosmet toiletries. 1991;106(8):45–50. [Google Scholar]
- 15.Reddy DN, Udupa N. Formulation and evaluation of oral and transdermal preparations of flurbiprofen and piroxicam incorporated with different carriers. Drug Dev Ind Pharm. 1993;19(7):843–852. doi: 10.3109/03639049309062986. [DOI] [Google Scholar]
- 16.Schreier H, Bouwstra J. Liposomes and niosomes as topical drug carriers: dermal and transdermal drug delivery. J Control Release. 1994;30(1):1–15. doi: 10.1016/0168-3659(94)90039-6. [DOI] [Google Scholar]
- 17.Van Hal D, Van Rensen A, De Vringer T, Junginger H, Bouwstra J. Diffusion of estradiol from non-ionic surfactant vesicles through human stratum corneum in vitro. STP Pharma Sci. 1996;6(1):72–78. [Google Scholar]
- 18.Bhalekar MR, Pokharkar V, Madgulkar A, Patil N. Preparation and evaluation of miconazole nitrate-loaded solid lipid nanoparticles for topical delivery. AAPS PharmSciTech. 2009;10(1):289–296. doi: 10.1208/s12249-009-9199-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chawla V, Saraf SA. Rheological studies on solid lipid nanoparticle based carbopol gels of aceclofenac. Colloids Surf B Biointerfaces. 2012;92:293–298. doi: 10.1016/j.colsurfb.2011.12.006. [DOI] [PubMed] [Google Scholar]
- 20.Park EK, Song KW. Rheological evaluation of petroleum jelly as a base material in ointment and cream formulations: steady shear flow behavior. Arch Pharm Res. 2010;33(1):141–150. doi: 10.1007/s12272-010-2236-4. [DOI] [PubMed] [Google Scholar]
- 21.Chow KT, Chan LW, Heng PWS. Formulation of hydrophilic non-aqueous gel: drug stability in different solvents and rheological behavior of gel matrices. Pharm Res. 2008;25(1):207–217. doi: 10.1007/s11095-007-9457-3. [DOI] [PubMed] [Google Scholar]
- 22.Jones DS, Lawlor MS, Woolfson AD. Examination of the flow rheological and textural properties of polymer gels composed of poly (methylvinylether-co-maleic anhydride) and poly (vinylpyrrolidone): rheological and mathematical interpretation of textural parameters. J Pharm Sci. 2002;91(9):2090–2101. doi: 10.1002/jps.10195. [DOI] [PubMed] [Google Scholar]
- 23.Wavikar P, Vavia P. Nanolipidgel for enhanced skin deposition and improved antifungal activity. AAPS PharmSciTech. 2013;14(1):222–233. doi: 10.1208/s12249-012-9908-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jenning V, Gysler A, Schafer-Korting M, Gohla SH. Vitamin A loaded solid lipid nanoparticles for topical use: occlusive properties and drug targeting to the upper skin. Eur J Pharm Biopharm. 2000;49(3):211–218. doi: 10.1016/S0939-6411(99)00075-2. [DOI] [PubMed] [Google Scholar]
- 25.Muller RH, Radtke M, Wissing SA. Solid lipid nanoparticles (SLN) and nanostructured lipid carriers (NLC) in cosmetic and dermatological preparations. Adv Drug Deliv Rev. 2002;54:S131–S155. doi: 10.1016/S0169-409X(02)00118-7. [DOI] [PubMed] [Google Scholar]
- 26.Mei Z, Chen H, Weng T, Yang Y, Yang X. Solid lipid nanoparticle and microemulsion for topical delivery of triptolide. Eur J Pharm Biopharm. 2003;56(2):189–196. doi: 10.1016/S0939-6411(03)00067-5. [DOI] [PubMed] [Google Scholar]
- 27.Ozguney IS, Karasulu HY, Kantarci G, Sumru S, Guneri T, Ertan G. Transdermal delivery of diclofenac sodium through rat skin from various formulations. AAPS PharmSciTech. 2006;7(4):39–45. doi: 10.1208/pt070488. [DOI] [PubMed] [Google Scholar]
- 28.Hamza YES, Aburahma MH. Design and in vitro evaluation of novel sustained-release double-layer tablets of lornoxicam: utility of cyclodextrin and xanthan gum combination. AAPS PharmSciTech. 2009;10(4):1357–1367. doi: 10.1208/s12249-009-9336-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kandasamy R, Veintramuthu S. Formulation and optimization of zidovudine niosomes. AAPS PharmSciTech. 2010;11(3):1119–1127. doi: 10.1208/s12249-010-9480-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Abdelbary G, El-gendy N. Niosome-encapsulated gentamicin for ophthalmic controlled delivery. AAPS PharmSciTech. 2008;9(3):740–747. doi: 10.1208/s12249-008-9105-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Balakrishnan P, Shanmugam S, Lee WS, Lee WM, Kim JO, Oh DH, et al. Formulation and in vitro assessment of minoxidil niosomes for enhanced skin delivery. Int J Pharm. 2009;377(1):1–8. doi: 10.1016/j.ijpharm.2009.04.020. [DOI] [PubMed] [Google Scholar]
- 32.Mali N, Darandale S, Vavia P. Niosomes as a vesicular carrier for topical administration of minoxidil: formulation and in vitro assessment. Drug Deliv Transl Res. 2012:1–6. [DOI] [PubMed]
- 33.Fang JY, Hong CT, Chiu WT, Wang YY. Effect of liposomes and niosomes on skin permeation of enoxacin. Int J Pharm. 2001;219(1):61–72. doi: 10.1016/S0378-5173(01)00627-5. [DOI] [PubMed] [Google Scholar]


