Abstract
The aim of the study was to investigate the potential of nanosuspension to enhance the bioavailability of SKLB610 (Biopharmaceutical Classification System class II drug), a bioactive anticancer compound synthesized in our labs. SKLB610 nanosuspensions were prepared using wet media milling. Physicochemical characteristics of the nanosuspensions were evaluated, including particle size and distribution, dissolution, transmission electron microscopy, atomic force microscopy, thermogravimetric analysis, and X-ray powder diffractometry. The dissolution rate of SKLB610 was greatly improved in nanosuspensions, compared to crude SKLB610. Pharmacokinetic studies in rats demonstrated that the oral bioavailability of SKLB610 in nanosuspension (89.4%) was 2.6-fold higher than in coarse suspension (34.1%). Stabilizer type, milling time, and milling speed had a significant effect on particle size of the SKLB610 nanosuspensions. Nanosuspensions effectively improved the dissolution rate and bioavailability of the water-insoluble drug SKLB610 by reducing the compound particle size to the nanoscale and employing a proper formulation.
Key words: bioavailability, dissolution, media milling, nanosuspension, SKLB610
INTRODUCTION
The poor water solubility of some drugs is a major problem in their drug formulation. Approximately 40% of new drug candidates in cancer treatment have low water solubility (1). This limits drug dissolution rate and impairs drug bioavailability when taken by mouth (2). The development of water-insoluble drugs is a great challenge (3). Co-solvents, salt formation, pro-drug forms, solid dispersions (4,5), cyclodextrins (6,7), microemulsions (8,9), liposomes (10), and micellar systems (11) have been used to improve drug solubility. The use of nanometer-sized drug suspensions is a novel formulation strategy.
We designed and synthesized a small-molecule compound SKLB610 [N-methyl-4-(4-(3-(trifluoromethyl) benzamido) phenoxy) picolinamide; Fig. 1] (12) that is a novel multitargeting inhibitor. SKLB610 is a potent inhibitor of vascular endothelial growth factor receptor 2 (VEGFR2), which mediates proliferation of tumor cells and the growth of several human tumor xenografts in BALB/c nude mice. Tumor growth inhibition rates were 70.2% (50 mg/kg) for A549 and 77.1% (50 mg/kg) for HCT116. Moreover, no adverse effects on clinical condition were observed (13). SKLB610 demonstrated potent antitumor and antiangiogenesis activity in in vitro and in vivo studies. SKLB610 is a water-insoluble compound (0.34 μg/ml solubility in water) and belongs to class II of the Biopharmaceutical Classification System (BCS). The oral bioavailability of SKLB610 delivered in suspension is lower than 40% (14). We focused on improving the oral bioavailability of SKLB610.
Fig. 1.
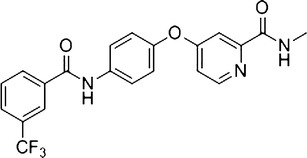
Chemical structure of SKLB610
Nanosuspensions have been found to be useful in the delivery of water-insoluble drugs. Nanosuspensions are submicron colloidal dispersions of pure particles of drug stabilized by surfactants (15). Reducing the particle size of the drug delivery particle increases the surface area to volume ratio and the associated bioavailability (16). There are two main approaches used to formulate nanosuspensions, “top down” and “bottom up”. In the “top down” approach, media milling is used. This approach is well developed and has been in commercial use for over 10 years (17,18).
The aim of our research was to enhance the oral bioavailability of SKLB610. We evaluated the effects of different types and concentrations of surfactant and polymeric excipients on the physicochemical characteristics of SKLB610 nanosuspensions. In vitro release behavior of prepared nanosuspensions was studied using dissolution testing. Finally, pharmacokinetic studies in rats were conducted to evaluate the impact of nanosuspension formulation on oral bioavailability.
MATERIALS AND METHODS
Materials
SKLB610 (purity >99.0% by high-performance liquid chromatography (HPLC)) was synthesized in our labs. Sodium lauryl sulfate (SLS) and polyvinylpyrrolidone (PVP) K-25 were obtained as gift samples from BASF (Ludwigshafen, Germany). Pluronic F-68 (Poloxamer 188) was purchased from BASF. Hydroxypropyl methylcellulose (HPMC) and Tween-80 were purchased from KeLong Chemical (Chengdu, China). HPLC-grade acetonitrile was purchased from Tedia Company Inc (Fairfield, OH, USA). Double-distilled deionized water was used in all experiments. All other chemicals were of analytical grade.
Preparation of Nanosuspensions Using Wet Media Milling
Nanosuspension preparation involved two major steps: the first one is uniform dispersion of drug and stabilizers in the dispersion medium and second one is particle size reduction in the milling chamber (19). A SKLB610 nanosuspension was produced using an agitator bead mill (PMQW, Nanjin Chishun, China). Two hundred milligram SKLB610 and the excipients PVP and SLS were dispersed in 20 ml deionized water using a magnetic stirrer at 500 rpm for 1 h. The weight ratio of SKLB610:PVP:SLS was 1:0.5:0.1. This suspension was mixed with 0.2–0.4 mm yttrium stabilized zirconium oxide beads in an agate jar. The agitator bead mill operating parameters were a horizontal speed of 450 rpm, vertical speed of half the horizontal speed, and milling time of 12 h at room temperature. After milling, zirconium oxide beads were filtered from the suspension (slurry). The resulting nanosuspension was frozen overnight at −20°C, and freeze-dried (FD-1A-50, Boyikang, China).
Lyophilization
A freshly prepared nanosuspension was immediately lyophilized after preparation. Mannitol was chosen as the cryoprotective agent for the freeze-drying process. Briefly, the SKLB610 nanosuspension was rapidly cooled to −80°C for 2 h and then transferred to a freeze-drier (FD-1000, EYELA, Japan) at −40°C for 48 h. The pressure was kept at 0.1 mbar.
Characterization of Nanosuspensions
Particle Size Determination
The particle size of fresh samples was determined using a laser Particle Size Analyzer (Nano-ZS90, Malvern Instrument, UK) at 25°C. This analysis determined the mean diameter (z average; measuring range, 20–10,000 nm) and polydispersity index (PDI). Measurements were performed within 48 h after preparation. All measurements were made in triplicate.
Transmission Electron Microscope
Prior to imaging, lyophilized powders were resuspended in deionized water and a droplet of the nanosuspension was dripped on a carbon-coated grid. The grid was blotted with filter paper and air dried. The morphologic characteristics of nanosuspensions were observed with a transmission electron microscope (TEM; H-6009IV, Hitachi, Japan).
Atomic Force Microscope
Nanosuspension lyophilized powders were resuspended in deionized water and placed on a mica surface. The suspension was dried at room temperature. The morphology of the nanosuspensions was examined using an atomic force microscope (AFM; SPA-400, Seiko Instruments Inc, Japan).
X-ray Powder Diffraction
Powder samples were examined with an X-ray powder diffractometer (XPRD; X'Pert Pro Philips, Netherlands). The radiation source was a Cu-Kɑ line (λ = 1.5406 Å). A 40-kV voltage 40-mA current, and 2θ range from 5° to 50° were used during testing.
Thermal Analysis
Thermogravimetric measurements (TG/DTA) were performed with a thermogravimetric analyzer (EXSTAR DMS 6000, Seiko Instruments Inc, Japan) coupled with a Perkin-Elmer computerized data station under a nitrogen atmosphere at a heating rate of 10°C/min in the range of 25–600°C.
Determination of SKLB610 Solubility
An excess of SKLB610 was added to each solvent; the resulting suspensions were stirred for 48 h at 25°C and filtered through a 0.22 μm dialysis membrane. The concentration of SKLB610 in the solvent was analyzed by HPLC, a C18 column (150 × 4.6 mm, 5 μm), and acetonitrile/water (55/45, v/v) as the eluent solution, at a flow rate of 1 ml/min. Each sample was measured three times.
In Vitro Dissolution
In vitro release behaviors of the nanosuspension dried powders was studied using the modified dialysis method. Of the SKLB610 nanosuspension solution, 0.5 ml was placed in a tube covered with a 0.22 μm dialysis membrane. Of the SKLB610 solution in DMSO (1 mg/ml), 0.5 ml was used as a control. Dialysis tubes were incubated in 30 ml of PBS (pH 7.4) containing Tween-80 (0.5%, w/v) at 37°C with gentle shaking (100 rpm). The media was displaced with fresh PBS at predetermined time points. The removed media was centrifuged at 13,000 rpm for 15 min. The concentration of SKLB610 in the supernatant was determined using HPLC, a C18 column (150 × 4.6 mm, 5 μm), and acetonitrile/water (55/45, v/v) as the eluent solution, at a flow rate of 1 ml/min.
In Vivo Pharmacokinetic Studies
All animal experiments complied with Guidelines for the Care and Use of Laboratory Animals and were approved by the Institutional Animal Care and Use Committee of Sichuan University. Healthy male Sprague–Dawley rats (weight, 220 ± 20 g) were supplied by the experimental animal center (Sichuang University, China). The rats were randomly divided into three groups of six. Rats were fasted overnight and had free access to water before each experiment. The three treatment groups consisted of intravenous administration of the coarse suspension, orally administrated nanosuspension and orally administrated coarse suspension. A dose of 50 mg/kg was used in each group. Blood samples (0.3–0.4 ml) were collected from the jugular vein at 0.25, 0.5, 0.75, 1, 2, 4, 6, 8, 10, 12, and 24 h after administration, and placed in heparinized Eppendorf tubes. The intravenous group also had a blood sample collected at 0.083 and 0.167 h. The tubes were immediately centrifuged at 4,000 rpm for 10 min to harvest the plasma. Plasma was stored in a new tube and stored at −20°C until analysis.
Blood samples were processed using the acetonitrile protein precipitation method. Of the plasma samples, 100 μl were mixed with 20 μl of Sorafenib solution in methanol (30 μg/ml) as the internal standard. Of acetonitrile, 300 μl was then added. The samples were vortexed for 3 min and centrifuged at 13,000 rpm for 10 min. The supernatant was transferred to a clean tube and evaporated to dryness using nitrogen gas at 40°C. The extraction residue was reconstituted in 50 μl mobile phase and centrifuged at 13,000 rpm for 3 min. Of the supernatant, 20 μl was injected into the HPLC system. The determined approach of HPLC was the same as the method of dissolution test.
RESULTS AND DISCUSSION
Particle Size Study
Screening Study of Polymer and Surfactant
Stabilizers are amphiphilic molecules were selected to promote the particle size reduction process. They are necessary for the preparation and stability of the nanosuspension. Stabilizers were used in nanosuspensions to provide wetting of the hydrophobic surfaces of the drug particles and to inhibit particles agglomeration in the medium, which alleviated the sedimentation issues. Different stabilizers were screened to identify the optimal formulation with the smallest size and PDI. Polymers were screened for HPMC and PVP K30 content and surfactant was screened for the content of SLS, F68, and Tween-80 (Table I). We found that a double-stabilizer formulation was better than a single stabilizer formulation in decreasing particle size. Formula 6 was associated with the smallest particle size and PDI (mean particle size of 204 nm, PDI of 0.210).
Table I.
Mean Particle Size (MPS) and the Polydispersity Index Value (PDI) of Different Nanosuspensions
| Formula | Drug (mg) | HPMC (mg) | PVP K30 (mg) | SLS (mg) | F68 (mg) | Tween-80 (μl) | Milling time (h) | Horizontal milling speed (rpm)a | MPS (nm) | PDI |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 200 | 100 | – | – | – | - | 6 | 450 | 3,465 ± 930 | 0.910 ± 0.321 |
| 2 | 200 | – | 100 | – | – | – | 6 | 450 | 472 ± 45 | 0.570 ± 0.180 |
| 3 | 200 | – | – | 100 | – | – | 6 | 450 | 335 ± 33 | 0.318 ± 0.151 |
| 4 | 200 | – | – | – | 100 | – | 6 | 450 | 645 ± 48 | 0.505 ± 0.189 |
| 5 | 200 | – | – | – | – | 100 | 6 | 450 | 582 ± 67 | 0.443 ± 0.170 |
| 6 | 200 | – | 100 | 20 | – | – | 6 | 450 | 204 ± 15 | 0.210 ± 0.015 |
| 7 | 200 | – | 100 | – | 20 | – | 6 | 450 | 292 ± 35 | 0.242 ± 0.027 |
| 8 | 200 | – | 100 | – | – | 20 | 6 | 450 | 298 ± 33 | 0.237 ± 0.031 |
− Mean zero
aVertical speed was half of the horizontal speed
Optimization of Milling Time and Milling Speed
Formula 6 was felt to be optimal and was used for milling studies. The effect of milling time and milling speed on particle size was evaluated (Table II). Particle size decreased as milling speed and milling time increased. Milling time longer than 12 h and milling speed greater than 450 rpm did not further reduce particle size. Formula 12 produced the best SKLB610 nanosuspension. SKLB610 coarse suspension (Formula 17) particle size is very big. The PDI of coarse suspension was recorded at 0.893 which indicated the drug particles distribution was too wide and the size data may not display the true situation.
Table II.
Effect of Milling Time and Milling Speed on Particle Size Z-AVE and PDI
| Formulation | Milling time (h) | Horizontal milling speed (rpm) | MPS (nm) | PDI |
|---|---|---|---|---|
| 9 | 0.5 | 450 | 439 ± 33 | 0.332 ± 0.046 |
| 10 | 1 | 450 | 328 ± 25 | 0.247 ± 0.020 |
| 11 | 2 | 450 | 225 ± 24 | 0.232 ± 0.015 |
| 6 | 6 | 450 | 204 ± 15 | 0.210 ± 0.015 |
| 12 | 12 | 450 | 174 ± 12 | 0.175 ± 0.018 |
| 13 | 24 | 450 | 172 ± 13 | 0.174 ± 0.011 |
| 14 | 12 | 250 | 598 ± 35 | 0.455 ± 0.022 |
| 15 | 12 | 350 | 302 ± 26 | 0.329 ± 0.018 |
| 16 | 12 | 600 | 170 ± 13 | 0.176 ± 0.015 |
| 17 | – | – | 2785 ± 315 | 0.893 ± 0.091 |
Characterization of Nanosuspensions
Transmission Electron Microscopy Analysis
The morphological characteristics of nanosuspensions are shown in Fig. 2. Drug particles were uniformly distributed in nanosuspension. Prepared nanoparticles had spheroid appearance with a mean particle size of about 174 nm. These findings support the use of the wet media milling method in preparing SKLB610 nanosuspensions.
Fig. 2.
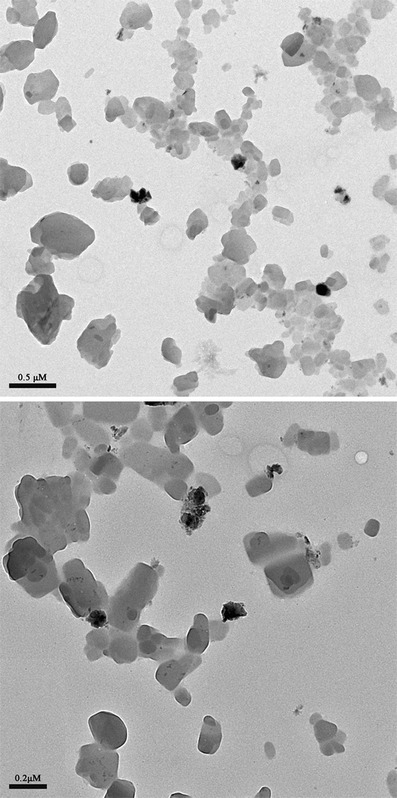
Transmission electron microscopy (TEM) micrographs of nanosuspension
Atomic Force Microscope Analysis
AFM demonstrated the spherical shape of the drug nanoparticles (Fig. 3). The diameters of the nanoparticles observed by AFM were in good agreement with those determined by TEM.
Fig. 3.
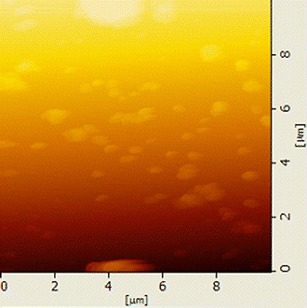
AFM image in trapping mode at 10 μm scan range of prepared SKLB610 nanosuspension
Thermal Analysis by DTA
Thermogravimetric analysis is used to study the crystalline state of drug nanoparticles. Thermogravimetric analysis curves of (a) SLS, (b) PVP K30, (c) SKLB610, (d) physical mixture, and (e) nanosuspension powders were obtained (Fig. 4). The DTA curve for pure SKLB610 (Fig. 4c) demonstrated the drug melting peak at 155.7°C. No such peak was observed with the nanoparticles. Pure SKLB610 was also associated with another peak at 132–133°C (Fig. 4e). The melting peak of SKLB610 in nanosuspension was not as sharp as the pure SKLB610, probably due to the preparation process. Amorphous domains were also generated on the particle surface (Fig. 4) (20).
Fig. 4.

Thermal analysis of nanosuspension. DTA thermograms for nanosuspension are presented at a SDS, b PVP, c SKLB610, d physical mixture, and e SKLB610 nanosuspension
Powder X-ray Diffraction Analysis
Powder X-ray diffraction analysis (XRPD) was used to study changes in the crystalline state of SKLB610 during the nanosuspension preparation process. The XRPD patterns of (a) SLS, (b) PVP K30, (c) SKLB610, (d) physical mixture, and (e) nanosuspension powders are presented in Fig. 5. The characteristic 2θ peaks of SKLB610 at 18.4°, 20.2°, 22.6°, and 27.4° were found (Fig. 5c). In the nanosuspension powder (Fig. 5e), peaks were found at 18.4°, 20.2°, and 27.4°. The loss of the peak at 22.6° was explained by a dilution effect related to the adjuvant. Relative peak intensity varied among the samples. SKLB610 had lower crystallinity after the milling process, probably due to the reduction in particle size. SKLB610 nanosuspension powders with low crystallinity were expected to have a higher dissolution rate and bioavailability.
Fig. 5.

X-ray powder diffraction patterns of a SDS, b PVP, c SKLB610, d physical mixture, and e SKLB610 nanosuspension
SKLB610 Solubility
SKLB610's solubility in water and other solvent was reported in Table III. The solubility of SKLB610 in water is very low, while solubility in organic solvent ethanol is very high. Saturation solubility of SKLB610 nanosuspension at room temperature was 103 ± 18 μg/ml. Reducing the particle size down to the submicron range markedly increased the saturation solubility of SKLB610. It is remarkable that the increase saturation might favorably affect dissolution rate and bioavailability.
Table III.
SKLB610 Solubility at 25°C. Data are Expressed as Mean ± SD (n = 3)
| Solvent | SKLB610 Solubility (μg/ml) |
|---|---|
| Water | 0.34 ± 0.07 |
| Ethanol | 16500 ± 800 |
| Ethyl acetate | 380 ± 30 |
| 0.5% Tween 80a | 23 ± 2 |
| Nanosuspension | 103 ± 18 |
aAqueous solution
In Vitro Dissolution Test
For BCS class II drugs like SKLB610 (poor solubility and high permeability), the poor dissolution rate very often controls the extent of oral absorption (21). In order to ascertain whether the rate of dissolution of SKLB610 was improved, different drug samples were evaluated (Fig. 6). The rate of dissolution of pure SKLB610 was very low. Only 6.5% of the drug dissolved in the first 15 min. Only 24.6% of the drug was dissolved after 60 min. Physical mixing group behaves like pure drug group. Only 10.3% dissolved in the first 15 min. Nanosuspension formulation of SKLB610 significantly improved the dissolution rate. Almost 84.1% of the drug dissolved in the first 15 min. The enhancement of the dissolution rate is described by the Noyes–Whitney principle (22). Drug dissolution rate was linearly dependent upon the surface area of the drug particles. For spherical drug particles, the surface area to volume ratio significantly increased as particle size decreased. Particle size reduction significantly improved dissolution rate. The lower crystallinity of the nanosuspension may also have contributed to the enhanced dissolution rate (23). We assumed that the increase in dissolution rate would improve the bioavailability.
Fig. 6.
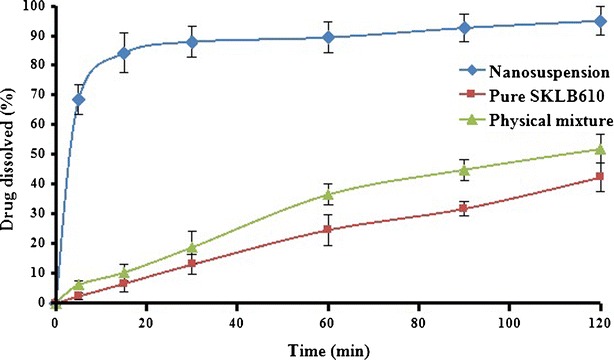
Mean dissolution profiles of SKLB610 nanosuspension, pure SKLB610, and physical mixture. Data are expressed as mean ± SD (n = 3)
Bioavailability Study in Rats
The plasma concentration–time curves of SKLB610 after oral administration of the coarse suspension, nanosuspension, and intravenous administration in rats are shown in Fig. 7. The pharmacokinetic parameters are displayed in Table IV. The plasma concentration after administration of the nanosuspension was significantly higher than after the coarse suspension. The nanosuspension had a higher mean Cmax (378.9% higher) and AUC0 − t (262.3% higher) than the course preparation. The absolute bioavailability of SKLB610 in nanosuspension was 89.4%. These findings demonstrate the advantage of using media milling to enhance bioavailability.
Fig. 7.
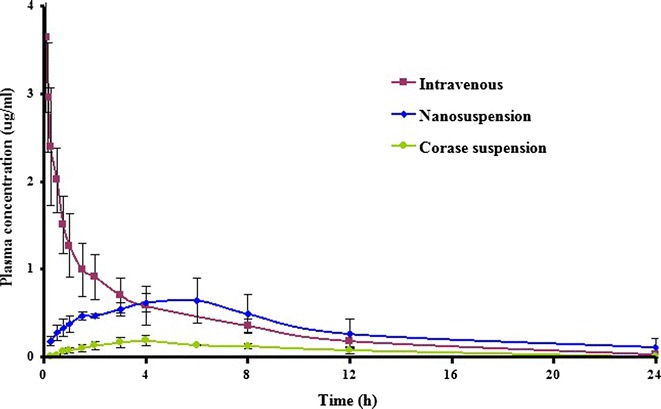
Plasma concentration-time profiles of SKLB610 after intravenous and oral administrations of 50 mg/kg in the rat. Two different oral formulations were tested: nanosuspension and coarse suspension. Data are expressed as mean ± SD (n = 6)
Table IV.
Pharmacokinetic Parameters in 3 Groups: Coarse Suspension of Oral SKLB610 (50 mg/kg), Nanosuspension of Oral SKLB610 (50 mg/kg) and Intravenous SKLB610 (50 mg/kg). Data are Expressed as Mean ± SD (n = 6)
| Parameter | Intravenous | Nanosuspension | Coarse suspensions |
|---|---|---|---|
| C max (μg/mL) | 3.65 ± 0.86 | 0.72 ± 0.21* | 0.19 ± 0.05 |
| T max (h) | – | 4.67 ± 1.51** | 3.67 ± 0.52 |
| AUC0 − t (μg h/mL) | 8.85 ± 2.39 | 7.92 ± 2.80* | 3.02 ± 0.45 |
| MRT0 − t (h) | 4.88 ± 0.80 | 8.34 ± 1.24** | 6.85 ± 1.07 |
| T 1/2 (h) | 4.14 ± 1.58 | 3.95 ± 0.73** | 3.35 ± 1.51 |
| CLz (L/h/kg) | 5.86 ± 1.63 | 6.67 ± 1.83* | 28.71 ± 9.08 |
| Vz (L/kg) | 33.10 ± 7.90 | 37.38 ± 10.75** | 127.94 ± 44.91 |
| F abs | 100% | 89.4%b | 34.1% |
* P < 0.05; ** P > 0.05, compared to the SKLB610 coarse suspension
The improvement in oral SKLB610 bioavailability was probably due to the increased dissolution rate. In addition, oral administration could increase gastrointestinal transit time, resulting in increased oral bioavailability (24). Decreasing particle size and increasing surface area could increase drug adhesion to gastrointestinal mucous surfaces, facilitating absorption (25).
CONCLUSION
SKLB610 nanosuspensions were prepared using wet media milling. Stabilizer species, milling time, and milling speed played a significant role in controlling particle size. Optimal milling time and milling speed for a suitable SKLB610 nanosuspension were identified. The crystalline state was examined using DTA and PXRD. Both results demonstrated the lower crystallinity of the drug particle after milling. Nanosuspensions exhibited a markedly enhanced dissolution rate and significantly improved oral bioavailability in rats compared to coarse suspensions. SKLB610 nanosuspensions can be expected to improve the therapeutic activity drugs administered in the treatment of cancer. Moreover, for BCS class II drugs, nanosuspensions appear as a promising approach for enhancing oral bioavailability.
Acknowledgments
This work was supported by the National Science and Technology Major Project of China (2009ZX09103-132). We are thankful to the State Key Laboratory of Biotherapy and Cancer Center for supporting this research project.
References
- 1.Gardner CR, Walsh CT, Almarsson Ö. Drugs as materials: valuing physical form in drug discovery. Nat Rev Drug Discov. 2004;3:926–934. doi: 10.1038/nrd1550. [DOI] [PubMed] [Google Scholar]
- 2.Lipinski CA, Lombardo F, Dominyl BW, Feeney PJ. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv Drug Deliv Rev. 2001;46:3–26. doi: 10.1016/S0169-409X(00)00129-0. [DOI] [PubMed] [Google Scholar]
- 3.Merisko-Liversidge E, Liversidge GG. Nanosizing for oral and parenteral drug delivery: a perspective on formulating poorly-water soluble compounds using wet media milling technology. Adv Drug Deliv Rev. 2011;63:427–440. doi: 10.1016/j.addr.2010.12.007. [DOI] [PubMed] [Google Scholar]
- 4.Christian L, Dressman J. Improving drug solubility for oral delivery using solid dispersions. Eur J Pharm Biopharm. 2000;50:47–60. doi: 10.1016/S0939-6411(00)00076-X. [DOI] [PubMed] [Google Scholar]
- 5.Shuxin W, Yingqian S, Xiuxiang Q, Fengping T. Improved bioavailability of poorly water-soluble drug curcumin in cellulose acetate solid dispersion. AAPS PharmSciTech. 2012;13:159–166. doi: 10.1208/s12249-011-9732-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Davis ME, Brewster ME. Cyclodextrin-based pharmaceutics: past, present and future. Nat Rev Drug Discov. 2004;3:1023–1035. doi: 10.1038/nrd1576. [DOI] [PubMed] [Google Scholar]
- 7.Yadav VR, Suresh S, Devi K, Yadav S. Effect of cyclodextrin complexation of curcumin on its solubility and antiangiogenic and anti-inflammatory activity in rat colitis model. AAPS PharmSciTech. 2009;10:752–762. doi: 10.1208/s12249-009-9264-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kawakami K, Yoshikawa T, Moroto Y, Kanaoka E, Takahashi K, Nishihara Y, et al. Microemulsion formulation for enhanced absorption of poorly soluble drugs: I. Prescription design. J Control Release. 2002;81(1–2):65–74. doi: 10.1016/S0168-3659(02)00049-4. [DOI] [PubMed] [Google Scholar]
- 9.Nandi I, Bari M, Joshi H. Study of isopropyl myristate microemulsion systems containing cyclodextrins to improve the solubility of 2 model hydrophobic drugs. AAPS PharmSciTech. 2003;4:71–79. doi: 10.1208/pt040110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Malam Y, Loizidou M, Seifalian AM. Liposomes and nanoparticles: nanosized vehicles for drug delivery in cancer. Trends Pharmacol Sci. 2009;30(11):592–599. doi: 10.1016/j.tips.2009.08.004. [DOI] [PubMed] [Google Scholar]
- 11.Hui D, Xiaojun W, Song Z, Xinli L. Applications of polymeric micelles with tumor targeted in chemotherapy. J Nanopart Res. 2012;14:1254–1267. doi: 10.1007/s11051-012-1254-1. [DOI] [Google Scholar]
- 12.Luoting Y, Yinglan Z, Yuquan W, Shengyong Y, Li Y. 4-(4-benzamido phenoxy)-2-(methylcarbamoyl) pyridine derivatives, preparation method and application thereof. CN Patent 101362718 (A); 2009.
- 13.Zhixing C, Renlin Z, Hongjun L, Shidong L, Yan Z, Youzhi X, et al. SKLB610: a novel potential inhibitor of vascular endothelial growth factor receptor tyrosine kinases inhibits angiogenesis and tumor growth in vivo. Cell Physiol Biochem. 2011;27:565–575. doi: 10.1159/000329978. [DOI] [PubMed] [Google Scholar]
- 14.Xun L, Shuangzhi L, Yongmei X, Jun H, Junming L, Hongjun L, et al. Pharmacokinetic studies of a novel multikinase inhibitor for treating cancer by HPLC-UV. J Chromatogr Sci. 2012;00:1–4. doi: 10.1093/chromsci/bms098. [DOI] [PubMed] [Google Scholar]
- 15.Na GC, Stevens HJ, Yuan BO, Rajagopalan N. Physical stability of ethyl diatrizoate nanocrystalline suspension in steam sterilization. Pharm Res. 1999;16:569–574. doi: 10.1023/A:1018883431970. [DOI] [PubMed] [Google Scholar]
- 16.Merisko-Liversidge E, Liversidge GG, Cooper ER. Nanosizing: a formulation approach for poorly-water-soluble compounds. Eur J Pharm Sci. 2003;18(2):113–120. doi: 10.1016/S0928-0987(02)00251-8. [DOI] [PubMed] [Google Scholar]
- 17.Muller RH, Jacobs C, Kayser O. Nanosuspensions as particulate drug formulations in therapy: rationale for development and what we can expect in the future. Adv Drug Deliv Rev. 2001;47:3–19. doi: 10.1016/S0169-409X(00)00118-6. [DOI] [PubMed] [Google Scholar]
- 18.van Eerdenbrugh B, Froyen L, van Humbeeck J, Martens J, Augustijns P, van den Mooter G. Top-down production of drug nanocrystals: nanosuspension stabilization, miniaturization and transformation into solid products. Int J Pharm. 2008;364:64–75. doi: 10.1016/j.ijpharm.2008.07.023. [DOI] [PubMed] [Google Scholar]
- 19.Singh SK, Srinivasan KK, Gowthamarajan K, Singare DS, Prakash D, Gaikwad NB. Investigation of preparation parameters of nanosuspension by top-down media milling to improve the dissolution of poorly water-soluble glyburide. Eur J Pharm Biopharm. 2011;78:441–446. doi: 10.1016/j.ejpb.2011.03.014. [DOI] [PubMed] [Google Scholar]
- 20.Chow AHL, Tong HHY, Chattopadhyay P, Shekunov BY. Particle engineering for pulmonary drug delivery. Pharm Res. 2007;24:411–437. doi: 10.1007/s11095-006-9174-3. [DOI] [PubMed] [Google Scholar]
- 21.Dressman JB, Fleisher D. Mixing-tank model for predicting dissolution rate control or oral absorption. J Pharm Sci. 1986;75:109–116. doi: 10.1002/jps.2600750202. [DOI] [PubMed] [Google Scholar]
- 22.Noyes AA, Whitney WR. The rate of solution of solid substances in their own solutions. J Am Chem Soc. 1897;19:930–934. doi: 10.1021/ja02086a003. [DOI] [Google Scholar]
- 23.Zhong J, Shen Z, Yang Y, Chen J. Preparation and characterization of uniform nanosized cephradine by combination of reactive precipitation and liquid anti-solvent precipitation under high gravity environment. Int J Pharm. 2005;30:286–293. doi: 10.1016/j.ijpharm.2005.06.005. [DOI] [PubMed] [Google Scholar]
- 24.Rabinow BE. Nanosuspensions in drug delivery. Nat Rev Drug Discov. 2004;3(9):785–796. doi: 10.1038/nrd1494. [DOI] [PubMed] [Google Scholar]
- 25.Jianjun Z, Huixia L, Kun J, Yuan G. Enhanced bioavailability after oral and pulmonary administration of baicalein nanocrystal. Int J Pharm. 2011;420:180–188. doi: 10.1016/j.ijpharm.2011.08.023. [DOI] [PubMed] [Google Scholar]


