Abstract
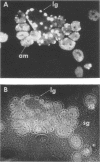
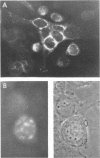
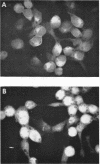
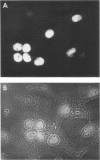
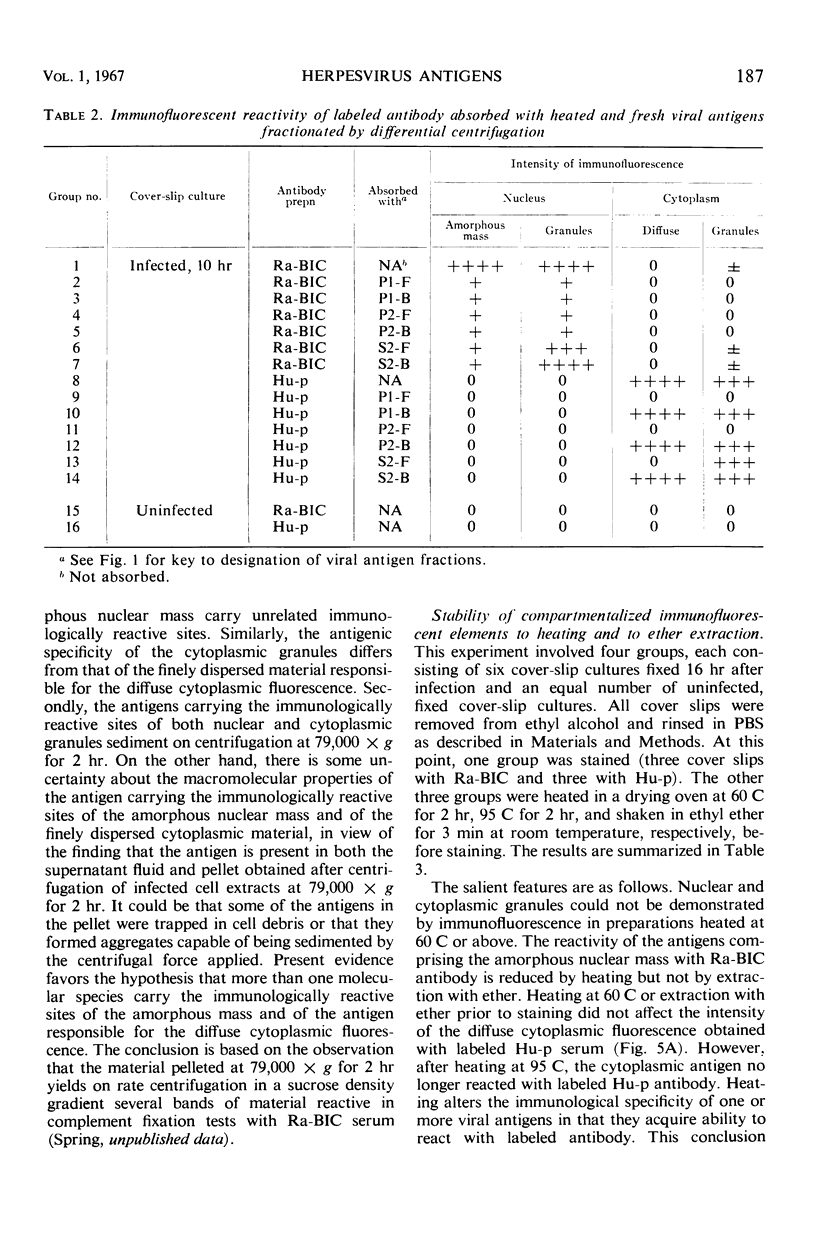
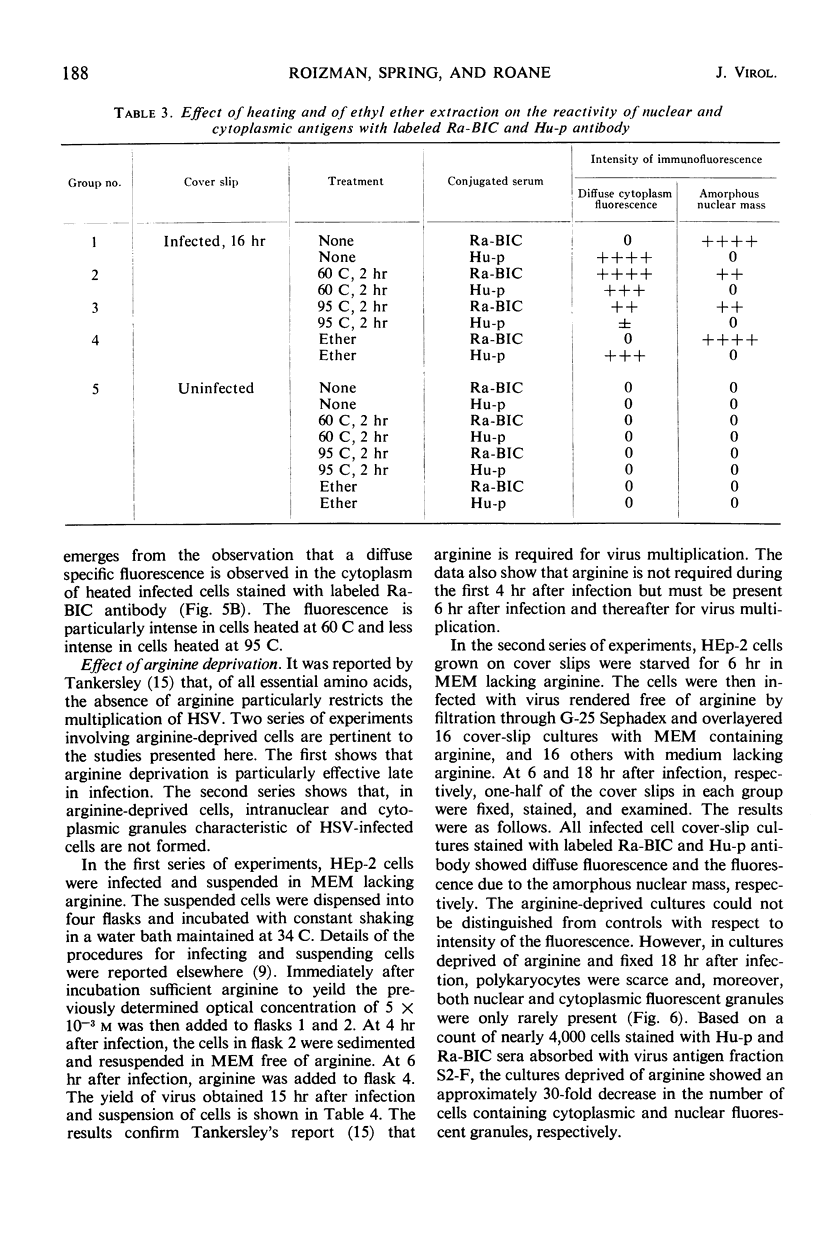
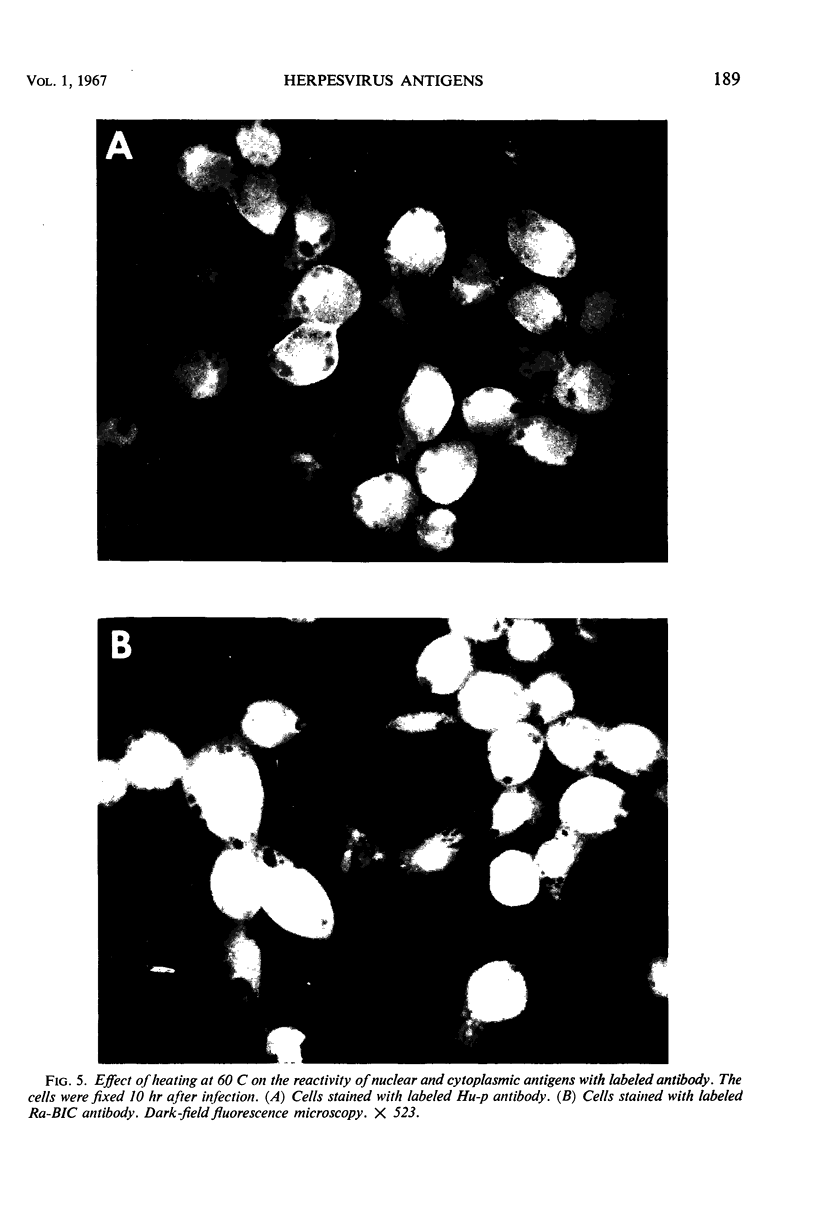
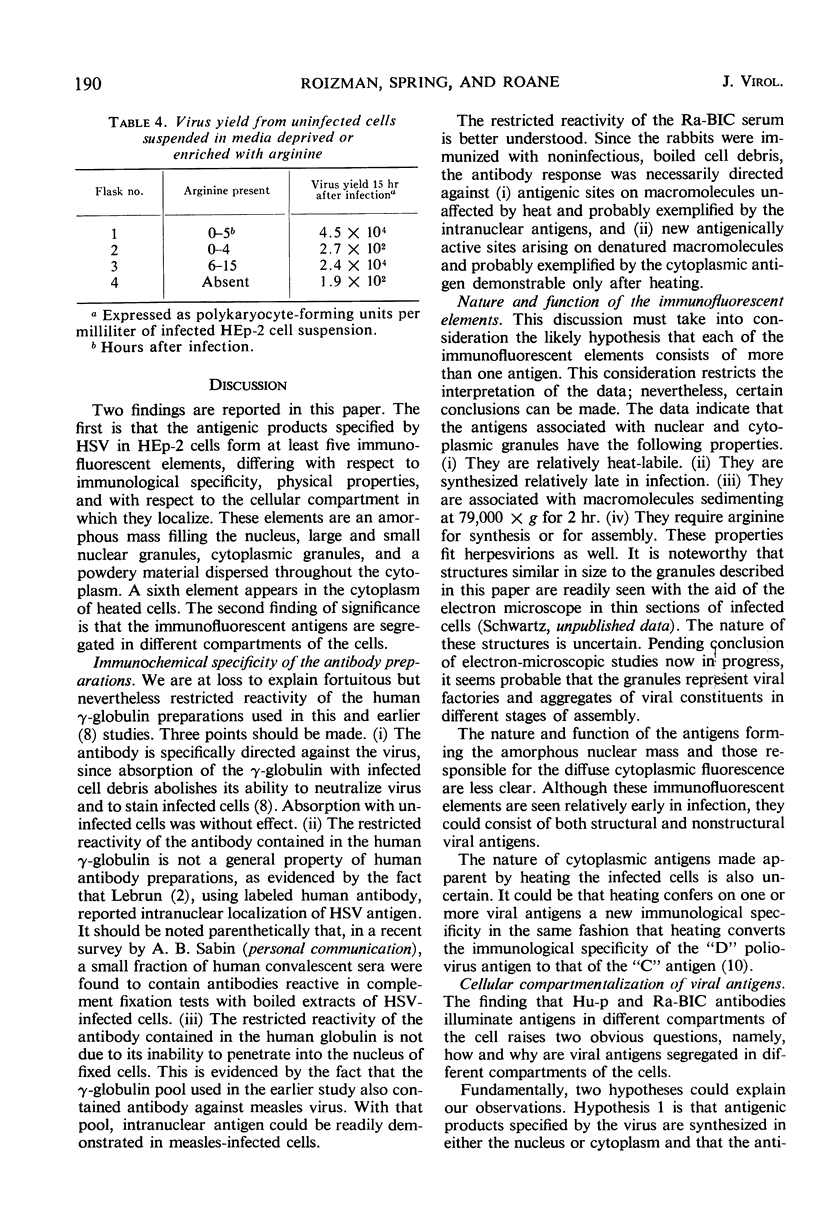
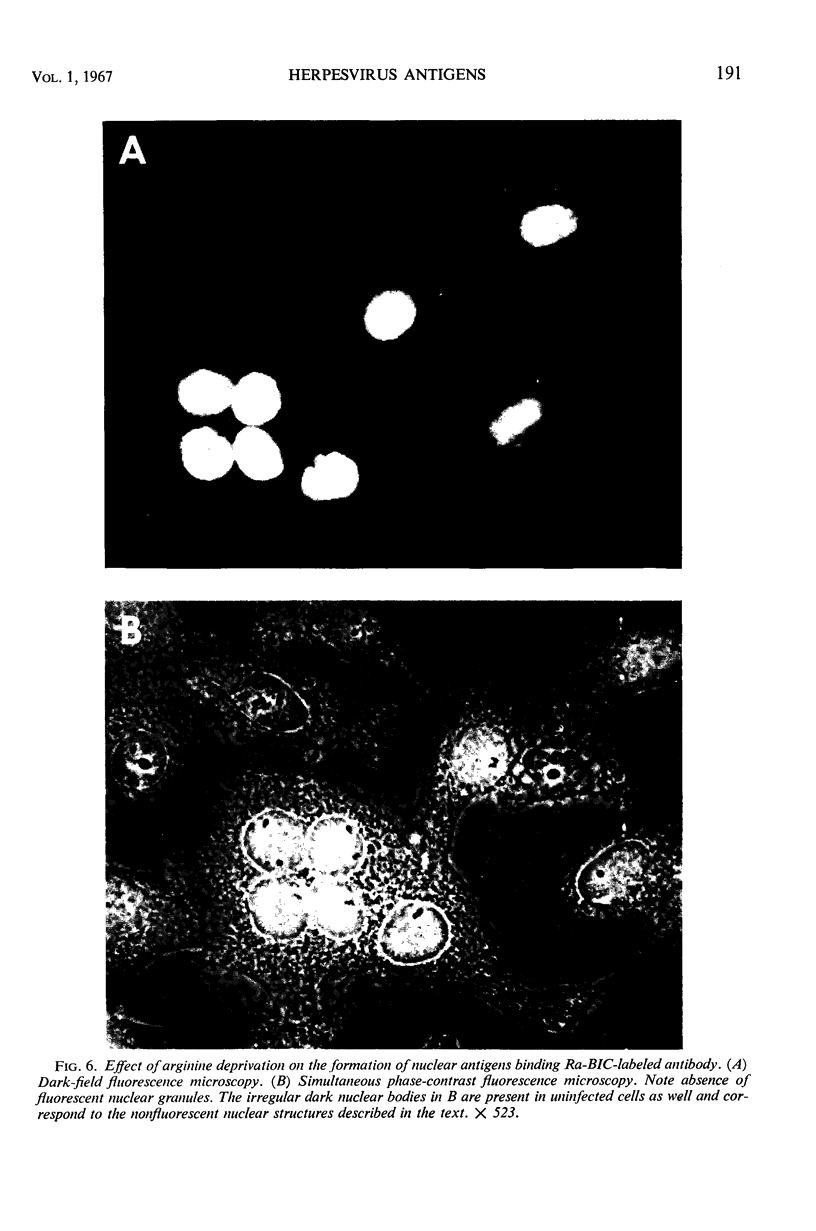
HEp-2 cells infected with herpes simplex virus develop five distinct immunofluorescent elements. Three (small nuclear granules, large nuclear granules, and an amorphous mass filling the nucleus) contain antigens which react with a rabbit serum prepared against boiled infected cell debris. A labeled pool of human antibody revealed antigens making up cytoplasmic granules and those responsible for a diffuse cytoplasmic fluorescence. All five immunofluorescent elements are demonstrable with a rabbit serum prepared against unheated infected cell debris. Viral antigens are segregated in the nucleus or in the cytoplasm; within the limits of detection, each antigen accumulates in one compartment only. The antigens responsible for the diffuse cytoplasmic fluorescence and for the amorphous nuclear mass are synthesized early in infection; they are formed in arginine-deprived cells and exist in a form which does not sediment on centrifugation at 79,000 × g for 2 hr. The antigens comprising the nuclear and cytoplasmic granules arise relatively late in infection; they are not formed in arginine-deprived cells, and they are readily sedimented on centrifugation at 79,000 × g for 2 hr. Heating (60 C for 2 hr) confers on one or more cytoplasmic viral antigens a new specificity; the altered antigens are demonstrable with labeled rabbit anti-boiled infected cell serum which normally does not combine with cytoplasmic antigens.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- EAGLE H. Amino acid metabolism in mammalian cell cultures. Science. 1959 Aug 21;130(3373):432–437. doi: 10.1126/science.130.3373.432. [DOI] [PubMed] [Google Scholar]
- LEBRUN J. Cellular localization of Herpes simplex virus by means of fluorescent antibody. Virology. 1956 Aug;2(4):496–510. doi: 10.1016/0042-6822(56)90006-x. [DOI] [PubMed] [Google Scholar]
- MARSHALL J. D., EVELAND W. C., SMITH C. W. Superiority of fluorescein isothiocyanate (Riggs) for fluorescent-antibody technic with a modification of its application. Proc Soc Exp Biol Med. 1958 Aug-Sep;98(4):898–900. doi: 10.3181/00379727-98-24222. [DOI] [PubMed] [Google Scholar]
- MORGAN C., ELLISON S. A., ROSE H. M., MOORE D. H. Structure and development of viruses as observed in the electron microscope. I. Herpes simplex virus. J Exp Med. 1954 Aug 1;100(2):195–202. doi: 10.1084/jem.100.2.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MORGAN C., ROSE H. M., HOLDEN M., JONES E. P. Electron microscopic observations on the development of herpes simplex virus. J Exp Med. 1959 Oct 1;110:643–656. doi: 10.1084/jem.110.4.643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROIZMAN B., AURELIAN L., ROANE P. R., Jr THE MULTIPLICATION OF HERPES SIMPLEX VIRUS. I. THE PROGRAMMING OF VIRAL DNA DUPLICATION IN HEP-2 CELLS. Virology. 1963 Nov;21:482–498. doi: 10.1016/0042-6822(63)90209-5. [DOI] [PubMed] [Google Scholar]
- ROIZMAN B., MAYER M. M., ROANE P. R., Jr Immunochemical studies of poliovirus. IV. Alteration of the immunologic specificity of purified poliomyelitis virus by heat and ultraviolet light. J Immunol. 1959 Jan;82(1):19–25. [PubMed] [Google Scholar]
- ROIZMAN B., ROANE P. R., Jr A physical difference between two strains of herpes simplex virus apparent on sedimentation in cesium chloride. Virology. 1961 Sep;15:75–79. doi: 10.1016/0042-6822(61)90079-4. [DOI] [PubMed] [Google Scholar]
- ROIZMAN B., ROANE P. R., Jr Demonstration of a surface difference between virions of two strains of herpes simplex virus. Virology. 1963 Feb;19:198–204. doi: 10.1016/0042-6822(63)90009-6. [DOI] [PubMed] [Google Scholar]
- ROIZMAN B., ROANE P. R., Jr Studies of the determinant antigens of viable cells. I. A method, and its application in tissue culture studies, for enumeration of killed cells, based on the failure of virus multiplication following injury by cytotoxic antibody and complement. J Immunol. 1961 Dec;87:714–727. [PubMed] [Google Scholar]
- ROIZMAN B. Virus infection of cells in mitosis. I. Observations on the recruitment of cells in karyokinesis into giant cells induced by herpes simplex virus and bearing on the site of virus antigen formation. Virology. 1961 Apr;13:387–401. doi: 10.1016/0042-6822(61)90269-0. [DOI] [PubMed] [Google Scholar]
- Roane P. R., Jr, Roizman B. Differentiation of nuclear and cytoplasmic herpesvirus antigens in infected cells. Virology. 1966 Aug;29(4):668–670. doi: 10.1016/0042-6822(66)90290-x. [DOI] [PubMed] [Google Scholar]
- Sydiskis R. J., Roizman B. Polysomes and protein synthesis in cells infected with a DNA virus. Science. 1966 Jul 1;153(3731):76–78. doi: 10.1126/science.153.3731.76. [DOI] [PubMed] [Google Scholar]
- TANKERSLEY R. W., Jr AMINO ACID REQUIREMENTS OF HERPES SIMPLEX VIRUS IN HUMAN CELLS. J Bacteriol. 1964 Mar;87:609–613. doi: 10.1128/jb.87.3.609-613.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]