Abstract
Soluplus® is a novel amphiphilic polymer that has been shown to enhance the solubility and drug dissolution rate of poorly soluble drugs. However, there still is a lack of information regarding the physical mechanical properties of Soluplus® with addition of the plasticizers. This study characterized the mechanical properties of Soluplus® with four different plasticizers. The plasticizers selected were polyethylene glycol 6, triethyl citrate, propylene glycol, and glycerin; they were studied at three different levels (15%, 20%, and 25% w/w). The effects of these plasticizers on the glass transition temperature, tensile strength, percent elongation, and Young’s modulus of free films made from Soluplus® were measured and the toughness and ratio of tensile strength to Young’s modulus were calculated. These results showed these four plasticizers are capable to plasticizing Soluplus® as indicated by the glass transition temperature lowering, tensile strength, and Young’s modulus while increasing the percent elongation and film toughness. Among the plasticizers tested, polyethylene glycol 6 showed greatest changed in the mechanical properties studied.
KEY WORDS: physical–mechanical properties, plasticizers, Soluplus® (polyvinyl caprolactam–polyvinyl acetate–polyethylene glycol grafted copolymer), solvent casting, tensile strength
INTRODUCTION
Soluplus® is a novel amphiphilic polymer that can be used as a polymeric solubilizer. It is a polyvinyl caprolactam–polyvinyl acetate–polyethylene glycol grafted copolymer (see Fig. 1). Enhanced solubility and dissolution rate of BCS Class II drugs such as Itraconazole, Meloxicam, and Spironolactone by hot melt extrusion; KinetiSol® dispersing; and electrospinning techniques were observed in recent studies (1,2).
Fig. 1.

The chemical structure of polyvinyl caprolactam–polyvinyl acetate–polyethylene glycol graft copolymer trade name Soluplus®
The functions of Soluplus® as a potential solubilizer and solid dispersion carrier have been studied in a few publications (1,3,4); to date, no commercial products have utilized this amphiphilic polymer due in part to its novelty and the lack of information regarding its chemical and physical mechanical properties. More characterization studies such as the physical–mechanical properties are needed to serve as reference for other formulation scientists to use this new polymer in the pharmaceutical industry.
In our solvent casting studies, we observed that it is difficult to remove the solvent cast Soluplus® film from the Teflon® mold due to the brittle properties of the cast films. Without plasticizer, the pure Soluplus® films always broke into little pieces when removed from the Teflon® mold making it impossible to produce intact films. Hence, it would be very challenging to develop pure Soluplus® film formulations without adding a plasticizer. In addition, plasticizers are often added to a hot-melt extrusion formulation to improve the processing conditions during the manufacturing of the extruded dosage form, improve the physical and mechanical properties of the final product, and lower the glass transition temperature (Tg) of the polymer to avoid drug degradation that can occur at higher processing temperatures (5–8). Thus, plasticizers will be needed to change thermal and mechanical properties in Soluplus® formulations to take full advantage of its potential as a solubilizer and solid dispersion carrier which has been demonstrated in other studies (1,2)
Plasticizers are generally nonvolatile, high boiling, low molecular weight compounds added to a polymer to improve its processability, flexibility, and stretchability by modifying the mechanical properties making the films more ductile, lowering the melt viscosity and the Tg of the product without altering the fundamental chemical character of the plasticized material (9,10). When incorporated into the polymer system, plasticizers can increase the free volume between the polymer chains which allows the chain segments to move and rotate more freely allowing for increased movement of polymer chains with respect to each other, consequently, decreasing the polymer Tg and melt viscosity (11,12).
Some of the commonly used methods to characterize the efficiency of the plasticizer are through the comparison of Tg depression and the mechanical properties of the polymer by using different plasticizer types and levels (6,13–16). The mechanical properties of the polymer that may be improved by the addition of plasticizers include decreased the tensile strength and Young’s modulus and increased in percent elongation, toughness and resistance to cracking, etc. (17–20).
The objective of this study is to characterize the effects of some commonly used plasticizers on the thermal and mechanical properties of Soluplus®. The plasticizers selected are polyethylene glycol 6 (PEG-6), triethyl citrate (TEC), propylene glycol (PPG), and glycerin (GLY). This study will determine how effective different plasticizers are in changing the thermal and mechanical properties of Soluplus®, and it will examine the effect of plasticizer concentration on its thermal and mechanical properties. To accomplish these objectives, the glass transition temperature of Soluplus® will be studied by Modulated Differential Scanning Calorimetry (MDSC). The tensile strength, strain at break, percent elongation, young modulus, toughness, and the ratio of tensile strength to Young’s modulus of Soluplus® with addition of plasticizers will also be characterized using mechanical testing.
MATERIALS AND METHODS
Materials
The graft copolymer polyvinyl caprolactam–polyvinyl acetate–polyethylene glycol trade name Soluplus® (lot no. 05016716k0 and lot no. 08358475L0) was obtained from BASF SE (Ludwigshafen, Germany; see Fig. 1). Methanol (HPLC grade) was purchase from Fisher Scientific (Fair Lawn, NJ, USA). Plasticizers chosen for the study were PEG-6, TEC, PPG, and GLY. PEG-6 (lot no.74629036WO) was obtained from BASF SE (Ludwigshafen; TEC (lot no. 86404)) was from VertellusTM (Greensboro, NC, USA; PPG (lot no. S41023J11)) was from Ruger Chemical Co., Inc. (Irvington NJ, USA) and GLY (lot no. YT0714) was from Spectrum Chemical Mfg. Corp. (Gardena, CA, USA).
Sample Preparation
The Soluplus® films with the four different plasticizers PEG-6, TEC, PPG, and GLY at different weight ratios were prepared by solvent casting. Films with 0% to a concentration of about 15% w/w of plasticizer could not be removed from the mold without breaking into pieces due to being very brittle; thus, we could not study films without at least a minimal concentration of plasticizer. For the study test parameters, films containing greater than 25% w/w were too ductile to have a defined fracture point during testing. Hence, only films containing plasticizers of 15%, 20%, and 25% w/w were tested. To cast the films, Soluplus® was dissolved in the methanol for an hour with continuous stirring using a magnetic stir bar following the addition of the plasticizer at the different weight ratios; mixing was continued for an additional hour. Before film casting, the polymer–plasticizer solution was sonicated for 5 min to prevent air bubble formation. Then, 12 mL of polymer–plasticizer solution was cast into a level Teflon Petri dish and dried at 50°C, for approximately 4.5 h, until the loss on drying of the solid dispersion was greater than 78% of the initial weight of the polymer–plasticizer solution prior the drying step. For the solutions used to cast the films, the amount of plasticizer and polymer was fixed at 20% (w/v) of the solution solids content, i.e., as more plasticizer was added polymer was removed to maintain the 20% (w/v) solids content. The dried solid dispersions were quenched cooling in the freezer (−20°C) for an hour. Immediately after quenching, the samples were stored in a desiccator at room temperature for 24 h before testing. The quench cooling was done to provide a consistent cooling processing history for the samples in a dry environment and storing the samples in a desiccator was to protect the samples from moisture, which is known to act as a plasticizer and affect the thermal and mechanical properties of polymer films.
Thermal Analysis
Thermal analyses were performed using a MDSC (TA Instruments Model 2920; New Castle, DE, USA) DSC, equipped with a refrigerated cooling system and analyzed using TA Universal Analysis 2000 Software. The instrument was calibrated using indium for temperature and cell constant, and sapphire for the specific heat capacity. Crimped hermetic aluminum pans were used for all sample tested. MDSC was used to study the solvent cast films to separate the total heat flow signal into its heat capacity and kinetic components. The MDSC experiments were conducted with heating rate of 2°C/min, from 0°C to 210°C using modulation temperature amplitude of 2°C and a modulation period of 100 s under nitrogen purge at a flow rate of 50 mL/min. All experiments were performed in triplicate. The Tg reported in this study was taken from the inflection point, which is the point on the curve with the steepest slope on the heat capacity increment from the reverse heat flows of the MDSC thermograms.
Mechanical Properties Testing
The solid solvent cast films are removed and cut into a dog bone shape using an ASTM D-638-V “dog bone” punch (see Fig. 2). The thickness of the film was measured at five different points along the small center portion with a micrometer and the average film thickness was used for all calculations. Films with nicked sides, cracks, or air bubbles were discarded.
Fig. 2.

ASTM dog bone punch (D-638-V) and the dimensions
The mechanical properties of the solvent cast film were determined using an Instron® 8521 System with a tension/compression 100 N Load Cell # 2530–427 (Instron®, Norwood, MA, USA). Film sample is held between a G227 Lightweight Screw Vise Grip and J227 Jaws for Film Testing (Test Resources, Shakopee, MN, USA) per ASTM D882 as shown in Fig. 3. The films were subjected to a tensile load at crosshead speeds of 10 to 75 mm/min rate during the method development process. To maintain same strain rate for the entire study, the rate of 75 mm/min was selected for the best reproducibility. Only films that broke at the center of the dog bone strip were used for the analysis, and films that broke near the grips were discarded. Six replicate measurements were conducted for each film. The load and displacement data were recorded using the Instron® system until the point of film failure.
Fig. 3.

Instron® 8521 System with sample held between a G227 Lightweight Screw Vise Grip and J227 Jaws for Film Testing
The mechanical properties of the films were characterized by the tensile strength, strain at break, percent elongation, young modulus, toughness, and the ratio of tensile strength to Young’s modulus. These mechanical properties were calculated using following equations:
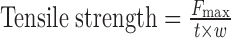 |
1 |
where Fmax is the load at failure (force at which the films breaks), t is the initial film thickness, and w is the initial film width.
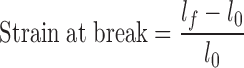 |
2 |
where lf is the final length of the film at failure and l0 is the initial length of the film between grips.
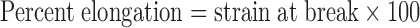 |
3 |
The Young’s modulus (elastic modulus) was calculated from the slope of the initial linear section on the stress–strain curve where the film undergoes elastic deformation. The toughness was calculated from the area under the stress–strain curve using the trapezoid rule.
RESULTS AND DISCUSSION
Thermal Analysis
The Tg of neat Soluplus® was observe at 68°C. For the first heating cycle, a typical MDSC trace of pure Soluplus® tested “as is” from the supplier is shown in Fig. 4. The data shows there is a strong enthalpic relaxation occurring in the same temperature range as the Tg. Using MDSC, to separate the total heat flow into reversing and nonreversing parts, the enthalpy of relaxation can be separated from the Tg events, which allows for the determination of the Tg.
Fig. 4.

Typical MDSC thermogram of Soluplus® showing the heat flow, reversing heat flow and nonreversing heat flow
For the plasticizers studied, the Tg of the solvent cast Soluplus® films showed a statistically significant decrease with plasticizer addition (see Fig. 5). The exception was 25% w/w of GLY where increasing the plasticizer from 20% to 25% did not significantly decreased the Tg of the Soluplus® film (p value > 0.05). A t test was used to make the statistical comparisons between the different plasticizer levels; for these tests 15% was compared to 20% and 20% was compared to 25%. As shown in Fig. 5, in general, all the plasticizers show similar trends and lowered the Tg by about the same amount. However, the lowest Tg was seen in films containing PEG-6 and GLY for plasticizer level of 15% and 20% w/w. At the highest plasticizer level (25% w/w), Tgs of TEC, PPG, and Gly are observed between 30°C and 31°C while the Tg of PEG-6 was observed at 14°C. This significant reduction in Tg values with addition of PEG-6 at 25% is an indication of the plasticizer effect that results in significant increase in polymer chain mobility. Since the Tg of a polymer is a fundamental parameter that can be used to determine the interactions between the polymer and additives; this data tells you that there is a special interaction between PEG-6 and Soluplus®. In addition, these results are confirmed by mechanical testing, see discussion below. The data also show there is a critical volume fraction where PEG-6 really affects the polymer properties, because at the lower plasticizer concentrations of 15% and 20% the difference between PEG-6 and the other plasticizers was much less.
Fig. 5.

Tg of Soluplus® with addition of different plasticizers (error bars within symbols if not shown)
Mechanical Testing
An idealized stress–strain curve for polymer film tensile testing is shown in Fig. 6. In this scenario, the strain is applied to the polymer film by moving the grips at a constant rate until fracture of the film occurs. In Fig. 6, regions A–B, B–C, and C–D represent the elastic deformation, plastic strain hardening deformation, and necking regions, respectively. The points B and D represent the yield point and fracture point (film breaking point), respectively. These regions are well known in the literature and a description can be found in any reference on the strength of materials.
Fig. 6.

A typical stress–strain curve for polymer film undergoing tensile strain testing
The maximum tensile strength of a film is the maximum stress that a film can withstand being stretched before necking or failing (21). As mentioned earlier, plasticizers can increase the free volume between the polymer chains leading to greater chain mobility and film flexibility; a plasticized polymer would therefore be less resilient and would deform at a lower force than without the plasticizer. Hence, lower tensile strength is expected with the addition of plasticizer. Similarly, the elongation is expected to be higher with the addition of plasticizer. The Young’s modulus is the slope of the linear section on the stress–strain curve where the film undergoes the elastic deformation, and because there is greater chain mobility with the addition of a plasticizer there is less resistance to deformation hence a lower Yong’s modulus is expected. Thus, by studying changes in tensile strength, percent elongation, and elastic modulus with different plasticizers and concentrations of plasticizer, the plasticizer efficiency on Soluplus® can be assessed.
The stress–strain curve from the tensile test can be used to characterize the mechanical properties of the cast films. A typical stress–strain curve of the solvent cast Soluplus® film is shown in Fig. 7; it should be noted that Soluplus® films had approximately the same general shape. The tensile strength was calculated from the stress–strain curve, using Eq. 1 and the force at which the film breaks (see Fig. 7). The percent elongation was calculated using the strain at break (indicated by the arrows shown in Fig. 7) and Eq. 3. The Young’s modulus was calculated from the slope of the initial linear section on the stress strain curve where the film undergoes the elastic deformation; Fig. 7 shows he points that were used for the calculation. The Tg reduction discussed in earlier section shows that all plasticizers tested are capable of plasticizing the films. Therefore, improved mechanical properties are expected with the addition of these plasticizers.
Fig. 7.

Example of stress–strain curve for plasticized Soluplus® film (graph from 20% w/w TEC)
Tensile Strength
The mechanical properties are useful indications of film strength. Films that show higher tensile strength correspond to stronger film as the tensile strength of a film is the maximum stress that a film can withstand being stretched before necking or cracking (21). Figure 8 shows the tensile strength at break of Soluplus® as a function of the plasticizers content. Tensile strength reductions were seen with the addition of plasticizers, which is consistent with the general expectation for a plasticized polymer (17). At (15% w/w) plasticizer level, films containing PPG yield the highest tensile strength (13.4 MPa) followed by GLY (10.5 MPa) and the lowest tensile strength films contained PEG-6 and TEC where the tensile strengths were 8.8 and 8.5 MPa, respectively (see Table I). At higher plasticizer levels (25% w/w), the film containing PPG still had the highest tensile strength (6.8 MPa). The greatest reduction of tensile strength was seen with the addition of PEG-6 among the plasticizers tested from 20% to 25% w/w. The tensile strength of the film containing 25% w/w of PEG-6 was not measured as the film stretched without fracture under the testing condition used.
Fig. 8.

Tensile strength of Soluplus® film containing different plasticizers (error bars within symbols if not shown)
Table I.
Summary of the Tensile Strength, Percent Elongation at Break (%E), Young’s Elastic Modulus with Increasing Plasticizer Content for PEG-6, TEC, PPG, and Gly
| Plasticizer | 15% (w/v) | 20% (w/v) | 25% (w/v) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| TS (MPa) | %E | Y (MPa) | TS (MPa) | %E | Y (MPa) | TS (MPa) | %E | Y (MPa) | |
| PEG-6 | 8.8 (0.98) | 66.4 (24) | 196.0 (18) | 2.2 (0.25) | 301.8 (52) | 29.9 (3.8) | 0.37 (0.09) | 461a | 1.4a |
| TEC | 8.5 (1.9) | 2.6 (0.46) | 472.4 (21) | 9.8 (1.3) | 57.6 (42) | 148.6 (4.5) | 5.1 (0.21) | 178.5 (31) | 70.0 (3.3) |
| PPG | 13.4 (2.1) | 25.9 (29) | 326.2 (19) | 8.9 (1.5) | 65.3 (47) | 180.7 (27) | 6.8 (0.77) | 119.6 (45) | 121.1 (6.1) |
| GLY | 10.5 (0.56) | 47.3 (26) | 279.1 (19) | 7.6 (0.61) | 85.0 (55) | 153.1 (3.7) | 4.7 (0.65) | 195.5 (47) | 77.5 (10) |
aThese values should only be considered a rough approximation because the PEG-6 at 25% (w/v) made the films so ductile that there was not clear break point and the actual values are difficult to determine
Elongation
Based on the definition of plasticization, the elongation should increase with increase in the plasticizer concentration, which is observed with the plasticized Soluplus® film shown in Fig. 9 (17). The percent elongation increased as the plasticizers content increased for all the plasticizers tested. Among the plasticizers tested, PEG-6 shows the most significant changed where the percent elongation increased by 235% when the PEG-6 content increased from 15% to 20% w/w. The percent elongation of the Soluplus® film was more than 460% with addition of 25% w/w of PEG-6. The film became too ductile and the measurement of the percent elongation above 460% was unable to record under the testing condition used. The percent elongation is a useful parameter for evaluating plasticizer effectiveness as a function of type and quantity of the plasticizer 9). By comparing the percent elongation for plasticizer content of 15% and 20% w/w, PEG-6 is the most effective plasticizer; follow by GLY, PPG, and TEC. Among the films containing 25% w/w plasticizers, PEG-6 again has the highest percent elongation followed by GLY, TEC, and PPG.
Fig. 9.

Percent elongation of Soluplus® film containing different plasticizers
Young’s Modulus
The Young’s modulus is the slope of the linear section on the stress–strain curve where the film undergoes the elastic deformation. It measures the resistance of the film to elastic deformation which can be used to reflects the stiffness and strength of the film (22,23). Higher values of Young’s modulus corresponding to stiffer film where it requires higher loads to elastically deform it whereas lower Young’s modulus corresponding to flexible films where it requires lower loads to elastically deform.
Figure 10 shows the Young’s modulus of the Soluplus® with different plasticizers. The Young’s modulus decreased with the addition of the plasticizer content, i.e., the Soluplus® loses its stiffness and becomes more flexible with the addition of the plasticizer. Among the plasticizers tested, PEG-6 is the most effective plasticizer in depressing the Young’s modulus of Soluplus® film with PEG-6 content of 15% to 25% w/w. At 15% w/w plasticizer content, Soluplus® films containing TEC are stiffest (highest tensile strength value) while films containing PEG-6 are the most flexible (lowest tensile strength value). At plasticizer content of 20% and 25% w/w, Soluplus® films containing PPG are stiffest while films containing PEG-6 are again the most flexible.
Fig. 10.

Young’s modulus of Soluplus® film with different plasticizers (error bars within symbols if not shown)
Toughness
Toughness of the film is the total area under the stress–strain curve, which is a measure of how much energy a sample can absorb before it fails. While the tensile strength gives indication of how much force is needed to break a sample, toughness gives indication of how much energy is needed to break a sample (i.e., material’s resistance to fracture) (24). Hence, high toughness materials can absorb more energy before fracturing while low toughness materials absorb less energy before fracturing. A film that is strong does not necessary make it tough; similarly, a film that is ductile does not necessary make it tough. The toughness of the film depends on both the strength and ductility of the film. Hence, a film that has higher strength and ductility will have higher toughness than a material with lower strength and ductility.
Figure 11 shows the toughness of the Soluplus® film with different plasticizers. The toughness of the Soluplus® film increased as the plasticizer content increased. Toughness of film containing 25% w/w PEG-6 was not calculated since the strain was not measured due to the high ductility of the film. Nevertheless, film containing PEG-6 show highest toughness with plasticizers content of 15% and 20% w/w. At 25% w/w plasticizer level, the toughness of TEC, PPG, and Gly are not significantly different (p value > 0.5).
Fig. 11.
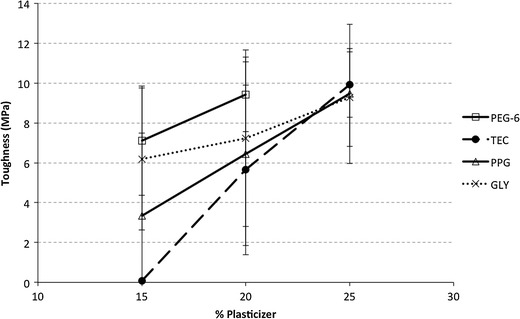
Toughness of Soluplus® film with different plasticizers
Tensile Strength/Young’s Modulus Ratio
The ratio of tensile strength to Young’s modulus is another useful parameter that predicts the crack resistance of a film (16). The resistance of a film to the initiation of the fracture process is a measure of the surface energy (γ),
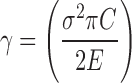 |
4 |
where σ is the tensile strength, C is the flaw or crack size before the initiation of the failure process, and E is the Young’s modulus. Since the crack size is difficult to measure, the ratio of tensile strength to Young’s modulus (σ2/2E) provides an estimate of crack resistance if C and π are ignored (16,25). Previous studies have used the crack resistance to predict the resistance of a film to cracking, with higher values exhibiting greater resistance to cracking (18,19). Figure 12 shows films containing 15–20% w/w PEG-6 yield highest ratio of tensile strength to Young’s modulus, exhibiting greatest resistance to cracking. The ratio of tensile strength to Young’s modulus for film containing 25% w/w PEG-6 was not calculated as the tensile strength value was not recorded as mentioned earlier. Soluplus® film containing TEC and PPG show increased resistance to cracking with higher ratio of tensile strength to Young’s modulus when the plasticizer content was increased from 15% to 20% w/w. However, a reduction of the ratio of tensile strength to Young’s modulus was observed when the plasticizer level increased from 20% to 25% w/w. This data suggest there is no benefit to increase the plasticizer content for TEC and PPG from 20% to 25% w/w if the aim is to increase the resistance to cracking. The resistance to cracking of the Soluplus films® are not significantly affected by increasing the GLY content from 15% to 25% w/w (p value > 0.5).
Fig. 12.

Tensile strength/young’s modulus ratio of Soluplus® film with different plasticizers
CONCLUSIONS
The mechanical properties of the Soluplus® film with addition of four different plasticizers were tested. All four plasticizers effectively decreased the Tg, tensile strength, and Young’s modulus and increased the percent elongation and toughness of the film. Among the plasticizers tested, PEG-6 is the most efficient plasticizer in changing the mechanical properties of the films. This study can serve as a reference for other researchers when selecting a plasticizer in their Soluplus® formulations.
REFERENCES
- 1.Hardung H, Djuric D. Soluplus®. A novel excipient for hot melt extrusion. Chim Oggi Chem Today. 2010;28(5):XIV–XV. [Google Scholar]
- 2.Hughey JR, Keen JM, Brough C, Saeger S, McGinity JW. Thermal processing of a poorly water-soluble drug substance exhibiting a high melting point: the utility of KinetiSol® dispersing. Int J Pharm. 2011;419(1–2):222–30. doi: 10.1016/j.ijpharm.2011.08.007. [DOI] [PubMed] [Google Scholar]
- 3.Nagy ZK, Balogh A, Vajna B, Farkas A, Patyi G, Kramarics Á, et al. Comparison of electrospun and extruded soluplus®-based solid dosage forms of improved dissolution. J Pharm Sci. 2011;101(1):322–32. doi: 10.1002/jps.22731. [DOI] [PubMed] [Google Scholar]
- 4.Onike O, Dhumal R, Kelly A, Paradkar A. Tadalafil-soluplus solid dispersion using melt extrusion for improved dissolution. J Pharm Pharmacol. 2010;62(10):1476–7. [Google Scholar]
- 5.Crowley MM, Zhang F, Repka MA, Thumma S, Upadhye SB, Battu SK, et al. Pharmaceutical applications of hot-melt extrusion: part I. Drug Dev Ind Pharm. 2007;33(9):909–26. doi: 10.1080/03639040701498759. [DOI] [PubMed] [Google Scholar]
- 6.Repka MA, Gerding TG, Repka SL, McGinity JW. Influence of plasticizers and drugs on the physical–mechanical properties of hydroxypropylcellulose films prepared by hot melt extrusion. Drug Dev Ind Pharm. 1999;25(5):625–33. doi: 10.1081/DDC-100102218. [DOI] [PubMed] [Google Scholar]
- 7.Repka MA, Battu SK, Upadhye SB, Thumma S, Crowley MM, Zhang F, et al. Pharmaceutical applications of hot-melt extrusion: part II. Drug Dev Ind Pharm. 2007;33(10):1043–57. doi: 10.1080/03639040701525627. [DOI] [PubMed] [Google Scholar]
- 8.Prodduturi S, Manek RV, Kolling WM, Stodghill SP, Repka MA. Solid-state stability and characterization of hot-melt extruded poly(ethylene oxide) films. J Pharm Sci. 2005;94(10):2232–45. doi: 10.1002/jps.20437. [DOI] [PubMed] [Google Scholar]
- 9.Meier L. Plasticizers. In: Gächter R, Müller H, editors. Plastics additives handbook. 4. Cincinnati: Hanser; 1996. pp. 327–422. [Google Scholar]
- 10.Wilson AS. Plasticizers. Principles and practices. Cambridge: The Institute of Materials; 1995. [Google Scholar]
- 11.Aharoni SM. Increased glass transition temperature in motionally constrained semicrystalline polymers. Polym Adv Technol. 1998;9(3):169–201. doi: 10.1002/(SICI)1099-1581(199803)9:3<169::AID-PAT740>3.0.CO;2-Z. [DOI] [Google Scholar]
- 12.Pradhan DK, Choudhary RNP, Samantaray BK, Karan NK, Katiyar RS. Effect of plasticizer on structural and electrical properties of polymer nanocompsoite electrolytes. Int J Electrochem Sci. 2007;2:861–71. [Google Scholar]
- 13.Mididoddi PK, Prodduturi S, Repka MA. Influence of tartaric acid on the bioadhesion and mechanical properties of hot-melt extruded hydroxypropyl cellulose films for the human nail. Drug Dev Ind Pharm. 2006;32(9):1059–66. doi: 10.1080/03639040600683410. [DOI] [PubMed] [Google Scholar]
- 14.Repka MA, McGinity JW. Influence of vitamin E TPGS on the properties of hydrophilic films produced by hot-melt extrusion. Int J Pharm. 2000;202(1–2):63–70. doi: 10.1016/S0378-5173(00)00418-X. [DOI] [PubMed] [Google Scholar]
- 15.Elgindy N, Samy W. Evaluation of the mechanical properties and drug release of cross-linked Eudragit films containing metronidazole. Int J Pharm. 2009;376(1–2):1–6. doi: 10.1016/j.ijpharm.2009.03.026. [DOI] [PubMed] [Google Scholar]
- 16.Okhamafe AO, York P. Stress crack resistance of some pigmented and unpigmented tablet film coating systems. J Pharm Pharmacol. 1985;37(7):449–54. doi: 10.1111/j.2042-7158.1985.tb03038.x. [DOI] [PubMed] [Google Scholar]
- 17.Wypych G. Handbook of plasticizers. Ontario, Canada: ChemTec; 2004. [Google Scholar]
- 18.Felton LA. Characterization of coating systems. AAPS PharmSciTech. 2007;8(4):E1–9. doi: 10.1208/pt0804112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rowe R. Correlations between the in-situ performance of tablet film coating formulations based on hydroxypropyl methylcellulose and data obtained from tensile testing of free films. Pharm Technol. 1983;29:205–7. [Google Scholar]
- 20.Heinamaki JT, Lehtola VM, Nikupaavo P, Yliruusi JK. The mechanical and moisture permeability properties of aqueous based hydroxypropyl methylcellulose coating systems plasticized with polyethylene-glycol. Int J Pharm. 1994;112(2):191–6. doi: 10.1016/0378-5173(94)90429-4. [DOI] [Google Scholar]
- 21.Aulton ME. Assessment of the mechanical properties of film coating materials. Int J Pharm Technol Prod Manuf. 1982;3:9–16. [Google Scholar]
- 22.Roberts RJ, Rowe RC. The Young’s modulus of pharmaceutical materials. Int J Pharm. 1987;37(1–2):15–8. doi: 10.1016/0378-5173(87)90004-4. [DOI] [Google Scholar]
- 23.Omelczuk MO, McGinity JW. The influence of polymer glass transition temperature and molecular weight on drug release from tablets containing poly(dl)-lactic acid) Pharm Res. 1992;9(1):26–32. doi: 10.1023/A:1018967424392. [DOI] [PubMed] [Google Scholar]
- 24.Davis JR. Tensile testing. 2. OH: ASM International; 2004. [Google Scholar]
- 25.Jackson WJ, Caldwell JR. Antiplasticization. III. Characteristics and properties of antiplasticizable polymers. J Appl Polym Sci. 1967;11(2):227–44. doi: 10.1002/app.1967.070110206. [DOI] [Google Scholar]


