Abstract
Pectin is a heteropolysaccharide which has been investigated for the development of colon-specific drug delivery systems. Polymers have been associated with pectin to reduce its aqueous solubility and improve the performance of drug delivery systems. Pectin–casein interaction is widely known in food research, but it has not been fully considered by pharmaceutical scientists. Thus, this study investigated the potential of casein–pectin microparticles as a drug delivery system and clarified the impact of cross-linking and drying methods on the in vitro release of indomethacin (IND) or acetaminophen (PCT) from microparticles. Microparticles were prepared by coacervation and dried by spray or spouted bed methods. Drug recovery, in vitro drug release, size, morphology, and the thermal and diffractometric properties of dried microparticles were determined. Spray-dried non-cross-linked microparticles were able to prolong IND release, and pectin was still degraded by pectinolytic enzymes. On the other hand, glutaraldehyde cross-linking prevented the enzymatic breakdown of pectin without improving IND release. Spouted bed drying reduced IND recovery from all microparticles when compared with spray drying, thus the successful spouted bed drying of microparticles depends on the chemical characteristics of both the drug and the polymer. Release data from PCT microparticles suggested that the microparticle formulation should be improved to bring about a more efficient delivery of water-soluble drugs. In conclusion, casein–pectin microparticles show great potential as a drug delivery system because casein reduces the water solubility of pectin. The drying method and cross-linking process had significant effects on the in vitro performance of these microparticles.
KEY WORDS: coacervation, polymeric biomaterials, polymeric drug carrier, spouted bed, spray drying
INTRODUCTION
Pectin is a non-starch natural heteropolysaccharide which is degraded by colonic bacterial enzymes but is resistant to enzymes of the stomach and small intestine (1). The selective digestibility of pectin makes it suitable for the development of colonic drug delivery systems (1,2). However, pectin on its own swells on contact with aqueous media and leads to drug release in the upper gastrointestinal tract and thereby prevents successful colonic delivery (1,2).
To reduce the aqueous solubility of pectin, several polymers have been associated with it, as presented by Liu et al. (3). Caseins are proline-rich milk proteins, which are insoluble in low pH aqueous media. Food researchers have long been interested in the ability of pectin to stabilize casein micelles during milk drink acidification, which prevents casein aggregation (4). Until recently, however, the potential of the casein–pectin interaction has not been considered by pharmaceutical scientists. Rediguiere et al. (4) studied the complexation of pectin and casein in a calcium-free, low pH aqueous mixture using pectin concentrations higher than those studied by food scientists. These authors reported the formation of a microparticulate system by coacervation under mild conditions.
Coacervation, as studied by Rediguiere and co-workers, is an attractive method for the production of microparticles because it reaches very high payloads (5), but the isolation and drying steps make the processing of this microencapsulation technique very expensive (5). Thus, drying methods for processing these coacervates must be either designed or adapted.
Coacervates are usually dried by spray, fluidized bed, or freeze drying. Spray drying has been widely used for this purpose because of its ability to dry heat sensitive materials and provide easy control over the particles' properties. Spouted bed drying is a fluidization technique which yields high thermal efficiency at low cost. The application of spouted beds overlaps with those of fluidized beds (6), and spouted beds are considered a low cost option (7,8) for processing low tonnage products typically found in the pharmaceutical industry (9). Drying paste-like materials in a spouted bed requires movement of the inert bodies added to the system, induced by direct contact of the hot air stream with the film formed by spreading the paste on the surface of the inert bodies. Once dried, the film is removed from the inert surface by the mechanical attrition caused by spouting (7). This technique has been neglected in the pharmaceutical literature. There are few studies showing its application in drying pharmaceutical pastes such as plant extracts and microcapsule dispersions (10–12). The impressive performance of a spouted bed in drying microparticles containing probiotics (13) shows the flexibility of this drying method. Applications of spouted beds for drying pharmaceuticals have been reported by Marreto et al. (14).
The aim of this study was to evaluate casein–pectin coacervates as a multiple-unit drug delivery system designed for the colonic drug delivery of two model drugs with different solubilities: acetaminophen (PCT) and indomethacin (IND). The effect of chemical cross-linking and the drying method (spouted or spray drying) on drug release, microcapsule digestibility, and structural integrity was investigated.
MATERIAL AND METHODS
Materials
Genus® USP type citric pectin (degree of esterification 68% and molar mass between 110 and 130 kg/mol) was obtained from CP Kelco S/A (São Paulo, Brazil). Bovine milk casein was purchased from Kauffman & Co. (Germany). Caseinate dispersion was analyzed by Rediguiere et al. (4) and was shown to be made up of αs-casein (32 kDa), β-casein (29 kDa), and κ-casein in a 4:1:4:1.3 (αs1/αs2/β/κ) ratio. Pectinex® Ultra SP-L was kindly donated by Novozymes S/A (Paraná, Brazil). Indomethacin and acetaminophen (pharmaceutical grade) were purchased from Henrifarma LTDA (São Paulo, Brazil). All other materials used were of analytical grade.
Methods
Preparation of Microparticles
Non-cross-linked microparticles were prepared by individually dispersing 8 g of pectin or casein in 100 mL of distilled water (solid content 8%, w/v) under constant mechanical stirring. To prepare casein, the dispersion pH was adjusted to 8.0 using 4.0 M sodium hydroxide. After complete dispersion, 100 mL of the pectin dispersion was slowly added to 100 mL of the casein dispersion, and the pH of the mixture was adjusted to 8.0 ± 0.1 using 4.0 M sodium hydroxide. Eight grams of IND or PCT was coarsely dispersed in distilled water (20 mL) and added to the casein–pectin dispersion (2:1, polymer/drug weight ratio). For the IND, 0.02% (w/v) sodium lauryl sulfate was added to the drug dispersion.
After 12 h of mechanical stirring (800 rpm), microparticles were obtained by slowly and gradually reducing the pH to 3.5 ± 0.1 with 1.0 M citric acid. After that, in some formulations, the microparticle walls were hardened by adding glutaraldehyde (50 μL/g polymer) with constant stirring for an additional 30 min (cross-linked microparticles).
Non-coacervated microparticles (IND–pectin or IND–casein microparticles) were prepared by dispersing the individual polymers in distilled water (16 g in 200 mL) under mechanical stirring (800 rpm). In both cases, the pH of the dispersion was adjusted to 8.0 with 4.0 M sodium hydroxide. This was followed by 12 h of mechanical stirring and a new adjustment of pH to 3.5 with 1.0 M citric acid. Drug incorporation was performed by adding an IND aqueous dispersion to the polymer dispersions at pH 8.0 (2:1, polymer/drug weight ratio). Drug dispersion was prepared by adding 8 g of the drug to 20 mL of a 0.02% (w/v) sodium lauryl sulfate aqueous solution.
Drying Procedures
The spouted bed unit consisted of a cylindrical column measuring 140 mm in diameter and 830 mm in height attached to a conical base with a 60° internal angle and an air inlet orifice with a diameter of 22 mm. The inert bodies were spherical beads with a narrow size distribution and an average diameter of 2.6 mm. Spouting air was supplied by a 30 HP compressor (120 SCFM, Wayne, Santa Catarina, Brazil), and its temperature was measured and kept constant by a digital PID device (HW2000, Coel, São Paulo, Brazil) connected to a 4-kW electric heater. Microparticle dispersions were not sprayed but dropped onto the spouting bed of inert bodies through a vertical tube centrally located above the particle fountain, with the help of a peristaltic pump (LS Masterflex, Illinois, USA). The dried powder was collected in a glass flask using a Lapple cyclone. Figure 1 presents a schematic diagram of the experimental apparatus, and Table I lists the details of the experimental conditions.
Fig. 1.

Schematic diagram of the spouted bed facility
Table I.
Summary of Experimental Conditions in Spray and Spouted Bed Drying
| Drying conditions | Spray drying | Spouted bed |
|---|---|---|
| Tgi (°C) | 220 | 110 |
| Tgo (°C) | 95 | 95 |
| Vms (m3/h) | 77.34 | 20.32 |
| WMEC (L/h) | 1.62 | 1.62 |
| We (L/h) | 0.870 | 0.168 |
| η | 0.4120 | 0.9084 |
| H/H max | – | 50 |
| V/V ms | – | 1.3 |
Tgi inlet temperature, Tgo outlet temperature, Vms minimum spouting air volume, V spouting air volume, WMEC maximum water evaporating capacity, We dispersion feed outflow, η thermal efficiency, H max maximum spoutable bed height (28 cm), H bed height
Spray drying was carried out with a laboratory scale mini spray drier model LM-MSD 1.0 (Labmaq LTDA, Ribeirão Preto, Brazil) using a double fluid type atomizer nozzle with an orifice of 0.7 mm. Table I lists the experimental conditions applied in this study. For a better comparison of the two drying procedures, the maximum capacities of water evaporation were calculated at the same outlet temperature and kept at the same level for both procedures.
Optical and Scanning Electron Microscopy
The structural features of microparticles were investigated by optical microscopy using a Leica DMLB microscope equipped with a Leica MPS 30 camera (Germany). Size analysis was performed using ImageJ software (NIH, Maryland, USA).
For the scanning electron microscopic analysis, microparticles were attached to an SEM stub using a two-sided adhesive tape. The specimens were coated with gold using a model SCD-050 Bal Tec sputter coater (Austria) and analyzed using a JSM 5200 Jeol scanning electron microscope (Japan).
Drug Recovery from Microparticles
Drugs were exhaustively extracted from spouted bed and spray-dried microparticles. For IND extraction, 40 mg of the microparticles was added to 4 mL of a 1:1 (v/v) mixture of methanol/sodium dibasic phosphate buffer (pH 8.0). The mixture was mechanically agitated for 15 min and then centrifuged. The supernatant was then quantified spectrophotometrically at 318 nm. The precipitate was subjected to four additional extraction steps, using the procedure described above. The sum of the material recovered from each of these extractions was considered the total drug recovery from the samples. The analyses were carried out in triplicate.
The same procedure was used for PCT extraction from dried microparticles, except that methanol was replaced by ethanol in the solvent system. The spectrophotometric detection was performed at 243 nm.
Mass Balance Study for IND Casein–Pectin Microparticles in the Spouted Bed Drying
A mass balance experiment was carried out while drying the IND microparticles in a spouted bed to assess the possibility of drug segregation during the drying operation. Microparticles from the collector flask were extracted as described above. Microparticles which adhered to the spouted bed walls, inert bodies, and filter bag were also analyzed. For assaying IND in the microparticles adhering to inert bodies, 200 g of inert bodies was rinsed with a solution of methanol–phosphate buffer (1:1, v/v). The rinsing solutions were centrifuged and the supernatant was assayed at 318 nm. The difference between the weight of the inert bodies before and after rinsing was considered the microparticle weight of the sample. Microparticles which had adhered to the drying wall chamber or those collected by the filter bag were extracted and quantified, as previously described. All analyses were carried out in triplicate.
Physicochemical Characterization of IND Microparticles
Differential scanning calorimetry analyses were performed in a Shimadzu calorimeter (DSC-50, Japan) from room temperature to 200°C. Each sample (±4.5 mg) was analyzed under nitrogen flux (50 mL/min) at 10°C/min after calibrating the temperature and heat of fusion using metallic indium and zinc standards. Baseline calibration was performed according to the manufacturer's instructions. All analyses were performed in triplicate.
Fourier transform infrared spectra were collected with a PerkinElmer RXIPF-IR (Massachusetts, USA). The samples were dispersed in KBr by grinding the powders in an agate mortar and compressing the sample. The compressed sample was then scanned in the range of 400–4,000 cm−1 at 2 cm−1 resolution.
In Vitro Release Studies
To examine the effects of the cross-linking process and drying method on IND and PCT release from microparticles, studies were carried out using polyethylene baskets covered with nylon and prepared with the same dimensions as the USP dissolution apparatus I (Sotax AT7, Germany). The apparatus was operated at 50 rpm and 37 ± 0.1°C.
At the beginning of the experiment, the dissolution medium was 375 mL of the 0.1 M HCl solution (pH 1.2 ± 0.05). After 2 h, 125 mL of the 0.2 M sodium tribasic phosphate buffer was added (pH 6.8 ± 0.05), and after 6 h, 15 mL of the same buffer was added to pH 7.4 ± 0.05. One milliliter of the pectinolytic enzymes (Pectinex® Ultra SP-L, Novozymes S/A) was added to the dissolution medium after 6 h. All analyses were performed under sink conditions (100 mg of the IND-loaded microcapsules and 300 mg of the PCT) and in triplicate.
Aliquots (5 mL) were collected during the experiment and replaced by an equal volume of the dissolution media. The material collected was filtered and spectrophotometrically assayed at 243 nm (PCT) or 318 nm (IND). PCT or IND analytical curves were prepared by diluting the drugs in the solvent system of the same composition as the dissolution media.
Data from in vitro release studies were fitted to Higuchi and first-order equations (Excel®, Microsoft Corporation). The expression in Eq. 1 was used to solve the Higuchi model.
| 1 |
where Ft is the amount of drug released in time t, K is the Higuchi dissolution constant, and t is time.
RESULTS
Microparticle Morphology and Size Analysis
The morphologic characteristics of the microparticles can be observed in Fig. 2. As prepared, the empty microparticles exhibited a predominantly spherical shape (Fig. 2a), which was maintained after spray drying (Fig. 2b). The incorporation of the poorly soluble drug, IND, produced more irregular spray-dried microparticles (Fig. 2c) with some large structures which could be drug crystals coated by polymer (Fig. 2d). The incorporation of PCT led to a similar effect but to a lesser degree (Fig. 2e).
Fig. 2.
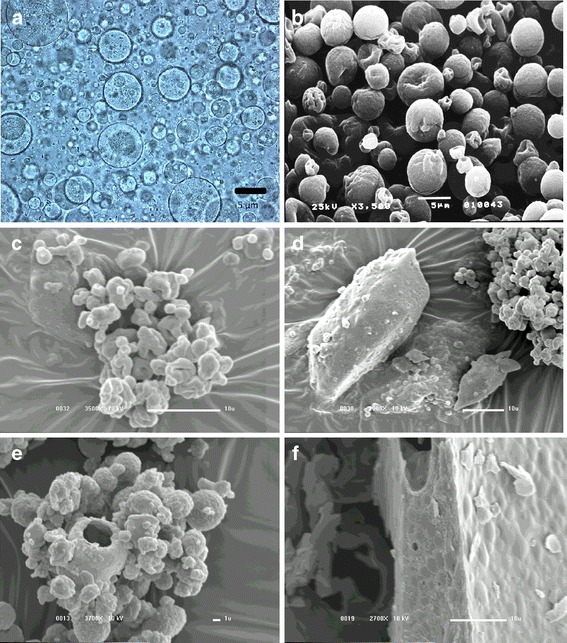
Outer morphology of casein–pectin microparticles observed by optical microscopy (a) or SEM (b–f). Empty (a), spray dried (b), indomethacin (c, d) and acetaminophen (e) loaded spray-dried microparticles, and empty spouted bed-dried microparticles (f). Magnification, ×400 (a); ×3,500 (b, c); ×2,000 (d); ×3,700 (e); ×2,700 (f)
Empty spouted bed microparticles (Fig. 2f) were irregular in shape with clusters which could be attributed to microparticle coalescence on the inert body surface. In contrast to spray-dried microparticles, drug incorporation did not affect the morphology of the spouted bed microparticles (data not shown). The cross-linking process did not result in any modifications to the morphology of the microparticles dried by either method studied here (data not shown).
The mean diameter (Dp) of the microparticles was affected by both drug incorporation and drying method (Table II). Spray drying slightly decreased the Dp of empty microparticles as a result of solvent losses. The incorporation of the poorly soluble drug IND increased the Dp in spray-dried microparticles to a greater degree than did the incorporation of PCT (Table II). On the other hand, the coalescence of empty microparticles increased the particle size after spouted bed drying, but in this case, Dp was not affected by drug incorporation. The cross-linking process did not affect the Dp for either the spray- or spouted bed-dried microparticles (Table II).
Table II.
Mean Particle Size of As-Prepared and Dried Microcapsules
| Formulation | Dp (μm) |
|---|---|
| EM | 7.4 |
| EM-SD | 6.2 |
| EMC-SD | 6.4 |
| IND-MC-SD | 9.9 |
| PCT-MC-SD | 7.3 |
| EMC-SB | 28.2 |
| EM-SB | 27.3 |
| IND-MC-SB | 25.4 |
| PCT-MC-SB | 27.5 |
EM as-prepared empty cross-linked microparticles, EM-SD empty spray-dried microparticles, EMC-SD empty cross-linked spray-dried microparticles, IND-MC-SD indomethacin cross-linked spray-dried microparticles, PCT-MC-SD acetaminophen cross-linked spray-dried microparticles, EMC-SB empty cross-linked spouted bed microparticles, EM-SB empty spouted bed microparticles, IND-MC-SB indomethacin cross-linked spouted bed microparticles, PCT-MC-SB acetaminophen cross-linked spouted bed microparticles
Dissolution Studies
Figure 3a shows the percentage of IND released as a function of time from non-cross-linked microparticles during conditions mimicking mouth to colon transit. In all curves presented, IND release in the first 2 h of the experiment was very low because of the poor solubility of this drug in the acidic environment. After 2 h, a sodium tribasic phosphate buffer was added to pH 6.8, after which the release profile of the IND from non-cross-linked spray-dried microparticles followed two phases. The first release phase (from 150 to 360 min) showed a lower IND dissolution when compared with the free IND crystals, and the Higuchi equation fits the data better than the first-order kinetics equation (Table III). The second release phase (from 360 to 450 min) started after adding pectinolytic enzymes, which resulted in a rapid drug release. The Higuchi equation fits the data from the second phase release better than the first-order equation, but it is important to note that the correlation coefficient values are very close in this case (Table III). In contrast, the release profile of the IND from spouted bed non-cross-linked microparticles (Fig. 3a) did not show bimodal behavior and exhibited rapid IND dissolution, similar to free IND. The Higuchi correlation coefficient was similar to that of the first order and the Higuchi K value was far superior (Table III) when compared with the values obtained for spray-dried microparticles in the first or second release phases.
Fig. 3.
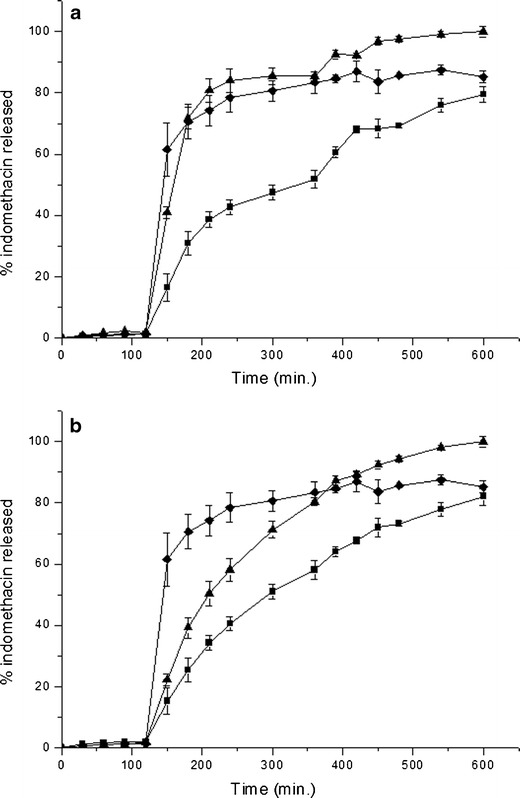
In vitro release profiles of indomethacin from non-cross-linked (a) and cross-linked (b) dried microparticles. Free indomethacin (black diamond); spray-dried microparticles (black square); spouted bed-dried microparticles (black triangle)
Table III.
Linearization of Release Data from Casein–Pectin Microparticles
| Microparticle formulation | Higuchi model | First-order kinetics | ||
|---|---|---|---|---|
| CD | K | CD | K | |
| IND non-cross-linked spray dried | 0.8787a | 4.86a | 0.7077a | 0.8790a |
| 0.8988b | 7.71b | n.a.b | n.a.b | |
| IND cross-linked spray dried | 0.9806 | 5.65 | 0.8480 | n.a. |
| IND non-cross-linked spouted bed dried | 0.8482 | 19.62 | 0.8122 | n.a. |
| IND cross-linked spouted bed dried | 0.9429 | 5.98 | 0.7979 | n.a. |
I ND indomethacin, n.a. not assessed, K rate constant, CD correlation coefficient
aFirst release phase
bSecond release phase
Figure 3b shows the release profiles of the IND from cross-linked microparticles. Spray-dried microparticles showed a gradual, monomodal IND release. The IND release from spouted bed cross-linked microparticles (Fig. 3b) showed similar profiles when compared with the spray-dried microparticles. In both cases, the Higuchi equation fits the data better than that of the first order (Table III). The analysis of Fig. 3a, b showed a higher IND release from spouted bed microparticles when compared to free IND or spray-dried microparticles. This could be attributed to the normalization performed on release data from cross-linked and non-cross-linked spouted bed microparticles, which showed a low IND recovery.
PCT release profiles from spray- and spouted bed-dried microparticles are shown in Fig. 4a, b (non-cross-linked microparticles and cross-linked microparticles, respectively). In both cases, the total drug release occurred within 210 min. The Higuchi equation fits these data better than that of the first order for all PCT formulations (correlation coefficients higher than 0.9610). Higuchi K values for cross-linked and non-cross-linked spouted bed microparticles were 6.05 and 5.91, respectively. For cross-linked and non-cross-linked spray-dried microparticles, Higuchi K values were 7.35 and 4.80, respectively.
Fig. 4.
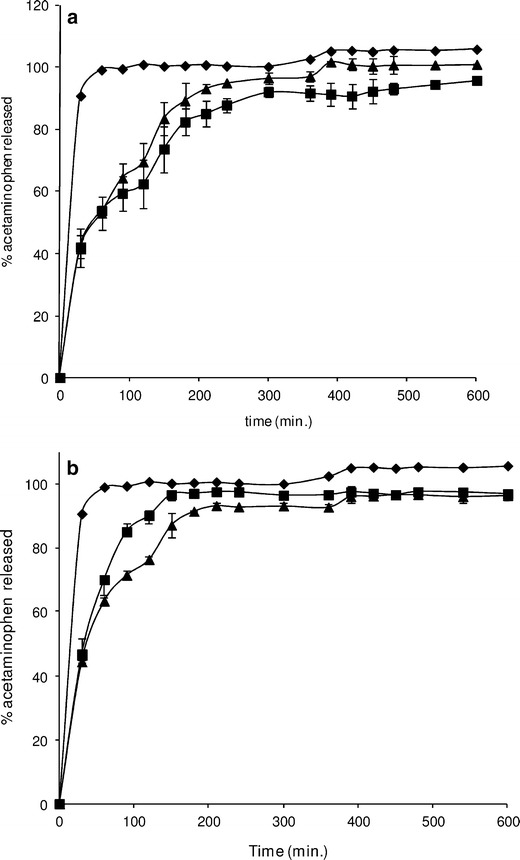
In vitro release profiles of acetaminophen from non-cross-linked (a) and cross-linked (b) dried microparticles. Free acetaminophen (black diamond); spray-dried microparticles (black square); spouted bed-dried microparticles black triangle)
Drug Recovery and Mass Balance Study
IND concentration in the assay solutions showed incomplete drug recovery from spouted bed microparticles (31.71 ± 0.28%) in contrast to spray-dried particles (99.04 ± 4.30%). On the other hand, total PCT recovery was achieved from microparticles dried by spouted bed drying or spray drying.
A mass balance was carried out to assess the possibility of IND crystals adhering to the spouted bed (on the surface of inert bodies or the wall of the chamber). Material collected from inert particles showed an IND content of 31.06% (±4.07%), similar to that in microparticles from the collector flask. Powder accumulation in the filter bag was negligible, demonstrating that drug drag did not occur in the air stream. In contrast, IND recovery from microparticles adhering to the wall chamber was complete (94.91 ± 6.09%) but not superior to the expected theoretical value, which would indicate that no drug segregation occurred on the wall.
Casein–IND and pectin–IND microparticles were prepared and dried in a spouted bed to determine which polymeric component was implicated in the low IND recovery. These dispersions were dried immediately after their preparation, and the dispersions were kept under constant mechanical stirring to prevent solid sedimentation. Drug recovery was only incomplete after IND–casein spouted bed drying (29.39%), which showed that casein was involved in the low drug recovery from casein–pectin microparticles. So, the IND–casein microparticles were subjected to additional physicochemical characterization.
Differential scanning calorimetry (DSC) was used for the thermal characterization of the free drug and IND–casein microparticles. The melting point of the non-processed IND crystals was 160.56°C (Table IV), which is typical of the γ polymorphic form (15). Free IND crystals dried by spray or spouted bed methods showed similar melting point temperatures with a decrease in the enthalpy of fusion (Table IV) when compared with non-processed IND crystals. This decrease denotes an increase in crystal energy and reactivity after drying, which is expressed by the entropy of processing (∆Ps) parameter (16). ∆Ps was more pronounced in spouted bed-dried crystals (∆Ps, 23.13) when compared with spray-dried samples (∆Ps, 10.78).
Table IV.
Melting Point and Enthalpy of Fusion of the Indomethacin
| Material | Melting point (°C) ± DP | ΔH (J/g) ± DP | % theoretical value expected |
|---|---|---|---|
| INDN | 160.53 ± 0.10 | −115.81 ± 3.20 | 100.00 |
| IND-SD | 159.96 ± 0.06 | −110.58 ± 2.23 | 95.50 ± 1.92 |
| IND-SB | 160.27 ± 0.15 | −105.26 ± 0.18 | 90.90 ± 0.15 |
| IND-CAS | 158.83 ± 0.19 | −28.83 ± 4.96 | 78.20 ± 13.4a |
| MP-SD | 159.26 ± 0.31 | −28.43 ± 4.31 | 77.13 ± 14.9a |
| MP-SB | 157.58 ± 0.22 | −4.25 ± 0.11 | 12.11 ± 0.38a |
INDN non-processed indomethacin free crystals, IND-SD spray-dried free indomethacin, IND-SB spouted bed-dried free indomethacin, IND-CAS physical indomethacin/casein mixture (1:2, w/w), MP-SD spray-dried microparticles, MP-SB spouted bed-dried microparticles
aRelated to the expected enthalpy of fusion of the spray-dried indomethacin crystals
Table IV also presents the IND melting points and the enthalpy of fusion of the IND in the IND–casein physical mixture, spouted bed, and spray-dried casein microparticles. These results show that spray drying has little effect on the melting point and enthalpy of fusion of the IND incorporated in the microparticles, which are close to those of the physical mixture. In contrast, the enthalpy of fusion of the drug was significantly reduced after spouted bed drying, although there was no significant shift in the peak of fusion (Table IV).
The infrared spectra (data not shown) supported the thermal findings, which would suggest the presence of IND γ crystals. This finding is in harmony with that of Taylor and Zografi (17). Furthermore, the FT-IR spectra did not show any solid-state changes in free IND crystals after processing. However, the acid carbonyl stretching vibration peak (1,717 cm−1) and benzoyl stretching peak (1,692 cm−1) which are typical of the γ IND (17) were not observed after the spouted bed drying of the microparticles. Physicochemical characterization of PCT microparticles indicated the absence of solid-state changes or drug–polymer interactions (data not shown).
DISCUSSION
Interactions between polysaccharides and proteins can enhance food quality and produce innovative cosmetic and pharmaceutical products (4). The interaction between casein and pectin was studied in calcium-free solutions with low pH values and high biopolymer concentrations. This interaction resulted in the formation of microparticles under acidic pH (4). In this study, these casein–pectin microparticles as a drug delivery system were evaluated as was the influence of the cross-linking process and drying method on the characteristics of these microparticles.
The shape and mean diameter (Dp) of the spray-dried casein–pectin microparticles were affected by the type of drug incorporated. PCT-loaded microparticles showed a more spherical shape and a lower Dp because PCT has a smaller crystalline fraction than IND due to the partial solubilization of PCT during the coacervation procedure. The shape and Dp of the microparticles were also affected by the drying method. Dispersions in spouted beds are dried by spreading the material on the surface of inert bodies, removing the water and then forming a dried film. The coalesced microparticles are removed as agglomerates of higher dimensions and irregular shape.
Release studies were carried out to determine the in vitro performance of casein–pectin microparticles dried by spray or spouted bed methods and to determine the effect of the cross-linking process on this performance. Rediguieri and co-workers (4) showed that complexation in casein–pectin mixtures is a reversible phenomenon and pectin molecules migrate from the particles to the bulk phase at high pH. The release profile of IND from spray-dried non-cross-linked microparticles (Fig. 3a) suggested that pectin migration to the bulk phase at pH 6.8 was not fast enough to lead the microparticles to lose their structural integrity. It also showed that pectinolytic enzymes were still able to degrade pectin on the surface of the non-cross-linked microparticles. In contrast, cross-linking inhibited enzymatic action without further prolonging IND release from the microparticles (Fig. 3b). Modifications in the enzymatic susceptibility of pectin may result from the reaction of the terminal aldehyde groups of the cross-linker with the hydroxyl groups of the pectin chains forming a compact cross-linked network (18). However, it is much more probable that the effect of the glutaraldehyde on microparticle properties is related to a chemical reaction to the amino groups of casein. This reaction may also inhibit enzyme access to pectin sites, thus maintaining the structural integrity of the microparticles. It should be noted that the maintenance of enzymatic susceptibility through microbial triggering is a basic characteristic of materials used for colon-specific drug delivery systems. This requirement suggests that the cross-linking process has a negative impact on the in vitro drug delivery performance of these casein–pectin microparticles.
Most of the multiple-unit systems based on pectin—designed for achieving colon delivery or for prolonging drug release—are pellets or beads cross-linked with calcium ions and prepared by inotropic gelation techniques. A comparison of the in vitro release performance of the casein–pectin microparticles developed here with the data in the literature was limited by the differences in particle size, composition (type of pectin, cross-linking agent, drug-to-polymer ratio), release test conditions, and the techniques used for particle preparation.
However, indomethacin-loaded pectin microparticles (≈10 μm) have previously been prepared by spray drying (19). Non-cross-linked microparticles released indomethacin very quickly (about 90% in less than 2 h at pH 7.4). In this study, indomethacin release from spray-dried non-cross-linked microparticles prepared by coacervation was much more prolonged, which would indicate a reduction in the solubility of pectin due to complexation with casein.
The behavior of the spouted bed-dried casein–pectin microparticles was very different from that of the spray-dried ones. A high release rate constant was calculated for spouted bed non-cross-linked microparticles, which could be attributed to microparticle rupture during the drying operation. This finding suggests that the cross-linking agent influences the mechanical properties of the microparticles, since the IND release rate constant for cross-linked spouted bed microparticles was similar to that observed for cross-linked spray-dried microparticles. This finding indicates that glutaraldehyde cross-linking allowed casein–pectin microparticles to resist the mechanical stress imparted by the movement of the inert bodies.
Despite the low processing costs of spouted bed technology and its potential for application in the pharmaceutical industry, this technique presented no advantages for drying IND casein–pectin microparticles. Only cross-linked microparticles could be successfully dried by this technique, without any improvement in the release profile. Spouted bed drying also led to low IND recovery from the microparticles; this finding is in harmony with Baracat et al. (11).
The investigation of low drug recovery must include studies on drug–excipient interactions, evaluations of drug segregation during processing, and a determination of the suitability of the extraction method (20). The high recovery of IND spray-dried microparticles showed the enhanced extraction capability of the binary solvent system used here. The polymeric component implicated in IND low recovery was casein, since total IND recovery was obtained from spouted bed-dried pectin microparticles.
According to Marini et al. (21), modifications in DSC curves, such as a reduction in the enthalpy of fusion and a shift of the peak of fusion, are considered to be indications of the physicochemical interactions between drugs and excipients. DSC curves from IND–casein microparticles dried by spouted bed showed a reduction in enthalpy without any significant modifications in melting temperatures. This finding in conjunction with the infrared findings would suggest the existence of an interaction between IND and casein under spouted bed drying conditions. This interaction seems to be irreversible under the conditions of the release test, since the total drug release at the end of the experiments was similar to that observed after the exhaustive extraction procedure.
Bed temperature and residence time distributions (RTD) determine the thermal resistance of the materials in drying operations (22). However, in this study, RTDs seem to have had secondary importance only, as total IND recovery was obtained from microparticles which had adhered to the wall of the spouted bed chamber. It is likely that microparticles adhering to the chamber wall are exposed to heat for a longer time than microparticles collected in the collector flask, and this difference suggests that there is a relationship between the mechanical stress caused by inert particle shocking and low IND recovery.
Total PCT recovery was obtained from all the formulations tested here. PCT release from casein–pectin microparticles was very rapid irrespective of the cross-linking process and drying method used (Fig. 4a, b). This finding was in harmony with previously reported results on the release behavior of small water-soluble compounds from pectin matrices (3).
CONCLUSIONS
Casein–pectin microparticles were able to prolong indomethacin release, and the casein–pectin interaction did not prevent an enzymatic attack on the polysaccharide. The results also showed that the drying method selected affected product quality and that successful spouted bed drying of microparticles depends on the mechanical strength of the particles and the chemical characteristics of the drug and polymer.
Acetaminophen-loaded microparticles were successfully processed by both spouted bed and spray drying methods, but the casein–pectin formulation must be improved if water-soluble drugs are to be delivered more efficiently. The drug-to-hydrocolloid ratio and ratio of pectin to casein could drastically affect the release profile and these formulation parameters should be investigated in order to improve microcapsule performance.
ACKNOWLEDGMENTS
Financial support from FAPESP (03/09447-6) and CNPQ (PQ-II) is gratefully acknowledged.
REFERENCES
- 1.Das S, Desmukh R, Jha AK. Role of natural polymers in the development of multiparticulate systems for colonic drug targeting. Syst Rev Pharm. 2010;1:79–85. doi: 10.4103/0975-8453.59516. [DOI] [Google Scholar]
- 2.Wong TW, Colombo G, Sonvico F. Pectin matrix as oral drug delivery vehicle for colon cancer treatment. AAPS PharmSciTech. 2011;12:201–214. doi: 10.1208/s12249-010-9564-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Liu LS, Fishman ML, Kost J, Hicks KB. Pectin-based systems for colonic-specific drug delivery via oral route. Biomaterials. 2003;24:3333–3343. doi: 10.1016/S0142-9612(03)00213-8. [DOI] [PubMed] [Google Scholar]
- 4.Rediguieri CF, Freitas O, Lettinga MP, Tuinier R. Thermodynamic incompatibility and complex formation in pectin/caseinate mixtures. Biomacromolecules. 2007;8:3345–3354. doi: 10.1021/bm7004438. [DOI] [PubMed] [Google Scholar]
- 5.Gouin S. Microencapsulation: industrial appraisal of existing technology and trends. Trends Food SciTechnol. 2004;15:330–347. doi: 10.1016/j.tifs.2003.10.005. [DOI] [Google Scholar]
- 6.Epstein N, Grace JR. Spouting of particulate solids. In: Fayed ME, Otten L, editors. Handbook of powder science and technology. New York: Chapman and Hall; 1997. pp. 532–567. [Google Scholar]
- 7.Passos ML, Massarani G, Freire JT, Mujumdar AS. Drying of pastes in spouted beds of inert particles: design criteria and modeling. Dry Technol. 1997;15:605–624. doi: 10.1080/07373939708917249. [DOI] [Google Scholar]
- 8.Markowski AS. Quality interactions in a jet spouted bed dryer for bio-products. Dry Technol. 1993;11:369–387. doi: 10.1080/07373939308916825. [DOI] [Google Scholar]
- 9.Pakowski Z, Mujumdar AS. Drying of pharmaceutical products. In: Mujumdar AS, editor. Handbook of industrial drying. New York: Marcel Dekker; 1995. pp. 743–773. [Google Scholar]
- 10.Shuhama IK, Aguiar ML, Oliveira WP, Freitas LAP. Experimental production of annatto powders in spouted bed dryer. J Food Eng. 2003;59:93–97. doi: 10.1016/S0260-8774(02)00433-8. [DOI] [Google Scholar]
- 11.Baracat MM, Nakagawa AM, Freitas LAP, Freitas O. Microcapsule processing in a spouted bed. Can J Chem Eng. 2004;82:134–141. doi: 10.1002/cjce.5450820117. [DOI] [Google Scholar]
- 12.Runha FP, Cordeiro DS, Pereira CAM, Vilegas J, Oliveira WP. Production of dry extracts of medicinal Brazilian plants by spouted bed process: development of the process and evaluation of thermal degradation during the drying operation. Trans Inst Chem Eng. 2001;79:1–9. doi: 10.1205/026387601528408. [DOI] [Google Scholar]
- 13.Oliveira AC, Moretti TS, Boschini C, Baliero JCC, Freitas LAP, Freitas O, et al. Microencapsulation of B. lactis (BI01) and L. acidophilus (LAC4) by complex coacervation followed by spouted bed drying. Dry Technol. 2007;25:1687–1693. doi: 10.1080/07373930701590939. [DOI] [Google Scholar]
- 14.Marreto RN, Freire JT, Freitas LAP. Drying of pharmaceuticals: the applicability of spouted beds. Dry Technol. 2006;24:327–338. doi: 10.1080/07373930600564324. [DOI] [Google Scholar]
- 15.Imaizumi H, Nambu N, Nagai T. Stability and several physical properties of amorphous and crystalline forms of indomethacin. Chem Pharm Bull. 1979;28:2565–2569. doi: 10.1248/cpb.28.2565. [DOI] [PubMed] [Google Scholar]
- 16.Duddu SP, Grant DJW. The use of thermal analysis in the assessment of crystal disruption. Thermochim Acta. 1995;248:131–145. doi: 10.1016/0040-6031(94)01951-C. [DOI] [Google Scholar]
- 17.Taylor LS, Zografi G. Spectroscopic characterization of interactions between PVP and indomethacin in amorphous molecular dispersions. Pharm Res. 1997;14:1691–1698. doi: 10.1023/A:1012167410376. [DOI] [PubMed] [Google Scholar]
- 18.Surajit DAS, Ka-Yun NG. Impact of glutaraldehyde on in vivo colon-specific release of resveratrol from biodegradable pectin-based formulation. J Pharm Sci. 2010;99:4903–4916. doi: 10.1002/jps.22212. [DOI] [PubMed] [Google Scholar]
- 19.Lee C-M, Kim D-W, Lee H-C, Lee K-Y. Pectin microspheres for oral colon delivery: preparation using spray drying method and in vitro release of indomethacin. Biotechnol Bioprocess Eng. 2004;9:191–195. doi: 10.1007/BF02942291. [DOI] [Google Scholar]
- 20.Cory W, Field K, Wu-Linhares D. Is the method or the process—separating causes of low recovery. Drug Dev Ind Pharm. 2004;30:891–899. doi: 10.1081/DDC-200034588. [DOI] [PubMed] [Google Scholar]
- 21.Marini A, Berbenni V, Moioli S, Bruni G, Cofrancesco P, Margheritis C, et al. Drug-excipient compatibility studies by physicochemical techniques. J Therm Anal Calorim. 2003;73:529–545. doi: 10.1023/A:1025426012578. [DOI] [Google Scholar]
- 22.Marreto RN, Peixoto MPG, Tacon LA, Freitas LAP. Paste residence time in a spouted bed dryer. I: The stimulus-response methodology. Dry Technol. 2007;25:821–830. doi: 10.1080/07373930701370191. [DOI] [Google Scholar]


