Abstract
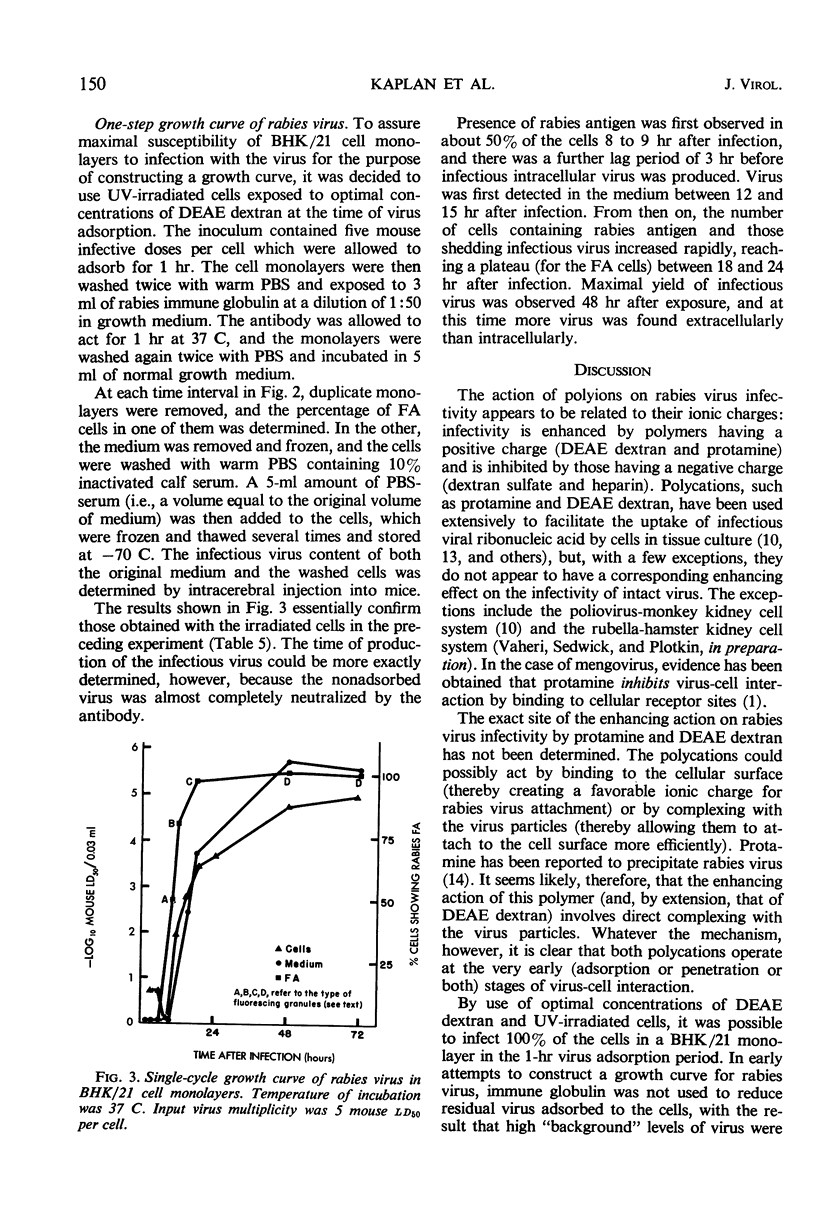
The infectivity of fixed rabies virus in a number of cell lines has been shown to be markedly enhanced by the addition of protamine or diethylaminoethyl dextran to the virus inoculum. The polycations appear to exert their influence at a very early stage (adsorption or penetration or both) of virus-cell interaction. Immune globulin blocked infection completely when added up to 5 min after exposure and almost completely when added 5 to 15 min after infection. Antibody had no effect on adsorption and penetration when added to the inoculum 30 min or more after cells were exposed to the virus. Irradiation of BHK/21 cell monolayers with ultraviolet light increased their sensitivity to rabies virus. The events occurring after synchronous infection of cells in both irradiated and nonirradiated cell monolayers were followed by means of fluorescent-antibody staining and by intracerebral titration in mice. Virus-specific fluorescent antigen first appeared between 8 and 9 hr after infection, and in irradiated cultures there was a further lag period of 3 hr before infectious virus was produced intracellularly. Virus was first detected in the medium 12 to 15 hr after infection, and maximal yield of infectious virus was observed 48 hr after exposure. In nonirradiated cultures, formation of infectious virus was delayed, and the final yield of virus was also reduced.
Full text
PDF

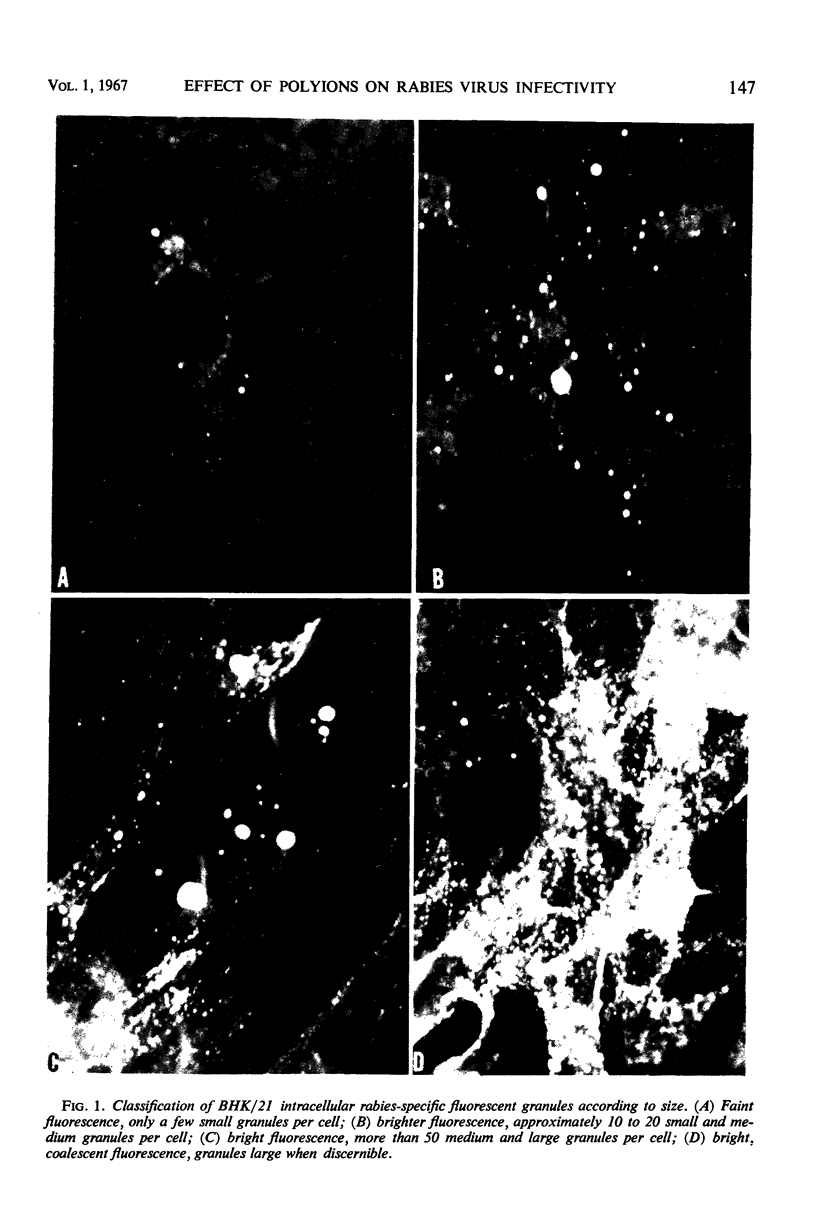
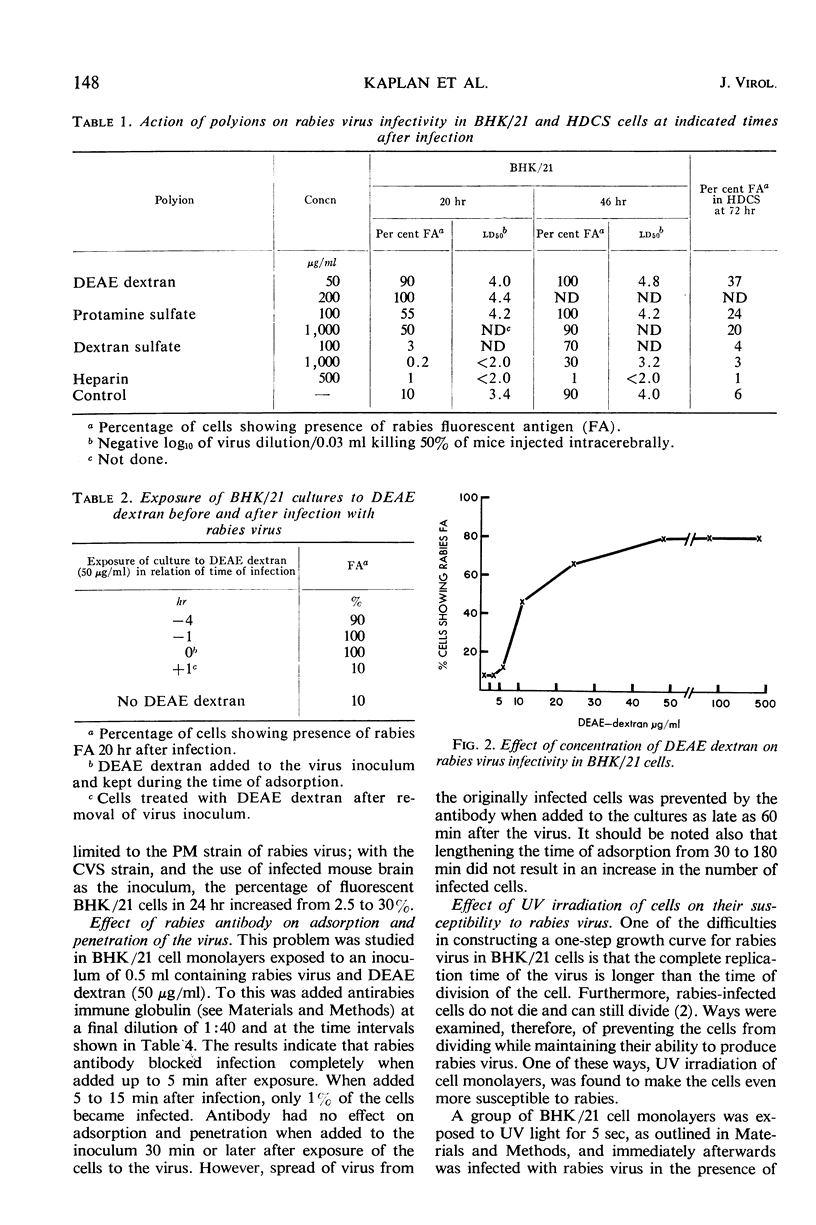
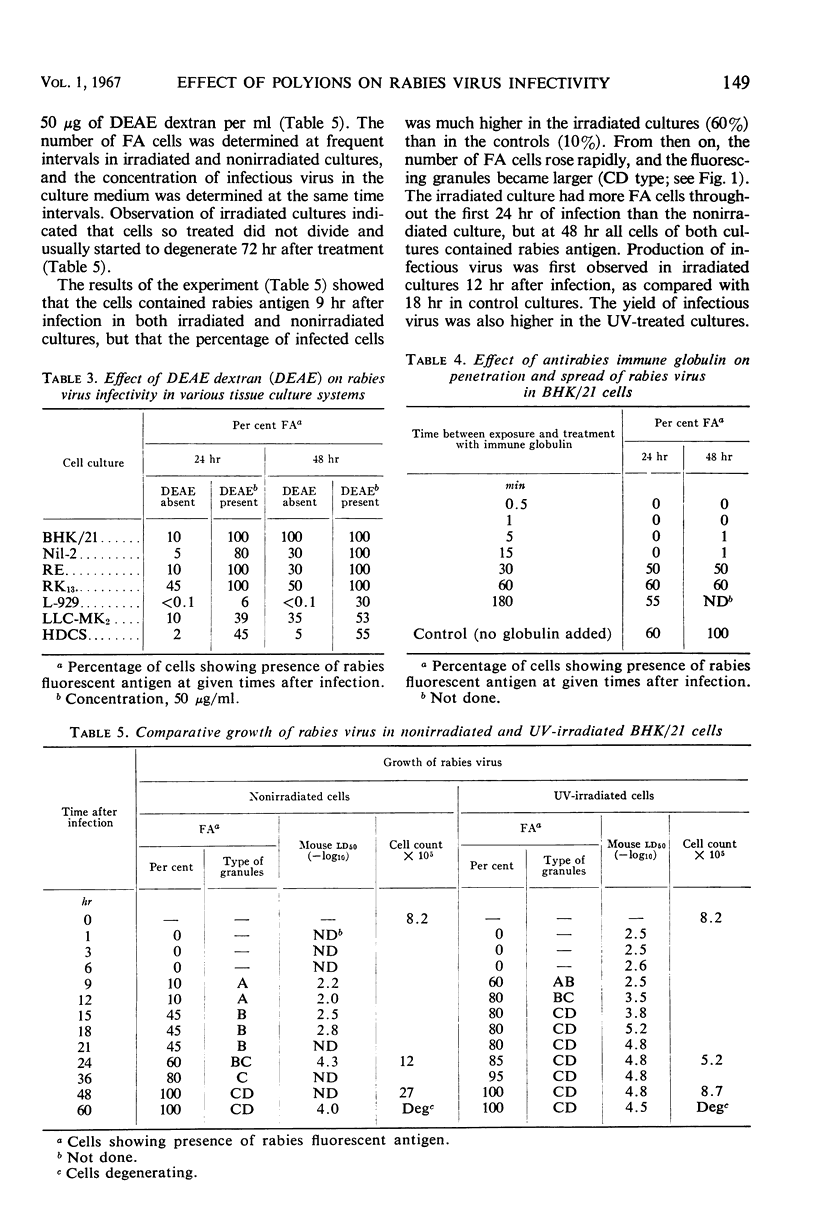

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- COLTER J. S., DAVIES M. A., CAMPBELL J. B. STUDIES OF THREE VARIANTS OF MENGO ENCEPHALOMYELITIS VIRUS. II. INHIBITION OF INTERACTION WITH L CELLS BY AN AGAR INHIBITOR AND BY PROTAMINE. Virology. 1964 Dec;24:578–585. doi: 10.1016/0042-6822(64)90210-7. [DOI] [PubMed] [Google Scholar]
- FERNANDES M. V., WIKTOR T. J., KOPROWSKI H. MECHANISM OF THE CYTOPATHIC EFFECT OF RABIES VIRUS IN TISSUE CULTURE. Virology. 1963 Sep;21:128–131. doi: 10.1016/0042-6822(63)90312-x. [DOI] [PubMed] [Google Scholar]
- HAYFLICK L., MOORHEAD P. S. The serial cultivation of human diploid cell strains. Exp Cell Res. 1961 Dec;25:585–621. doi: 10.1016/0014-4827(61)90192-6. [DOI] [PubMed] [Google Scholar]
- HULL R. N., CHERRY W. R., TRITCH O. J. Growth characteristics of monkey kidney cell strains LLC-MK1, LLC-MK2, and LLC-MK2(NCTC-3196) and their utility in virus research. J Exp Med. 1962 May 1;115:903–918. doi: 10.1084/jem.115.5.903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hummeler K., Koprowski H., Wiktor T. J. Structure and development of rabies virus in tissue culture. J Virol. 1967 Feb;1(1):152–170. doi: 10.1128/jvi.1.1.152-170.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kondo A. Growth characteristics of rabies virus in primary chick embryo cells. Virology. 1965 Oct;27(2):199–204. doi: 10.1016/0042-6822(65)90160-1. [DOI] [PubMed] [Google Scholar]
- MACPHERSON I., STOKER M. Polyoma transformation of hamster cell clones--an investigation of genetic factors affecting cell competence. Virology. 1962 Feb;16:147–151. doi: 10.1016/0042-6822(62)90290-8. [DOI] [PubMed] [Google Scholar]
- MCCARTHY K., TAYLOR-ROBINSON C. H., PILLINGER S. E. ISOLATION OF RUBELLA VIRUS FROM CASES IN BRITAIN. Lancet. 1963 Sep 21;2(7308):593–598. doi: 10.1016/s0140-6736(63)90393-3. [DOI] [PubMed] [Google Scholar]
- POMERAT C. M., SLICK W. C. Isolation and growth of endothelial cells in tissue culture. Nature. 1963 Jun 1;198:859–861. doi: 10.1038/198859a0. [DOI] [PubMed] [Google Scholar]
- Pagano J. S., Vaheri A. Enhancement of infectivity of poliovirus RNA with diethylaminoethyl-dextran (DEAE-D). Arch Gesamte Virusforsch. 1965;17(3):456–464. doi: 10.1007/BF01241201. [DOI] [PubMed] [Google Scholar]
- SMULL C. E., LUDWIG E. H. Enhancement of the plaque-forming capacity of poliovirus ribonucleic acid with basic proteins. J Bacteriol. 1962 Nov;84:1035–1040. doi: 10.1128/jb.84.5.1035-1040.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WARREN J., WEIL M. L. Purification of certain viruses by use of protamine sulfate. Proc Soc Exp Biol Med. 1949 Dec;72(3):662–664. doi: 10.3181/00379727-72-17535. [DOI] [PubMed] [Google Scholar]
- WIKTOR T. J., FERNANDES M. V., KOPROWSKI H. CULTIVATION OF RABIES VIRUS IN HUMAN DIPLOID CELL STRAIN WI-38. J Immunol. 1964 Sep;93:353–366. [PubMed] [Google Scholar]
- Wiktor T. J., Kaplan M. M., Koprowski H. Rabies and lymphocytic choriomeningitis virus (LCMV) infection of tissue culture; enhancing effect of LCMV. Ann Med Exp Biol Fenn. 1966;44(2):290–296. [PubMed] [Google Scholar]
- Wiktor T. J., Koprowski H. Propagation of rabies virus in tissue culture. Monogr Ser World Health Organ. 1966;23:173–178. [PubMed] [Google Scholar]