Abstract
Artemisinin, a poorly water-soluble antimalarial drug, presents a low and erratic bioavailability upon oral administration. The aim of this work was to study an agglomerated powder dosage form for oral administration of artemisinin based on the artemisinin/β-cyclodextrin primary microparticles. These primary microparticles were prepared by spray-drying a water–methanol solution of artemisinin/β-cyclodextrin. β-Cyclodextrin in spray-dried microparticles increased artemisinin water apparent solubility approximately sixfold. The thermal analysis evidenced a reduction in the enthalpy value associated with drug melting, due to the decrease in drug crystallinity. The latter was also evidenced by powder X-ray diffraction analysis, while 13C-NMR analysis indicated the partial complexation with β-cyclodextrin. Agglomerates obtained by sieve vibration of spray-dried artemisinin/β-cyclodextrin primary microparticles exhibited free flowing and close packing properties compared with the non-flowing microparticulate powder. The in vitro dissolution rate determination of artemisinin from the agglomerates showed that in 10 min about 70% of drug was released from the agglomerates, whereas less than 10% of artemisinin was dissolved from raw material powder. Oral administration of agglomerates in rats yielded higher artemisinin plasma levels compared to those of pure drug. In the case of the agglomerated powder, a 3.2-fold increase in drug fraction absorbed was obtained.
Key words: agglomerates, artemisinin, cyclodextrin, malaria, microparticles
INTRODUCTION
The therapy of malaria involves complex dosage regimens that are often difficult for patient adherence; this is likely the most important cause of the emergence of multi-drug resistant strains of microorganisms causing malaria (1,2). WHO policy regarding malaria treatment advocates the use of artemisinin or its derivatives in combination with other antimalarial drugs (3). Artemisinin is the active principle of the Chinese medicinal plant Artemisia annua (Quinghaosu) (4). It is the precursor of an important class of antimalarial drugs, structurally characterised by the presence of a sesquiterpene lactone with a peroxide bridge, which determines the antimalarial activity (5). Many derivatives have been synthesised from dihydroartemisinin, the active metabolite of artemisinin; among others, artemether, artesunate and artelinic acid are either currently in use or undergoing clinical evaluation (6). Artemisinin and its derivatives have a very fast action and the clearance time of parasites is shorter than with other antimalarial drugs, such as chloroquine (7).
The use of artemisinin in combinations is not restricted in children or pregnant women and this is a valuable advantage of this antimalarial drug because children and pregnant women have the highest risk for malaria-associated morbidity and mortality (8). Paediatric administration requires dosage forms suitable for different ages and abilities. Moreover, a range of strengths or concentrations enabling administration of the correct age-related dose is needed. Agglomerated powders can be employed as extemporaneous preparations, useful for children or patients with swallowing difficulties.
Owing to its low water solubility, artemisinin is characterised by poor and erratic bioavailability that, if improved, would give to artemisinin a more significant role in therapy. A previous study (9) in patients with uncomplicated falciparum malaria demonstrated the therapeutic equivalence of a 150-mg dose of artemisinin/β-cyclodextrin granules prepared by the “slurry method”, compared to a commercial preparation of artemisinin (250 mg). However, these hard granules were less suitable for the preparation of a fine dispersion to be administered to children. Agglomeration is a process in which a powder of relatively large size is obtained by tumbling or vibrating primary microparticles, which aggregate while keeping their original size and structure (10). Thus, agglomerates are clusters of primary microparticles held together by weak bonds. Improved packing and flow characteristics are the main features of these agglomerated preparations. Once the agglomerates enter in contact with water, the primary microparticles are released by disintegration or deagglomeration. Owing to its agreeable softness, the agglomerates can also be directly poured in the mouth.
Previous studies (11,12) have shown that a spray-drying technique could enable the preparation of drug/β-cyclodextrin complexes as micronized particles. So far, primary microparticles used for agglomeration have been prepared from mannitol/lecithin spray-dried solutions (10,13,14). The spray-dried β-cyclodextrin primary microparticles can be envisaged as a base for agglomerated dosage form for manufacturing. In the case of artemisinin, beside the technological aspects, a substantial biopharmaceutical advantage could be achieved by making primary microparticles with β-cyclodextrin (βCD), since artemisinin, as a poorly soluble drug, has its solubility increased by complexation with βCD (9,15–17). Phase solubility studies carried out to investigate the complexation capacity of the cyclodextrins with artemisinin in aqueous solution have shown a significant increase in drug solubility with linear phase solubility diagram classified as AL type (18–20).
The aim of this work was to study an agglomerated dosage form for oral administration of artemisinin based on artemisinin/β-cyclodextrin primary microparticles prepared by spray-drying. Up to now, none of the inclusion/complexation studies of artemisinin with cyclodextrins have involved the spray-drying technique. βCD was chosen for its frequent use in oral formulations (21). The artemisinin/βCD primary microparticles were agglomerated by sieve vibration. Solid-state characteristics of spray-dried primary microparticles and agglomerates were analysed by optical microscopy, scanning electron microscopy (SEM), differential scanning calorimetry (DSC), powder X-ray diffraction (PXRD), Fourier-transform infrared spectroscopy (FTIR) and solid-state nuclear magnetic resonance. In vitro drug dissolution rate and oral bioavailability in rats of both agglomerates and artemisinin raw material were measured and compared.
MATERIAL AND METHODS
Materials
Artemisinin raw material was purchased from Kunming Pharmaceutical Co. (Yunnan, China). β-Cyclodextrin (Kleptose R) was obtained from Roquette (Alessandria, Italy) and amlodipine from Cadila Pharmaceuticals Ltd. (Ahmedabad, India). All other reagents and chemicals used in the study were of analytical or high-performance liquid chromatography (HPLC) grade.
Methods
Artemisinin/β-Cyclodextrin Primary Microparticle Preparation
Artemisinin/β-cyclodextrin primary microparticles were obtained by spray-drying water–methanol solutions of artemisinin and βCD in 1:1 molar ratio. β-Cyclodextrin (4 g) was dispersed in 200 ml of water at 40°C to obtain a solution, whereas artemisinin (1 g) was dissolved in 200 ml of methanol. The organic solution was slowly added to the βCD water solution. The final solution of artemisinin and βCD was maintained at 40°C under continuous stirring while pumped into the spray-dryer. The total solid concentration was 1.25% (w/v). The spray-dryer equipment employed was a Büchi Mini Spray Dryer B-191 (Büchi Laboratoriums-Tecnik, Flawil, Switzerland). Spray-drying conditions were as follows: inlet temperature 130°C, outlet temperature 49–52°C, feed rate 4 ml/min, nozzle diameter 0.7 mm and drying air flow 600 L/h.
In the case of artemisinin alone, 0.6 g of drug was dissolved in 250 ml of methanol and 250 ml of water was then slowly added. The solution was spray-dried using the same conditions as those applied for the preparation of artemisinin/βCD primary microparticles.
A physical mixture of artemisinin and βCD (1:1 molar ratio) was also prepared by carefully mixing the two substances in a 10-ml glass vial. In addition, samples of artemisinin and βCD mixture were prepared by the slurry method for comparative purposes (15).
Agglomerate Preparation
Agglomerates were prepared by vibrating 4 g of artemisinin/βCD spray-dried powder on two stacked sieves (20 cm diameter) with mesh sizes 800 and 100 μm, respectively. The sieve pile was vibrated for 10 min at a vibration amplitude of 5–6 (Analysette 3 Fritz model, Fritsch GMBH, Idar-Oberstein, Germany). Agglomerates retained on the 100-μm sieve were collected. Agglomerates retained on the 800-μm sieve and the non-agglomerated powder (below 100 μm) were re-processed up to five times.
Microparticle and Agglomerate Characterization
Size Distribution Analysis
Size distribution analysis of artemisinin and artemisinin/βCD spray-dried microparticles was performed using a Spraytech® laser diffractometer (Malvern Instruments, Malvern, UK). Artemisinin was suspended in distilled water with 0.1% (w/v) of Tween 80 and directly analysed. In contrast, saturated cyclohexane solutions of βCD or artemisinin/βCD spray-dried microparticles, previously prepared, were used as suspending medium for the analysis of βCD and artemisinin/βCD spray-dried microparticles, respectively. Nearly 50 mg of each powder was dispersed in a 10-ml flask with the suitable suspending medium. The sample was then sonicated for a few minutes to eliminate the aggregates. Some drops of the suspension obtained were introduced in the diffractometer analysis chamber filled with the appropriate medium. The experiment was performed in triplicate for each powder.
Drug Loading
Drug loading in the spray-dried powder was determined by HPLC in accordance with the method reported by Sahoo et al. (22). The solubility of artemisinin and artemisinin/βCD spray-dried microparticles was measured in distilled water. Briefly, an excess of artemisinin raw material or of spray-dried microparticles was added to 5 ml of water in rubber-stoppered vials. The samples were left under magnetic stirring for 24 h at room temperature (25°C); 1 ml of the suspension was then centrifuged at 4,500 rpm for 10 min (Heraeus Labofuge 200, Thermo-Fisher Scientific, Waltham, Massachusetts, USA). After filtration of the supernatant with a 0.45-μm PTFE membrane (ReZist Syringe Filter, Maidstone, UK), the artemisinin concentration in the samples was determined by HPLC. Each analysis was performed in triplicate.
Scanning Electron Microscopy
The morphology of artemisinin and artemisinin/βCD spray-dried microparticles was examined by SEM (Leica Cambridge S-360, Leica Microsystem GMBH, Wetzlar, Germany). The samples were fixed onto an aluminium stub with double-sided tape before being coated with gold to a thickness of approximately 20 nm (Polaron SSC-515, VG Microtech, Uckfield, UK). For the artemisinin/βCD agglomerate images, the apparatus used was Zeiss SUPRA 40, Carl Zeiss (Oberkochen, Germany). The magnifications selected were ×500–4,000. The agglomerates were also examined under an optical stereomicroscope (magnification ×20) (Citoval 2, Jena, Germany) connected to a video camera (JVC, Tokyo, Japan).
Differential Scanning Calorimetry
DSC (PerkinElmer Pyris 6, Beaconsfield, UK) was performed on artemisinin, βCD, artemisinin–βCD physical mixture and artemisinin/βCD spray-dried microparticles to evaluate the degree of artemisinin complexation. Melting temperature and the enthalpy of fusion (ΔHf) of artemisinin were measured. DSC thermograms were recorded by placing accurately weighed amount of the sample (about 4–6 mg) in a 40-μl aluminium pan, sealed and pierced. The samples were heated from 25 to 180°C at a rate of 10°C/min under a dynamic nitrogen atmosphere (100 ml min−1). Each analysis was done in triplicate.
Powder X-ray Diffraction
PXRD analysis was carried out with a Miniflex X-Ray Diffractometer (Rigaku, Tokyo, Japan) using a Cu Kα radiation source (λ = 1.5418 Å) generated with 30 kV voltage over the scanning range (2θ) 5–50° (scanning speed of 0.05°/min). The sample size was about 500 mg.
Fourier-Transform Infrared Spectroscopy
FTIR analyses were performed using a Nicolet Nexus 7 FTIR (Thermo Nicolet, Madison, Wisconsin, USA). The scanning range was 4,000–400 cm−1 at the resolution of 1 cm−1. Each sample was mixed with potassium bromide at the ratio of 1:99 and then moulded in a disc with a hydraulic press. Each analysis was done in triplicate.
Solid-State NMR Analysis
Solid-state 13C CP-MAS NMR analysis of artemisinin, βCD, artemisinin–βCD physical mixture and artemisinin/βCD spray-dried microparticles was performed on a Bruker Avance 300 (Billerica, Massachusetts, USA) operating at 75 MHz.
Bulk and Tapped Density Determination
Bulk and tapped densities of artemisinin/βCD spray-dried microparticles and agglomerates were measured according to Ph. Eur. 7th Ed., whereas Carr’s Index was calculated by the equation reported by Carr (23).
In Vitro Dissolution Test
The drug dissolution rate from artemisinin/βCD agglomerates and artemisinin raw material was determined in 1,000 ml of degassed water at 37 ± 0.5°C, using a USP 34 Apparatus 1 (DT6 R, Erweka, Heusenstamm, D) with basket rotating at 100 rpm. Artemisinin and artemisinin/βCD agglomerates were introduced in hard gelatin capsules (size 00, Coni Snap, Capsugel®, Bornem, Belgium). Samples withdrawn at fixed times were filtered through a nylon membrane with polypropylene housing (pore size 0.2 μm, Puradisc™, Whatman, Maidstone, UK).
In Vivo Studies
The bioavailability study, approved by the Animal Ethics Committee of Universiti Sains Malaysia (approval #USM/PPSF/50 (079) Jld.2), involved adult male Sprague-Dawley rats weighing 372.0 ± 23.3 g. The animals were orally administered by gavage with artemisinin/βCD agglomerates or pure artemisinin powder (raw material). Just before administration, each preparation was dispersed in water under magnetic stirring, and 2 ml of drug dispersion was then collected with a syringe and administered to rats.
Artemisinin administered dose was 10 mg/kg body weight. Blood samples obtained by tail-clipping were collected at 20, 40, 60, 90, 120, 150, 180, 240, 360, and 600 min and stored in heparinised microcentrifuge tubes. The blood samples were centrifuged at 12,800×g for 10 min; subsequently, the plasma samples obtained were stored at −80°C until analysis.
Liquid chromatography separation module Alliance 2695 (Waters, Milford, Massachusetts, USA) coupled with a tandem mass spectrometry (MS/MS; Quattro Micro, Waters) was employed using atmospheric pressure ionisation source with electrospray ionisation operated in positive mode. The chromatographic separation was obtained using an X-Terra C8 (150 × 2.1 mm; 5 μm) analytical column (Waters, Milford, Massachusetts, USA). Mobile phase consisted of 1:1 acetonitrile/formic acid (0.1% v/v) aqueous solution. Flow rate was set at 0.2 ml/min. The fragmentation transitions surveyed were m/z 282.3 to 209.0 for artemisinin and m/z 408.9 to 237.7 for amlodipine, used as internal standard. Plasma samples were prepared by the liquid/liquid extraction method with 5 ml of ethyl ether and shaking for 2.5 min before centrifugation at 3,500×g for 10 min. The upper organic phase was transferred into an Eppendorf vial and evaporated to dryness at 40°C under a gentle stream of nitrogen. The dried samples were reconstituted with 100 μl of mobile phase prior to injection. A 20-μl aliquot of each sample was injected into the LC-MS/MS system for analysis.
Statistical Analysis
The in vitro drug release profiles of artemisinin raw material, artemisinin/βCD slurry powder and artemisinin/βCD agglomerates were compared using the similarity factor (f2). f2 value larger than 50 indicates that two dissolution profiles are similar (24,25).
One-way ANOVA test on in vivo data was performed by means of Kaleida Graph, Version 4.1.3 programme (Synergy Software, Reading, Pennsylvania, USA).
RESULTS AND DISCUSSION
Primary Microparticle Preparation and Characterization
Artemisinin/βCD primary microparticles were prepared by spray-drying a methanol solution of artemisinin mixed with an aqueous solution of βCD (1:1 molar ratio). Here, βCD is proposed as excipient for the manufacturing of microparticles to be used for the first time for agglomeration, as an alternative to the described mannitol/lecithin combination (10,13,14). It was expected that the artemisinin/βCD primary microparticles could exhibit sufficient cohesion properties to allow for the construction of the agglomerate structure, with a further expected advantage of an enhanced artemisinin apparent solubility (15,17).
The spray-dried powder yield was 60 ± 5% (n = 4) calculated on the total amount of artemisinin and βCD processed. The artemisinin loaded in the artemisinin/βCD primary microparticles was 21 ± 1% (w/w), in agreement with the expected value.
The volume median diameter D(v,0.5) of artemisinin/βCD spray-dried primary microparticles determined by laser light scattering was 8.9 ± 0.9 μm, while the D(v,0.5) of artemisinin and βCD starting raw materials was 38.7 ± 1.8 and 7.0 ± 0.1 μm, respectively. The data reported in Table I show a narrow size distribution of spray-dried microparticles compared with the artemisinin raw material. Moreover, the mean size of the artemisinin/βCD spray-dried microparticles had the same order of magnitude as βCD particles, significantly lower than artemisinin raw material.
Table I.
Volume median diameters (micrometre) of each powder (mean and standard deviation; n = 3)
| Sample | D(v,0.1) | D(v,0.5) | D(v,0.9) |
|---|---|---|---|
| Artemisinin (raw material) | 14.3 ± 0.4 | 38.7 ± 1.8 | 89.7 ± 1.9 |
| βCD | 2.7 ± 0.1 | 7.0 ± 0.1 | 13.4 ± 1.1 |
| Artemisinin/βCD spray-dried primary microparticles | 2.8 ± 0.4 | 8.9 ± 0.9 | 18.0 ± 1.1 |
SEM images of artemisinin raw material exhibited crystal particles (Fig. 1a), while those of artemisinin/βCD spray-dried powders exhibited aggregated roundish-shaped microparticles (Fig. 1b). In the case of artemisinin/βCD powder prepared according to the described slurry method (15), the original structure of artemisinin was still present in the particles; some of them appeared to be like a granule in structure and size (Fig. 1c).
Fig. 1.

SEM images of a artemisinin raw material; b artemisinin/βCD spray-dried primary microparticles and c artemisinin/βCD powder by slurry method
DSC analysis of artemisinin/βCD (1:1 molar ratio) spray-dried primary microparticles (Fig. 2), compared to artemisinin raw material crystals, showed a reduced drug melting peak at about 153°C (for the DSC analysis of βCD, see the reference (26)). The enthalpy of fusion (ΔHf) of artemisinin in artemisinin/βCD spray-dried primary microparticles was significantly lowered (Table II). A residual crystallinity of 30.6% for artemisinin/βCD spray-dried microparticles was calculated from the ratio between the value of enthalpy of fusion of this powder and that of crystalline artemisinin raw material. In the case of the artemisinin/βCD powders obtained by the slurry method, the value of the degree of crystallinity was 88.1% (see Table II). In contrast, no quantitative differences were found between the values of ΔHf of artemisinin raw material and those of artemisinin–βCD physical mixture. Thus, the spray-drying of the artemisinin solution in the presence of β-cyclodextrin caused a more significant reduction in the degree of crystallinity of the drug compared with the other method (15). Furthermore, a solution of artemisinin was spray-dried in the absence of cyclodextrin. The DSC trace of artemisinin spay-dried powder did not show a significant variation in the drug enthalpy of fusion, thus supporting the hypothesis that the modification of drug solid state stemmed mainly from the drug interaction with βCD.
Fig. 2.

DSC curves of a artemisinin raw material, b artemisinin–βCD physical mixture, c 1:1 artemisinin/βCD spray-dried microparticles and d 1:2 artemisinin/βCD spray-dried microparticles
Table II.
DSC data of the artemisinin and derived powders (mean and standard deviation; n = 3)
| Sample | Melting peak (°C) | ΔHf (J/g) | Crystalline degree (%) |
|---|---|---|---|
| Artemisinin (raw material) | 153.4 ± 0.1 | 86.4 ± 0.9 | 100 |
| Artemisinin–βCD (physical mixture) | 153.2 ± 0.2 | 85.6 ± 0.5 | 99.1 |
| Artemisinin/βCD (slurry method) | 152.6 ± 0.1 | 76.1 ± 0.2 | 88.1 |
| Artemisinin/βCD spray-dried primary microparticles | 155.2 ± 0.2 | 26.4 ± 0.1 | 30.6 |
The PXRD patterns of artemisinin raw material confirmed the crystalline nature of the drug. No variation in drug crystallinity was observed in the artemisinin–βCD physical mixture, while the PXRD analysis of artemisinin/βCD (1:1) spray-dried primary microparticles showed a diffraction pattern similar to that of the physical mixture, but with substantially lower peak intensities (Fig. 3). Both the thermal and the diffraction analyses pointed to the fact that spray-drying a 1:1 molar ratio solution of artemisinin and βCD significantly reduced artemisinin crystallinity and that an interaction between drug and βCD might occur, suggesting a partial drug complexation in βCD. This assumption would be in agreement with previous studies that have reported on the capacity of cyclodextrin to accommodate artemisinin (17,27).
Fig. 3.

PXRD of a artemisinin raw material, b artemisinin–βCD physical mixture and c artemisinin/βCD spray-dried microparticles
The strong reduction in artemisinin crystallinity in the artemisinin/βCD spray-dried microparticles led to an increase in drug apparent solubility. The solubility of artemisinin in the artemisinin/βCD spray-dried microparticles (246.7 ± 4.1 μg/ml) was sixfold higher than that of artemisinin raw material (44.7 ± 0.7 μg/ml).
The FTIR analysis of artemisinin/βCD (1:1) primary spray-dried microparticles evidenced that the characteristic peak of C–H stretching of artemisinin shifted from 2,950 to 2,980 cm−1, supporting the existence of an interaction between artemisinin and βCD.
The 13C NMR spectrum of artemisinin/βCD spray-dried primary microparticles, compared with those of artemisinin and βCD, showed the broadening of the signals corresponding to βCD indicating the interaction with artemisinin (Fig. 4). The NMR signals of artemisinin, although less intense, were present in the artemisinin/βCD spray-dried microparticle spectrum and no shifting was observed, suggesting the existence of drug in uncomplexed form in the spray-dried powder. However, the presence of new “broad” signals in the regions around 96, 50 and 45 ppm of the spectrum of artemisinin/βCD spray-dried microparticles could support a partial complexation of the drug with βCD. Moreover, by overlapping the 13C NMR spectra of βCD and artemisinin/βCD spray-dried microparticles, an absence of chemical shift of βCD signals between 75 and 70 ppm was observed. This chemical shift is assumed as a characteristic indication of the formation of a drug/βCD inclusion complex. Therefore, it must be postulated that the artemisinin/βCD interaction observed here would be different from the classic inclusion phenomenon.
Fig. 4.

NMR 13C spectrum of a artemisinin raw material, b β-cyclodextrin, and c artemisinin/βCD spray-dried microparticles
Complete drug complexation in the cyclodextrin cavity could be obtained by increasing the amount of βCD (28). Thus, 1:2 molar ratio artemisinin/βCD spray-dried primary microparticles were prepared. The DSC trace of these latter microparticles did not present the melting peak of artemisinin (Fig. 2).
Agglomerate Preparation and Characterization
Agglomerates of both 1:1 and 1:2 molar ratio artemisinin/βCD spray-dried microparticles were prepared by sieve vibration. The artemisinin/βCD primary microparticles obtained by spray-drying did not show acceptable packing and flow properties for their direct usage as dosage form. Granulation or compression procedures for dosage form manufacturing were considered to be too impactive on the integrity of artemisinin/βCD microparticles, affecting their properties and performance. Thus, agglomerates were manufactured employing artemisinin/βCD spray-dried primary microparticles since sufficient cohesion forces existed between these spray-dried primary microparticles. Capillary or electrostatic forces have to be evoked in order to give an explanation for the favourable formation of agglomerates from artemisinin/βCD spray-dried microparticles without the addition of other excipients.
The efficiency of the agglomeration process yielded 88.1 ± 0.9 and 60.2 ± 2.9% for 1:1 and 1:2 molar ratio artemisinin/βCD primary microparticles, respectively. In both cases, agglomerates significantly ameliorated the technological properties of the original spray-dried powders. However, considering the high amount of βCD for the same dose of artemisinin and the minor propensity to agglomerate, no further studies were carried out on the 1:2 molar ratio artemisinin/βCD spray-dried primary microparticles.
The agglomerates obtained from 1:1 artemisinin/βCD primary microparticles showed a Carr’s Index value of 19.1 ± 0.8%, whereas that of the Carr’s Index obtained for the spray-dried powder was 44.8 ± 1.6%. These values illustrate the evident advantage of agglomeration in terms of flowability and packing properties.
Optical microscopy and SEM images of 1:1 artemisinin/βCD agglomerates and details of their surface are shown in Fig. 5. The shape of the agglomerates was globular with measured size range of 100 μm–1 mm.
Fig. 5.

Optical microscope picture of 1:1 artemisinin/βCD spray-dried microparticle agglomerates a and SEM images of artemisinin/βCD agglomerate b and surface detail of the agglomerate c
Agglomerates de-agglomerated quickly in water, reconstituting a dispersion of the 1:1 artemisinin/βCD primary microparticles. The USP 34 Apparatus 1 (basket) was used for the determination of the dissolution rate of powders loaded into capsules. The in vitro dissolution tests were carried out in sink condition. Dissolution profiles of artemisinin, artemisinin/βCD agglomerates and artemisinin/βCD powder prepared by the slurry method are presented in Fig. 6. The dissolution rate of artemisinin crystals was very slow, owing to the poor solubility and hydrophobic nature of the drug. The increased apparent solubility of artemisinin stemming from the interaction of the drug with βCD led to an evident improvement in the dissolution rate. Dissolution of artemisinin from the artemisinin/βCD agglomerates was complete in less than 1 h and faster than that obtained with the particles prepared with the slurry powder. The similarity factors (f2) between the couples artemisinin/βCD agglomerates–artemisinin raw material and artemisinin/βCD agglomerates–slurry powder were 14.17 and 41.50, respectively, indicating that the dissolution profiles were significantly different from each other.
Fig. 6.

Dissolution profiles of artemisinin raw material (circles), 1:1 artemisinin/βCD spray-dried microparticle agglomerates (squares) and artemisinin/βCD powder by slurry method (rhombi) (mean ± standard deviation; n = 6)
In Vivo Studies
Artemisinin/βCD agglomerates and artemisinin raw material were administered by gavage to rats. Artemisinin plasma levels in rats were quantified up to 3 h when administered as agglomerates. Artemisinin and the internal standard peaks were detected at retention times of approximately 6.8 and 2.1 min, respectively. The total run time for each analysis was 12 min. The mean extraction recoveries for internal standard and artemisinin were 70 and 56%, respectively. Relative standard deviation for a plasma sample containing 20.32 ng/ml of artemisinin was found to be 10.3% (n = 6). The method was linear in concentration range 5.08 ng/ml ± 7.5%–81.28 ng/ml ± 11.4% (R2 = 0.9989). The in vivo plasma profiles of artemisinin are shown in Fig. 7.
Fig. 7.
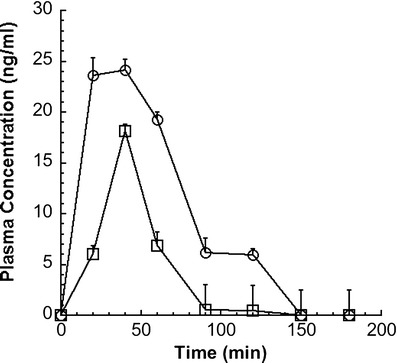
Plasma concentration time profile of 1:1 artemisinin/βCD spray-dried microparticle agglomerates (circles) and artemisinin powder (squares) orally administered both as aqueous dispersion in rats (mean ± standard error; n = 6); dose 10 mg/kg of artemisinin
Artemisinin/βCD agglomerates showed a mean AUC0−3h of 116.5 ± 0.95 versus 36.4 ± 0.68 ng ml−1 h for artemisinin raw material. Thus, a 3.2-fold increased in fraction absorbed was obtained. The AUC0−3h of artemisinin/βCD agglomerates was significantly higher (P < 0.05) than that obtained with the artemisinin suspension.
In summary, in vivo studies showed that the artemisinin/βCD agglomerates gave higher artemisinin plasma concentrations in shorter times compared to raw material.
CONCLUSIONS
βCD was shown to be a promising excipient for the preparation of drug/βCD primary microparticles capable of direct agglomeration. The technological characteristics of the agglomerated powder improved the packing and flow of the formulation.
Agglomerates of artemisinin/βCD powders dissolved more rapidly in vitro than artemisinin raw material. The increased apparent solubility and the fast dissolution rate of artemisinin from artemisinin/βCD agglomerates gave rise to a significant improvement in the bioavailability of artemisinin upon oral administration in rats.
As a consequence of the higher bioavailability, the use of artemisinin/β-cyclodextrin primary microparticles obtained by spray-drying could lead to a reduction in the artemisinin dose to be administered.
Acknowledgments
The financial support is acknowledged of the “Ministero dello Sviluppo Economico” within the project “Accordo Quadro MiSE-ICE-CRUI, progetto N.071” for collaboration between the University of Parma and Universiti Sains Malaysia. Artemisinin was a gift from Hovid Sdn Bhd (Ipoh, Malaysia), while β-cyclodextrin was kindly donated by Lisapharma S. p. A. (Erba, Italy). Moreover, the authors wish to thank Consortium TEFARCO Innova for its research support.
References
- 1.Patz JA, Olson SH. Malaria risk and temperature: influences from global climate change and local land use practices. PNAS. 2006;103(Suppl 15):5635–6. doi: 10.1073/pnas.0601493103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Reiter P. Global warming and malaria: knowing the horse before hitching the cart. Malar J. 2008;7(1):S3. doi: 10.1186/1475-2875-7-S1-S3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.WHO . World Malaria Report. Geneva: World Health Organization; 2008. [Google Scholar]
- 4.Klayman D. Quinghaosu (artemisinin): an antimalarian drug from China. Science. 1985;228:1049–55. doi: 10.1126/science.3887571. [DOI] [PubMed] [Google Scholar]
- 5.Agtmael MAV, Eggelte TA, Boxtel CJV. Artemisinin drugs in the treatment of malaria: from medicinal herb to registered medication. TiPS. 1999;20:199–205. doi: 10.1016/s0165-6147(99)01302-4. [DOI] [PubMed] [Google Scholar]
- 6.Olliaro P, Wells TNC. The global portfolio of new antimalarial medicines under development. Clin Pharmacol Ther. 2009;85(6):585–95. doi: 10.1038/clpt.2009.51. [DOI] [PubMed] [Google Scholar]
- 7.Meshnick S. Artemisinin: mechanisms of action, resistance and toxicity. Int J Parasitol. 2002;32:1655–60. doi: 10.1016/S0020-7519(02)00194-7. [DOI] [PubMed] [Google Scholar]
- 8.Nosten F, White NJ. Artemisinin-based combination treatment of falciparum malaria. Am J Trop Med Hyg. 2007;77(6):181–92. [PubMed] [Google Scholar]
- 9.Wong JW, Yuen KH, Nagappan S, Shahul WS, David Ho SS, Gan EK, et al. Therapeutic equivalence of a low dose artemisinin formulation in falciparum malaria patients. J Pharm Pharmacol. 2003;55(2):193–8. doi: 10.1211/002235702441. [DOI] [PubMed] [Google Scholar]
- 10.Russo P, Buttini F, Sonvico F, Bettini R, Massimo G, Sacchetti C, et al. Chimeral agglomerates of microparticles for the administration of caffeine nasal powders. J Drug Del Sci Tech. 2004;14(6):449–54. [Google Scholar]
- 11.Bootsma HPR, Frijlink HW, Eissens A, Proost JH, Van Doorne H, Lerk CF. β-Cyclodextrin as an excipient in solid oral forms: in vitro and in vivo evaluation of spray-dried diazepam-β-cyclodextrin products. Int J Pharm. 1989;51:213–23. doi: 10.1016/0378-5173(89)90194-4. [DOI] [Google Scholar]
- 12.Lin S-Y, Kao Y-H. Solid particulates of drug–β-cyclodextrin inclusion complexes directly prepared by a spray-drying technique. Int J Pharm. 1989;56:249–59. doi: 10.1016/0378-5173(89)90022-7. [DOI] [Google Scholar]
- 13.Russo P, Sacchetti C, Pasquali I, Bettini R, Massimo G, Colombo P, et al. Primary microparticles and agglomerates of morphine for nasal insufflation. J Pharm Sci. 2004;95(12):2553–61. doi: 10.1002/jps.20604. [DOI] [PubMed] [Google Scholar]
- 14.Raffin RP, Colombo P, Sonvico F, Polleto FS, Colombo G, Rossi A, et al. Soft agglomerates of pantoprazole gastro-resistant microparticles for oral administration and intestinal release. J Drug Del Sci Tech. 2007;17(6):407–13. [Google Scholar]
- 15.Wong JW, Yuen KH. Improved oral bioavailability of artemisinin through inclusion complexation with β- and γ-cyclodextrins. Int J Pharm. 2001;227:177–85. doi: 10.1016/S0378-5173(01)00796-7. [DOI] [PubMed] [Google Scholar]
- 16.Loftsson T, Hreinsdóttir D, Másson M. Evaluation of cyclodextrin solubilization of drugs. Int J Pharm. 2005;302:18–28. doi: 10.1016/j.ijpharm.2005.05.042. [DOI] [PubMed] [Google Scholar]
- 17.Kakran M, Gopal SN, Li L, Judeh Z. Dissolution enhancement of artemisinin with β-cyclodextrin. Chem Pharm Bull. 2011;59:646–52. doi: 10.1248/cpb.59.646. [DOI] [PubMed] [Google Scholar]
- 18.Usada M, Endo T, Nagase H, Tomono K, Ueda H. Interaction of antimalarial agent artemisinin with cyclodextrins. Drug Dev Ind Pharm. 2000;26:613–9. doi: 10.1081/DDC-100101276. [DOI] [PubMed] [Google Scholar]
- 19.Illapakurthy AC, Sabnes YA, Avery BA, Avery MA, Wyandt C. Interaction of artemisinin and its related compounds with hydroxypropyl-β-cyclodextrin in solution state: experimental and molecular-modeling studies. J Pharm Sci. 2003;92:649–55. doi: 10.1002/jps.10319. [DOI] [PubMed] [Google Scholar]
- 20.Wong JW, Yuen KH. Inclusion complexation of artemisinin with α-, β-, and γ-cyclodextrins. Drug Dev Ind Pharm. 2003;29:1035–44. doi: 10.1081/DDC-120025460. [DOI] [PubMed] [Google Scholar]
- 21.Davis ME, Brewster ME. Cyclodextrin-based pharmaceutics: past, present and future. Nat Rev Drug Discov. 2004;3:1023–35. doi: 10.1038/nrd1576. [DOI] [PubMed] [Google Scholar]
- 22.Sahoo NG, Abbas A, Judeh Z, Li CM, Yuen KH. Solubility enhancement of a poorly water-soluble anti-malarial drug: experimental design and use of a modified multifluid nozzle pilot spray drier. J Pharm Sci. 2009;98:281–96. doi: 10.1002/jps.21399. [DOI] [PubMed] [Google Scholar]
- 23.Carr RL. Evaluating flow properties of solids. Chem Eng. 1965;72:163–8. [Google Scholar]
- 24.US Department of Health and Human Services, CDER. Guidance for industry. Dissolution testing of immediate release solid oral dosage forms. Rockville: US Department of Health and Human Services, CDER; 1997.
- 25.Moore J, Flanner H. Mathematical comparison of curves with an emphasis on in vitro dissolution profiles. Pharm Tech. 1996;20:64–74. [Google Scholar]
- 26.Giordano F, Novak C, Moyano JR. Thermal analysis of cyclodextrins and their inclusion compounds. Thermochim Acta. 2001;380:123–51. doi: 10.1016/S0040-6031(01)00665-7. [DOI] [Google Scholar]
- 27.Marconi G, Monti S, Manoli F, Degli Esposti A, Mayer B. A circular dichroism and structural study of the inclusion complex artemisinin-β-cyclodextrin. Chem Phys Lett. 2004;383:566–71. doi: 10.1016/j.cplett.2003.11.084. [DOI] [Google Scholar]
- 28.Loftsoon T, Jarho P, Másson M, Järvinen T. Cyclodextrins in drug delivery. Expert Opin Drug Deliv. 2005;2:335–51. doi: 10.1517/17425247.2.1.335. [DOI] [PubMed] [Google Scholar]


