Abstract
Brinzolamide (BLZ) is a drug used to treat glaucoma; however, its use is restricted due to some unwanted adverse events. The goal of this study was to develop BLZ-loaded liquid crystalline nanoparticles (BLZ LCNPs) and to figure out the possibility of LCNPs as a new therapeutic system for glaucoma. BLZ LCNPs were produced by a modified emulsification method and their physicochemical aspects were estimated. In vitro release study revealed BLZ LCNPs displayed to some extent prolonged drug release behavior in contrast to that of BLZ commercial product (Azopt®). The ex vivo apparent permeability coefficient of BLZ LCNP systems demonstrated a 3.47-fold increase compared with that of Azopt®. The pharmacodynamics was checked over by calculating the percentage fall in intraocular pressure and the pharmacodynamic test showed that BLZ LCNPs had better therapeutic potential than Azopt®. Furthermore, the in vivo ophthalmic irritation was evaluated by Draize test. In conclusion, BLZ LCNPs would be a promising delivery system used for the treatment of glaucoma, with advantages such as lower doses but maintaining the effectiveness, better ocular bioavailability, and patient compliance compared with Azopt®.
Key words: brinzolamide, liquid crystalline nanoparticles, ocular bioavailability, ocular irritation, ophthalmic delivery
INTRODUCTION
Glaucoma is one of the major causes of blindness worldwide and is more prevalent among women and Asians. In 2010, there were around 60.5 million people with angle-closure glaucoma and open-angle glaucoma (OAG). This figure is expected to reach up to 80 million by 2020, out of which three fourth will have OAG. The number of blindness in both eyes caused by primary glaucoma was about 8.4 million in 2010. This will rise to 11.1 million by 2020 (1). Glaucoma is pathologically characterized by optic nerve and optic disk injury, visual field loss, and high intraocular pressure (IOP) which is caused by an imbalance between the production and clearance of aqueous humor. In clinic, carbonic anhydrase inhibitors based on the decrease of aqueous humor formation from ciliary body have been used for treating glaucoma since 1954 (2). Brinzolamide (BLZ) is a novel topically active carbonic anhydrase inhibitor derived from a unique family of heterocyclic sulfonamides used to lower and control elevated IOP (3). However, because of the poor aqueous solubility of BLZ, the clinical application is extremely limited. The commercial preparation of BLZ is called Azopt®, an aqueous suspension composed of 1% (w/v) BLZ. Unfortunately, this formulation is associated with side effects, such as blurred vision, pain, discomfort (stinging and burning), eye discharge, blepharitis, dry eye, and taste perversion (4–6).
The rapid and severe pre-corneal losses resulting from drainage and high tear fluid turnover lead to low drug ocular bioavailability, which is the most challenging in ocular formulation. To ameliorate ocular bioavailability, many ocular drug delivery systems have been proposed, for example, liposomes, nanoparticles, emulsion, and cubosomes (7–10).
The use of monoolein or glycerol monooleate (GMO)-based liquid crystalline (LC) phases, especially reverse hexagonal (HII) and bicontinuous cubic phase, in the drug delivery field has been reported in many works (10,11). These LC phases have the potential of controlling release rates, low toxicity, and utility in a variety of administration systems, including oral, transdermal, and parenteral delivery. An important aspect of cubic and hexagonal HII systems is that they are thermodynamically stable in water. As a result of their special structural properties, liquid crystalline phases of GMO, such as hexosomes (dispersed HII phases), have the potential to be utilized as a drug delivery carrier for active ingredients. Hexosome is formed by the emulsification of reverse hexagonal phases in water, which can be defined as nanoparticle dispersion systems. It has been proved that the dispersed particles preserved the internal structure of the bulk phase and its properties (12). Therefore, hexosomes have been proposed for solubilizing, encapsulating, and delivering active pharmaceutical elements including huge molecules such as peptide and proteins (13–15). Besides, the two-dimensional symmetry hexagonal phases can provide a complex diffusion pathway for controlled release of entrapped molecules (16).
Even though hexosomes can improve permeation of drug through skin and mucosal membrane for transdermal, dermal, and transmucosal delivery, little research has been done so far to show their potential as ophthalmic drug delivery systems (17,18).
Therefore, the goal of this study was to design an innovative carrier built on liquid crystalline nanoparticles (LCNPs) as an ocular delivery system of BLZ that would decrease ocular irritancy and promote bioavailability. BLZ LCNPs were prepared, and their internal configuration was further analyzed by transmission electron microscopy (TEM) and small angle X-ray scattering (SAXS). In vitro and in vivo profiles were studied finally taking Azopt® as a positive control.
MATERIALS AND METHODS
Materials
Brinzolamide, of 99% purity, was bought from Jinan Chenghui Shuangda Chemical Co. Ltd (Shandong, China). Azopt® was bought from Alcon (Puurs, Belgium). GMO was a mixed glyceride (RYLO MG 90, Danisco Ingredients, Brabrand, Denmark) with the following fatty acid composition: oleic acid (90 wt%), linoleic and saturated acids (6 wt%), and glycerol trioleate (GTO) (4 wt%). Pluronic F127 was bought from BASF Corp. (Ludwigshafen, Germany). Double-distilled water was prepared by Hitech-K flow Water Purification System (Hitech Instruments Co. Ltd., Shanghai, China). All other reagents were of analytical purity or higher.
Adult New Zealand rabbits (2.5–3.0 kg) were supplied by the Animal Experimental Center of Nanjing Medical University (Nanjing, China). They were conformed at 25°C and 55% of humidity under natural light/dark conditions for 1 week before procedure. All animal experimentations were performed according to the guidelines evaluated and approved by the ethics committee of Nanjing Medical University (Nanjing, China).
Preparation of BLZ LCNPs and 1% BLZ Solution
Preparation of the LCNP formulations was based on the modified emulsification of GMO and Poloxamer 407 in water as reported. The ratio of GMO/Poloxamer was 9:1 (w/w) in every experiment (11,19). GMO and Poloxamer were heated to 70°C firstly, and then, BLZ (0.5%) was placed in the molten GMO/Poloxamer solution and solubilized before adding to the aqueous phase. After that, the oil phase was dropped into the water phase and the mixture was emulsified using a high-shear dispersing emulsifier (T25 Basic, IKA Guangzhou, China) at 10,000 rpm for 5 min. Following equilibration for 12 h at room temperature, the crude emulsion was homogenized seven times (Panda 2000, GEA Niro Soavi S.P.A, Italy) at 350 bar (19). Lastly, glycerol was utilized to tune the osmotic pressure to physiological environment. Blank LCNPs were prepared in the same way without the drug.
BLZ solution (1%) was prepared by dissolving 100 mg BLZ in 10 mL simulated tear fluid (STF), using glycerol to adjust the osmotic pressure. The freshly prepared STF was manufactured using CaCl2·2H2O, 0.0084 g; KCl, 0.138 g; NaHCO3, 0.218 g; NaCl, 0.678 g; and water up to 100 g (20).
Characterization of BLZ LCNPs
HPLC Methodology
HPLC determination of BLZ concentration was carried out utilizing a Shimadzu LC-10AT, SPD-10A HPLC system (Shimadzu, Japan) at 254 nm. The column used was Hanbon Phecda C18, 250 × 4.6 mm column (Hanbon Sci. & Technology CO., Ltd, Jiangsu, China) with this solvent system: methanol/distilled water (60:40, v/v). Twenty microliter volume was injected at the flow rate of 0.8 mL/min. BLZ could be identified at a retention time of 6.3 min. Linear correlation between peak area and BLZ concentration was achieved within the concentration range of 1–100 μg/mL, with a limit of quantification of 0.25 μg/mL. The equation characterizing the calibration curves for BLZ was y = 40,112 x + 29,803 (R2 = 0.9999), where x is the concentration of BLZ and y is the peak area.
Particle Size, Osmotic Pressure, and pH
To investigate the particle size distribution of the LCNPs, photocorrelation spectroscopy (Malvern Zetasizer 3000, Malvern Instruments, Malvern, Worcestershire, UK) was used, based on quasi-elastic light scattering. The nanoparticle size was measured in triplicate after a dilution of the nanoparticle dispersion for ten times in distilled water at 25°C.
Osmotic pressure was determined by the freezing point method using a Model FM-9X Osmometer (Instrumental Factory of Shanghai Medical University, Shanghai, China).
The pH was established at 25°C employing a standardized PHS-3C pH meter (Shanghai Precision & Scientific Instrument Co., Ltd, China).
TEM
TEM was employed to determine the shape and morphology of LCNP dispersion. Preparation of the samples was carried out by inserting 5 μl droplet of the LCNPs onto a 300-mesh carbon-coated copper grid and allowing settlement of LCNPs for 3–5 min. Afterwards, the excess fluid was taken off and the grid was dried carefully in air. Analysis of the samples using a JEOL Model JEM 1010 80 kV transmission electron microscope (JEOL USA, Wilmington, DE, USA) was performed followed by digital photography on a Gatan axis-mounted 2k × 2k digital camera.
Drug Encapsulation Efficiency
For the drug encapsulation efficiency (EE) test, LCNP formulations were divided into two separate samples. The drug percentage captured by the LCNPs was indirectly calculated, following centrifugation in a membrane concentrator (Amicon Ultra 15, molecular weight cutoff (MWCO) 100 K, Millipore, Ireland) for 10 min at 21,000×g at 4°C, in a Sigma 3K30 centrifuge (Sigma-Aldrich, Germany). For the measurement of the drug concentration in the aqueous continuous phase, HPLC method described above was employed. This method was also utilized to analyze the total quantity of the drug in the LCNPs by dissolving the sample in methanol. The quantity of drug present within the LCNPs was computed by subtracting the bulk of drug in the aqueous continuous phase from the total quantity of the drug in the LCNPs. The EE of BLZ in LCNPs was concluded using the following equation:
| 1 |
where Winitial drug and Wfree drug were the total amount of the drug in LCNPs and the amount of the drug in the filtrate, respectively.
SAXS Measurement
NanoStar (Bruker AXS GmbH, Germany) was used to measure SAXS. The sample was transferred to a 0.5-mm diameter quartz capillary and sealed. The experiment was performed for 30 min in vacuum at 25°C and with a sample-to-detector interval of 10.7 cm.
In Vitro Release
Dynamic dialysis method (21) was utilized to measure the in vitro release of BLZ from LCNPs and a ZRS-8G Drug Dissolution Tester (Tianjin Medical Instrumental Factory, Tianjin, China) was used. LCNP formulations (2 mL) to be studied were pipetted into the dialysis bag (MWCO 14,000 Da; Sigma, USA) and placed into 100 mL release medium of STF and then stirred at 50 rpm in a 37 ± 0.5°C water bath. Samples (2 mL) were collected at specific time intervals (10, 20, 30, 40, 60, 120, 180, 240, 300, 360, 420, and 480 min) and instantly refilled with an identical volume of STF to keep up a sink condition (22). Samples were examined using the HPLC method as explained above.
The cumulative quantity of drug (Qn, in milligram) was plotted as a function of time (t, in minute) and computed based on the following equation:
| 2 |
where Cn stands for the drug concentration of the dissolution media at each sampling time, Ci is the drug concentration of the ith sample, and V0 and Vi stand for the volumes of the dissolution medium and the sample, respectively.
Ex Vivo Corneal Penetration Study
Corneas were obtained from New Zealand rabbits which were exterminated by injecting an excess of air into the marginal ear vein. The corneas were immediately excised, weighed, and preserved in glutathione bicarbonate ringer (GBR) buffer (19,23,24). The corneal permeation experiments were performed using modified Franz-type cells with a diffusion area of 0.68 cm2. Each formulation (500 μl) was placed into the donor chamber, and 10.5 mL GBR solution pre-adjusted to a temperature of 37°C was introduced in the receptor chamber with magnetic stirring throughout the whole experiment.
Samples (500 μl) were picked up at appropriate time intervals (30, 60, 90, 120, 150, 180, 210, 240, 270, 300, 330, and 360 min) after starting the experiment and restored with an equal volume of fresh GBR buffer. The concentration of drug that passed across the cornea was estimated by HPLC as described above.
The corneal condition was measured by the hydration level in accordance with a method previously described (23).
The amount of BLZ that permeated the corneal epithelium was plotted versus time and the slope of the linear portion of the graph was determined. The evident corneal permeability coefficient (Papp, in centimeters per second) was evaluated as follows:
| 3 |
where ΔQ/Δt is the linear portion of the slope (in micrograms per minute), 60 is the conversion of minutes to seconds, A is the corneal surface area (in this study, 0.68 cm2), and C0 is the initial drug concentration (in micrograms per cubic centimeter).
IOP Measurements
A single-dose crossover experiment was performed to evaluate the pharmacodynamic action of BLZ LCNPs, 1% BLZ solution, and Azopt®. Filtration through sterile 0.22 μm pore size pyrogen-free cellulose filters was carried out for the sterility of formulations.
The study was started only after acclimatization of the rabbits. Following instillation of a single drop of 0.2% (w/v) lidocaine hydrochloride which acted as local anesthetic, an indentation tonometer (YJI, Suzhou Mingren Medical Apparatus and Instruments Co. Ltd., Suzhou, China) was used to calculate IOP. Eye drops were administered locally into the lower cul-de-sac of the eye, and then, the eye was blinked thrice manually. Fifty microliters of the preparation was instilled in one eye and the other eye was treated with physiological saline as control. IOP evaluation was done at an interval of 1 h after treatment. All measurements were done thrice at each interval and the average reading was taken. The following equation (25) was taken into consideration to estimate the percentage decline in IOP:
| 4 |
All procedures were performed in a single lab by the same person using the same device. One dose was experienced daily on an animal, which was washed out at least 2 days between experiments. The pharmacodynamic figures taken into consideration were utmost percentage fall in IOP, time for maximal response (Tmax), area under percentage decrease in IOP versus time curve (AUC0 – 8 h), and mean residence time (MRT). These parameters were calculated using the 3P87 software.
Ocular Irritation Test
A single application of 50 μl preparation to one eye was used to estimate the ocular tolerance and irritation of the LCNP formulation in line with a modified Draize test (26). Physiological saline and blank LCNPs were applied to the other eye which served as control, respectively. Analysis of the ophthalmic tissue condition was executed 8 h after application. The congestion, swelling, discharge, and redness of the conjunctiva were sorted on a scale from 0 to 3, 0 to 4, 0 to 3, and 0 to 3, respectively. Irritation and corneal opacity were graded on a scale from 0 to 4 (27).
Statistical Analysis
Statistical analysis of the results was done using one-way analysis of variance (28), referring to a level of p < 0.05. Statistical analysis was enumerated with the Origin® program.
RESULTS AND DISCUSSION
Characterization of BLZ LCNPs
Particle Size, Osmolality, pH, and EE
Particle size is a vital parameter for absorption or transportation through the ocular barriers and should not be more than 10 μm. In our study, the mean particle size of LCNPs [185 ± 20 nm (mean ± SD, n = 6)] was calculated by six batches of samples. Figure 1 is only one of the representative figures.
Fig. 1.
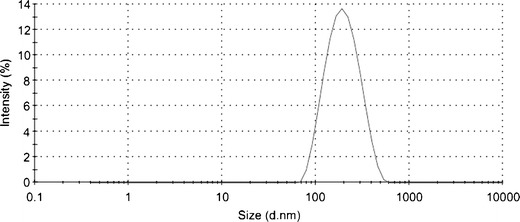
Size distribution of BLZ LCNPs
The osmolality of lacrimal fluid is between 280 and 293 mOsm/L and solutions with an osmolality lower than 100 mOsm/L or higher than 640 mOsm/L are considered as irritants. Unsuitable osmotic pressure can stimulate tear secretion and accelerate the outflow of therapeutic substances. In this study, the osmolality of the prepared LCNPs ranged from 280 to 285 mOsm/L, indicating that it was compatible of the BLZ LCNPs.
The pH of ophthalmic preparations varies from 4 to 9. Unsuitable pH value can cause irritation of eyes, augmentation of tear secretion, and can increase the efflux of therapeutic substances. In this experiment, the pH values of the prepared formulations were 6.4, suggesting that it was good compatibility of the BLZ LCNP systems.
Encapsulation efficiency of BLZ in the LCNPs was higher than 94% because of its lipophilicity.
TEM
Classic electron micrograph was shown in Fig. 2 demonstrating that LCNPs were actually formed and the particle size was consistent with dynamic light scattering data.
Fig. 2.
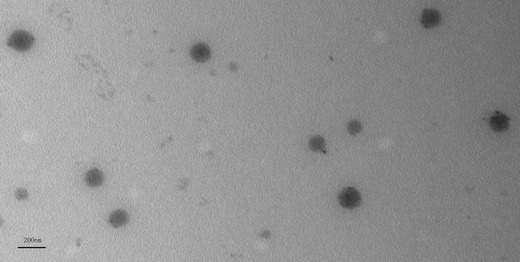
Typical TEM image of BLZ LCNPs (scale bar 200 nm)
SAXS
SAXS was usually carried out to provide quantitative details of phase structural dimensions and to confirm the internal structure of liquid crystalline system. Diffraction peaks were assigned to the different lyotropic liquid crystalline phases using the characteristic spacing ratios (lameller type, 1:2:3:4; hexagonal type, 1:√3:√4:√7; cubic type, √2:√4:√6:√8). In our study, diffraction peaks with reciprocal spacing ratio of 1:√3:√4 were observed in Fig. 3, which represented the reverse hexagonal phase (H2) (29). In this study, the purity of commercially available GMO used was 90%, and it contained a little amount of GTO, which was diluted to form H2 phase of colloidal particles (30).
Fig. 3.
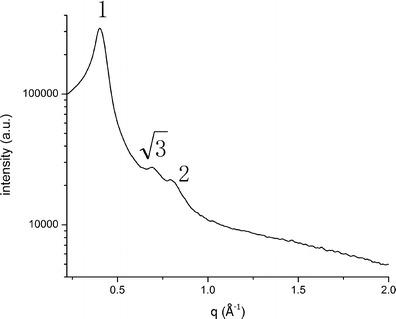
SAXS profiles of BLZ LCNPs
Many studies have indicated that H2 phase as a promising candidate for the special structural properties of densely packed, infinitely long, and straight water-filled rods could accommodate drugs within the lipidic regions (31).
In Vitro Release Studies
Maintaining the sink condition in the release experiments was an essential issue for in vitro release studies of poorly soluble drugs such as BLZ. In this study, the solubility of BLZ in STF was 1.54 mg/mL. Therefore, it was obvious that BLZ in the samples can absolutely dissolve in 100 mL STF. It indicated a perfect sink condition throughout the experiments.
The in vitro release behavior of BLZ LCNPs was shown in Fig. 4. We can see that the amount of drug released into the medium after 1 h was around 80.31% and 93.53% after 2 h for the Azopt®, in comparison to 55.41% and 72.32% for the LCNPs, respectively, which indicated that BLZ LCNPs displayed to some extent prolonged drug release behavior compared with Azopt®. However, there was also a rapid releasing pattern of LCNPs which could be explained by the following:
Fig. 4.
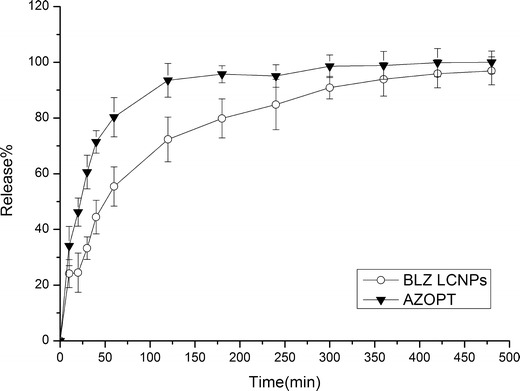
In vitro release profiles of BLZ LCNPs and Azopt® (mean ± SD, n = 6)
One likely reason of the burst release was especially owing to spreading of the free drug in external phase or the fact that the encapsulated drug was engrossed in the outer shell of the LCNPs and/or on the surface of the particles. The lipid might be crystallized prior to forming inner core, resulting in BLZ surrounding the lipid core and aqueous medium as well as entrapped in the core of the lipid nanoparticles.
The second reason was that the extensive distinct surface of the small particles could boost the initial drug release. In the preparation of BLZ LCNPs, the presence of lipid supplied an additional surface area. Thus, BLZ LCNPs had a large total external surface and high diffusion coefficient resulting in more intense interaction with the medium, leading to accelerated drug release.
Ex Vivo Corneal Penetration
Figure 5 demonstrates the ex vivo corneal penetration procedural observation of the BLZ LCNPs and Azopt®. After a delay time, a linear relationship between accumulative permeated BLZ and time could be seen. The apparent permeability coefficients (Papp) of BLZ LCNPs and Azopt® were 3.44 × 10−6 and 0.99 × 10−6 cm/s, respectively. In comparison to Azopt®, BLZ LCNPs displayed 3.47-fold increase of Papp, which suggested the enhanced penetration accomplished with LCNPs.
Fig. 5.
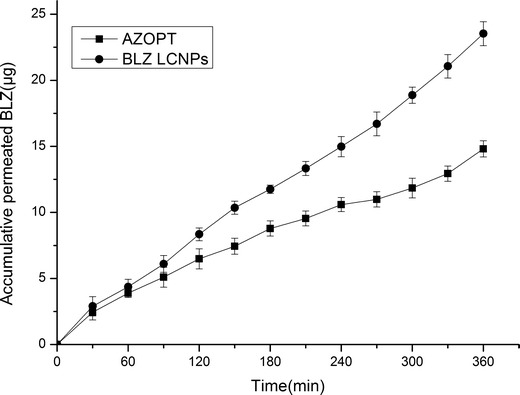
Ex vivo trans-corneal permeation profiles of BLZ LCNPs and Azopt® (n = 3)
It was reported that the cubosome-based GMO, a long-chain monoacyl lipid, exhibited very fast and complete lipid diffusion with cell membrane (32) and GMO has been described to be a transdermal enhancer (33). It was reasonable to speculate that LCNPs generated from GMO may serve as an enhancer for improved corneal permeability. The data of the corneal penetration supported the assumption that the BLZ LCNPs may serve as corneal penetration enhancer, which also might be the mechanism for the enhanced penetration by BLZ LCNPs.
Careful manipulation of the isolated cornea and preservation of its physical characteristic throughout the procedure were primordial for reproduction of the results. The corneal hydration status is a criterion commonly used to estimate the injury of this tissue. In general, the normal cornea has a hydration level of 76–80%, while an 83–92% hydration level means injury of the epithelium and/or endothelium (34). In this study, the hydration level of the corneas exposed to the test samples was 79.05% (BLZ LCNPs) and 81.31% (Azopt®) and did not surpass 83.0%. It displayed the integrity of the corneas during the experiments.
IOP Measurements
The results of therapeutic efficacy after instillation of a single dose of BLZ LCNPs, Azopt®, the blank LCNPs, BLZ solution, and physiological saline were shown in Table I and Fig. 6.
Table I.
Pharmacodynamic Parameters After Administration of BLZ LCNPs and Azopt®
| Samples | Pharmacodynamic parameters | |||
|---|---|---|---|---|
| Maximum percentage decrease in IOP | T max (h) | AUC0 – 8 h | MRT | |
| Azopt® | 33.75 ± 4.35 | 2 ± 0.45 | 152.11 ± 10.08 | 3.57 ± 0.08 |
| 1% BLZ solution | 24.65 ± 1.52 | 2 ± 0.55 | 177.90 ± 7.83 | 4.55 ± 0.15 |
| BLZ LCNPs | 47.67 ± 3.58a, b | 2 ± 0.20a, b | 283.48 ± 8.52a, b | 6.67 ± 0.16a, b |
IOP intraocular pressure, BLZ brinzolamide, LCNPs liquid crystalline nanoparticles, T max time for maximal response, AUC 0 – 8 h area under percentage decrease in IOP versus time curve, MRT mean residence time
a p < 0.05, statistically significant difference from Azopt®
b p < 0.05, statistically significant difference from 1% BLZ solution
Fig. 6.
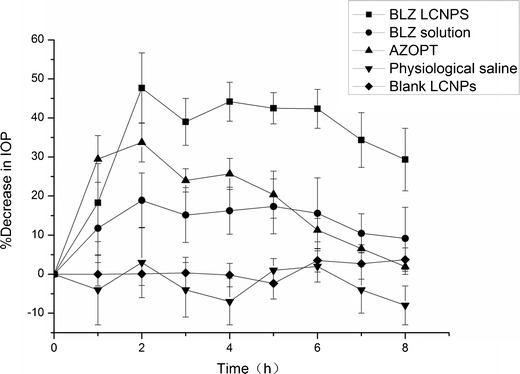
Percentage decrease in IOP after administration of different BLZ formulations, taking physiological saline and blank LCNPs as control. (Mean ± SD, n = 6)
From Fig. 6, we observed that blank LCNPs and physiological saline did not cause any significant change in IOP. BLZ LCNPs induced a remarkable decrease in IOP in contrast to Azopt® (p < 0.05) and a mean reduction of 15% or higher was considered to be effective in IOP control (35,36). It was also observed that the mean maximal percentage in IOP falls by 47.67% and 33.75% which occurred 2 h after the application of BLZ LCNPs and Azopt®, respectively. In this respect, BLZ LCNPs exerted a stronger effect on IOP reduction compared to Azopt® (p < 0.05).
Table I displayed some pharmacodynamic criteria for tested formulations. It was obvious that the area under the percentage decrease in IOP–time curve (AUC0 – 8 h) of BLZ LCNPs and Azopt® was 283.48 ± 8.52 and 152.11 ± 10.08, respectively, which demonstrated that the ocular bioavailability of BLZ LCNPs was greater than that of Azopt®. In consideration to the duration of IOP reduction, it was evident that the effect of LCNPs continued over 8 h, while Azopt® lasted for only 8 h. Table I denoted that BLZ LCNPs had a longer residence time (MRT) for percentage decrease in IOP than Azopt® (p < 0.05). This confirmed that LCNPs have more prolonged effect on decreased IOP in contrast to Azopt®. These data were compatible with earlier observations. The in vitro release study showed that BLZ LCNPs which could keep a sustained-release manner might enhance drug absorption to the anterior ocular tissues. In addition, the ex vivo cornea penetration study indicated that the Papp of BLZ LCNPs exhibited a 3.47-fold increase relative to Azopt®. Due to the slower tear washout and enhanced corneal penetration of BLZ LCNP, a gradual rise in percentage decrease of IOP was found in the BLZ LCNPs.
Ocular Irritation Evaluation
Eye irritation is a common side effect in the clinical use of ophthalmic drugs. It may cause decreased patient compliance to drugs and even stoppage of their use. Modified Draize test was used to determine the in vivo ocular irritancy of BLZ LCNPs using Azopt® as a positive control (Table II). The results indicated almost no sign of irritation in rabbit eyes. The scores were zero for iris hyperemia and conjunctival swelling for both formulations. LCNP score for corneal opacity and discharge was zero which was evidently lower than those of Azopt®. Therefore, the potential clinical safety of BLZ LCNPs was supported by minimal irritancy effects in vivo.
Table II.
Data for Draize Eye Irritation Test
| Degree of irritationa | ||||
|---|---|---|---|---|
| Azopt® | BLZ LCNPs | |||
| Control | Test | Blank LCNPs | Test | |
| Cornea | ||||
| Corneal opacity | 0 | 2 | 0 | 0 |
| Iris | ||||
| Irritation value | 0 | 0 | 0 | 0 |
| Conjunctiva | ||||
| Degree of flare | 0 | 1 | 0 | 1 |
| Degree of swelling | 0 | 0 | 0 | 0 |
| Degree of redness | 0 | 1 | 0 | 1 |
| Congestion | 0 | 1 | 0 | 1 |
| Secretion (discharge) | 0 | 1 | 0 | 0 |
aIrritation and corneal opacity were estimated on a scale from 0 to 4. Congestion, flare, swelling, discharge, and redness of the conjunctiva were graded on a scale from 0 to 3, 0 to 4, 0 to 4, 0 to 3, and 0 to 3, respectively
CONCLUSION
In this study, LCNPs were investigated as an ocular drug delivery system. BLZ LCNPs provided appropriate particle size with better tolerability, good IOP-lowering strength as Azopt® in lower doses, and longer duration of action. Consequently, the ophthalmic bioavailability of BLZ has been greatly improved. It proposed that LCNPs are favorable therapeutic system for local administration in the treatment of glaucoma.
Acknowledgments
We are grateful for the financial support from the National Natural Science Foundation of China (81273457); National Natural Science Foundation of Jiangsu Province (BK2012445, BK2012843); and Science and Technology Development Foundation of Nanjing Medical University (2011NJMU271).
Conflict of Interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
Contributor Information
Qunwei Xu, Phone: +86-25-86868468, Email: qunweixu@njmu.edu.cn.
Hongliang Xin, Phone: +86-25-86868468, Email: xhl@njmu.edu.cn.
References
- 1.Quigley H, Broman A. The number of people with glaucoma worldwide in 2010 and 2020. Br J Ophthalmol. 2006;90:262–7. doi: 10.1136/bjo.2005.081224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Becher B. Decrease in intraocular pressure in man by a carbonic anhydrase inhibitor, Diamox: a preliminary report. Am J Ophthalmol. 1954;37:13–5. doi: 10.1016/0002-9394(54)92027-9. [DOI] [PubMed] [Google Scholar]
- 3.Silver LH. Clinical efficacy and safety of brinzolamide (Azopt™), a new topical carbonic anhydrase inhibitor for primary open-angle glaucoma and ocular hypertension. Am J Ophthalmol. 1998;126:400–8. doi: 10.1016/S0002-9394(98)00095-6. [DOI] [PubMed] [Google Scholar]
- 4.Michaud J, Friren B. Comparison of topical brinzolamide 1% and dorzolamide 2% eye drops given twice daily in addition to timolol 0.5% in patients with primary open-angle glaucoma or ocular hypertension. Am J Ophthalmol. 2001;132:235. doi: 10.1016/S0002-9394(01)00974-6. [DOI] [PubMed] [Google Scholar]
- 5.March WF, Ochsner KI. The long-term safety and efficacy of brinzolamide 1.0% (Azopt) in patients with primary open-angle glaucoma or ocular hypertension. Am J Ophthalmol. 2000;129:136–43. doi: 10.1016/S0002-9394(99)00343-8. [DOI] [PubMed] [Google Scholar]
- 6.Shin D. Adjunctive therapy with brinzolamide 1% ophthalmic suspension (Azopt®) in patients with open-angle glaucoma or ocular hypertension maintained on timolol therapy. Surv Ophthalmol. 2000;44:S163–8. doi: 10.1016/S0039-6257(99)00106-X. [DOI] [PubMed] [Google Scholar]
- 7.Meisner D, Mezei M. Liposome ocular delivery systems. Adv Drug Deliv Rev. 1995;16:75–93. doi: 10.1016/0169-409X(95)00016-Z. [DOI] [Google Scholar]
- 8.Zimmer A, Kreuter J. Microspheres and nanoparticles used in ocular delivery systems. Adv Drug Deliv Rev. 1995;16:61–73. doi: 10.1016/0169-409X(95)00017-2. [DOI] [Google Scholar]
- 9.Yamaguchi M, Yasueda S, Isowaki A, Yamamoto M, Kimura M, Inada K, et al. Formulation of an ophthalmic lipid emulsion containing an anti-inflammatory steroidal drug, difluprednate. Int J Pharm. 2005;301:121–8. doi: 10.1016/j.ijpharm.2005.05.036. [DOI] [PubMed] [Google Scholar]
- 10.Han S, Shen J, Gan Y, Geng H, Zhang X, Zhu C, et al. Novel vehicle based on cubosomes for ophthalmic delivery of flurbiprofen with low irritancy and high bioavailability. Acta Pharmacol Sin. 2010;31:990–8. doi: 10.1038/aps.2010.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Esposito E, Cortesi R, Drechsler M, Paccamiccio L, Mariani P, Contado C, et al. Cubosome dispersions as delivery systems for percutaneous administration of indomethacin. Pharm Res. 2005;22:2163–73. doi: 10.1007/s11095-005-8176-x. [DOI] [PubMed] [Google Scholar]
- 12.Hirlekar R, Jain S, Patel M, Garse H, Kadam V. Hexosomes: a novel drug delivery system. Curr Drug Deliv. 2010;7:28. doi: 10.2174/156720110790396526. [DOI] [PubMed] [Google Scholar]
- 13.Lopes LB, Ferreira DA, de Paula D, Garcia MTJ, Thomazini JA, Fantini MCA, et al. Reverse hexagonal phase nanodispersion of monoolein and oleic acid for topical delivery of peptides: in vitro and in vivo skin penetration of cyclosporin A. Pharm Res. 2006;23:1332–42. doi: 10.1007/s11095-006-0143-7. [DOI] [PubMed] [Google Scholar]
- 14.Swarnakar NK, Jain V, Dubey V, Mishra D, Jain N. Enhanced oromucosal delivery of progesterone via hexosomes. Pharm Res. 2007;24:2223–30. doi: 10.1007/s11095-007-9409-y. [DOI] [PubMed] [Google Scholar]
- 15.Boyd BJ, Whittaker DV, Khoo SM, Davey G. Hexosomes formed from glycerate surfactants—formulation as a colloidal carrier for irinotecan. Int J Pharm. 2006;318:154–62. doi: 10.1016/j.ijpharm.2006.03.010. [DOI] [PubMed] [Google Scholar]
- 16.Amar-Yuli I, Wachtel E, Shoshan EB, Danino D, Aserin A, Garti N. Hexosome and hexagonal phases mediated by hydration and polymeric stabilizer. Langmuir. 2007;23:3637–45. doi: 10.1021/la062851b. [DOI] [PubMed] [Google Scholar]
- 17.Lee J, Kellaway IW. Buccal permeation of [D-Ala2, D-Leu5] enkephalin from liquid crystalline phases of glyceryl monooleate. Int J Pharm. 2000;195:35–8. doi: 10.1016/S0378-5173(99)00357-9. [DOI] [PubMed] [Google Scholar]
- 18.Lee J, Kellaway IW. Combined effect of oleic acid and polyethylene glycol 200 on buccal permeation of [D-Ala2, D-Leu5] enkephalin from a cubic phase of glyceryl monooleate. Int J Pharm. 2000;204:137–44. doi: 10.1016/S0378-5173(00)00490-7. [DOI] [PubMed] [Google Scholar]
- 19.Gan L, Han S, Shen J, Zhu J, Zhu C, Zhang X, et al. Self-assembled liquid crystalline nanoparticles as a novel ophthalmic delivery system for dexamethasone: improving preocular retention and ocular bioavailability. Int J Pharm. 2010;396:179–87. doi: 10.1016/j.ijpharm.2010.06.015. [DOI] [PubMed] [Google Scholar]
- 20.Hägerström H, Paulsson M, Edsman K. Evaluation of mucoadhesion for two polyelectrolyte gels in simulated physiological conditions using a rheological method. Eur J Pharm Sci. 2000;9:301–9. doi: 10.1016/S0928-0987(99)00070-6. [DOI] [PubMed] [Google Scholar]
- 21.Ye J, Wang Q, Zhou X, Zhang N. Injectable actarit-loaded solid lipid nanoparticles as passive targeting therapeutic agents for rheumatoid arthritis. Int J Pharm. 2008;352:273–9. doi: 10.1016/j.ijpharm.2007.10.014. [DOI] [PubMed] [Google Scholar]
- 22.Huo D, Deng S, Li L, Ji J. Studies on the poly(lactic-co-glycolic) acid microspheres of cisplatin for lung-targeting. Int J Pharm. 2005;289:63–7. doi: 10.1016/j.ijpharm.2004.10.017. [DOI] [PubMed] [Google Scholar]
- 23.Suhonen P, Jarvinen T, Peura P, Urtti A. Permeability of pilocarpic acid diesters across albino rabbit cornea in vitro. Int J Pharm. 1991;74:221–8. doi: 10.1016/0378-5173(91)90241-F. [DOI] [Google Scholar]
- 24.Liu J, Fu S, Wei N, Hou Y, Zhang X, Cui H. The effects of combined menthol and borneol on fluconazole permeation through the cornea ex vivo. Eur J Pharmacol. 2012;688:1–5. doi: 10.1016/j.ejphar.2011.12.007. [DOI] [PubMed] [Google Scholar]
- 25.Ammar HO, Salama H, Ghorab M, Mahmoud A. Nanoemulsion as a potential ophthalmic delivery system for dorzolamide hydrochloride. AAPS PharmSciTech. 2009;10:808–19. doi: 10.1208/s12249-009-9268-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Draize JH, Woodard G, Calvery HO. Methods for the study of irritation and toxicity of substances applied topically to the skin and mucous membranes. J Pharmacol Exp Ther. 1944;82:377–90. [Google Scholar]
- 27.Das S, Suresh PK, Desmukh R. Design of Eudragit RL 100 nanoparticles by nanoprecipitation method for ocular drug delivery. Nanomedicine. 2010;6:318–23. doi: 10.1016/j.nano.2009.09.002. [DOI] [PubMed] [Google Scholar]
- 28.Palma SD, Tartara LI, Quinteros D, Allemandi DA, Longhi MR, Granero GE. An efficient ternary complex of acetazolamide with HP-ss-CD and TEA for topical ocular administration. J Control Release. 2009;138:24–31. doi: 10.1016/j.jconrel.2009.04.035. [DOI] [PubMed] [Google Scholar]
- 29.Seddon JM. Structure of the inverted hexagonal (H II) phase, and non-lamellar phase transitions of lipids. Biochim Biophys Acta. 1990;1031:1–69. doi: 10.1016/0304-4157(90)90002-T. [DOI] [PubMed] [Google Scholar]
- 30.Monduzzi M, Ljusberg-Wahren H, Larsson K. A 13C NMR study of aqueous dispersions of reversed lipid phases. Langmuir. 2000;16:7355–8. doi: 10.1021/la0000872. [DOI] [Google Scholar]
- 31.Libster D, Ishai PB, Aserin A, Shoham G, Garti N. Molecular interactions in reverse hexagonal mesophase in the presence of Cyclosporin A. Int J Pharm. 2009;367(1):115–26. doi: 10.1016/j.ijpharm.2008.09.048. [DOI] [PubMed] [Google Scholar]
- 32.Barauskas J, Cervin C, Jankunec M, Špandyreva M, Ribokaitė K, Tiberg F, et al. Interactions of lipid-based liquid crystalline nanoparticles with model and cell membranes. Int J Pharm. 2010;391:284–91. doi: 10.1016/j.ijpharm.2010.03.016. [DOI] [PubMed] [Google Scholar]
- 33.Taskovich LT. Skin permeation enhancer compositions using glycerol monooleate. Google Patents. 1989.
- 34.Saettone MF, Chetoni P, Cerbai R, Mazzanti G, Braghiroli L. Evaluation of ocular permeation enhancers: in vitro effects on corneal transport of four β-blockers, and in vitro/in vivo toxic activity. Int J Pharm. 1996;142:103–13. doi: 10.1016/0378-5173(96)04663-7. [DOI] [Google Scholar]
- 35.Akman A, Cetinkaya A, Akova Y, Ertan A. Comparison of additional intraocular pressure-lowering effects of latanoprost vs brimonidine in primary open-angle glaucoma patients with intraocular pressure uncontrolled by timolol-dorzolamide combination. Eye. 2004;19:145–51. doi: 10.1038/sj.eye.6701428. [DOI] [PubMed] [Google Scholar]
- 36.Boyd BJ, Whittaker DV, Khoo SM, Davey G. Lyotropic liquid crystalline phases formed from glycerate surfactants as sustained release drug delivery systems. Int J Pharm. 2006;309:218–26. doi: 10.1016/j.ijpharm.2005.11.033. [DOI] [PubMed] [Google Scholar]


