Abstract
A novel biomass was prepared from Pichia anomala KCCM 11473, which grew well in ginseng-steaming effluent (GSE), and its physiological functionalities and enzyme activities were determined. When the strain was cultured in the GSE (pH 6.0) at 30℃ for 48 h, 1.6 mg of biomass per ml-cultures was produced. The cell-free extract of the biomass showed high antihypertensive angiotensin I-converting enzyme inhibitory activity of 72.0% and anticholesteromia HMG-CoA reductase inhibitory activity of 46.5%. The cell-free extract also showed 13.0 U per ml and 8.5 U per ml of neutral protease activity and alkaline protease, respectively.
Keywords: Enzyme activity, Functionality, Ginseng-steaming effluents, Pichia anomala
Ginseng and its extracts have recently been used in the production of health foods, such as ginseng tea, ginseng drinks, and the extract itself. A large amount of ginseng steaming effluent (GSE) is discharged during the preparation of the ginseng extract at processing companies (Kim et al., 2000). GSE contains useful substances, such as ginsenosides, sugars, and protein. However, only a small amount of GSE protein is used in the extraction of useful ginsenosides or in the production of maltooligosaccharides (Kim et al., 2000) or bioactive compounds such as chitosan (Kim et al., 2002) and ribonucleotides (Kim et al., 2002). The bulk of GSE is discharged in the sewage, causing environmental pollution. It is imperative, therefore, to improve the efficiency of GSE utilization and to develop high value products from GSE.
Yeast generally offers some industrial advantages, including rapid growth, ease of cultivation, and the capacity to be grown in a cheap medium containing agricultural byproducts (Kim et al., 2004). Various yeasts have been used for the production of high-value bio-ingredients in food and animal feed, as well as for medicinal purposes with respect to ribonucleotides, industrial enzymes, and vitamins (Kim et al., 2004). Recently, bioactive compounds such as an antihypertensive angiotensin I-converting enzyme (ACE) inhibitor (Kim et al., 2004), an antiangiogenic compound (Jeong et al., 2006), and antidementia β-secretase inhibitor (Lee et al., 2006) were produced and characterized from Saccharomyces cerevisiae.
In a previous study, we described the production of ribonucleotides by the autolysis of Hansenula anomala (Kim et al., 2002), a Pichia anomala mutant (Lee et al., 2004), and chitosan by Mucor miehei (Kim et al., 2002) grown on ginseng-steaming effluent. We now describe the physiological functionality and some enzyme activity of biomass from Pichia anomala grown on ginseng-steaming effluent for the efficient utilization of ginseng-steaming effluent.
Materials and Methods
Strain, ginseng-steaming effluent (GSE), and chemicals
Pichia anomala KCCM 11473, which was mentioned in a previous paper (Kim et al., 2002) to have grown well in GSE, was used in this study. The GSE was obtained from a ginseng processing plant in Geumsan, in Chungnam province, South Korea. The GSE contained 64.0% total sugar, 34.2% crude protein, and 1.8% ash with 1125.4 mg/l CODMn, and it had a pH of 4.95. The angiotensin I-converting enzyme (ACE) was extracted from rabbit lung acetone powder (Sigma Chemical Co., St. Louise, Mo., USA), and hippuric acid-histidine-leucine, fibrin, pyrogallol, and DPPH were also purchased from Sigma Chemical Co. (St. Louise, Mo., USA). Unless otherwise specified, all the chemicals were of analytical grade.
Preparation of biomass
Pichia anomala was inoculated in the GSE (pH 4.95) and cultured at 30℃ for 72 h. After centrifugation of the culture broth at 10,000 ×g for 15 min, the yeast cells were harvested and subsequently dried with a vacuum freeze dryer.
Assay of physiological functionality
A cell-free extract of the biomass was prepared from the selected strain of yeast by cell disruption and centrifugation. We then used the following means to determine the cardiovascular and anti-aging functionalities. The antihypertensive ACE inhibitory activity was assayed by means of Cushman and Cheung's method (Cushman and Cheung, 1971). A mixture containing 100 mM sodium borate buffer (pH 8.3), 300 mM NaCl, 3 units of ACE, and an appropriate amount of the cell-free extract was preincubated for 10 min at 37℃. The reaction was initiated by adding 50 µl of Hip-His-Leu at a final concentration of 5 mM and terminated after 30 min of incubation by the addition of 250 µl of 1.0 N HCl. The liberated hippuric acid was extracted with 1 ml of ethyl acetate, and 0.8 ml of the extract was dried with a Speed Vac Concentrator (EYELA Co., Japan). The residue was then dissolved in 1 ml of the sodium borate buffer. The absorbance at 228 nm was measured in order to estimate the ACE inhibitory activity.
The fibrinolytic activity was assayed by the method of Fayek et al. (1980) Each sample of 0.5 ml was added to 3 ml of a substrate solution (0.6% fibrin in 0.1M McIlvaine buffer, pH 7.0) and incubated at 40℃ for 10 min. The reaction was stopped by adding 3 ml of 0.4M TCA for 30 min. The mixture was then filtered with Whatman filter paper No. 2. A reaction mixture of 1 ml of the filtrate, 5 ml of 0.4M Na2CO3, and 1 ml of 1 N Folin reagent was placed at room temperature for 30 min. The amount of tyrosine released from the fibrin as a substrate was determined from the tyrosine standard curve by measuring the absorbance at 660 nm.
The HMG-CoA reductase inhibitory activity was assayed spectrophotometrically by measuring the rate of decrease in the absorbance at 340 nm due to the oxidation of NADPH (Kim et al., 2005). A 0.5 ml volume of the reaction mixture contained the following: 50 µM of a potassium phosphate buffer with a pH of 7.0, 2 µM of dithiothiothreitol, NADPH, 0.3 µM, 0.15 µM of HMG-CoA, and 100 µg of protein enzyme. Two reaction mixtures were preincubated in a 2 mm light path glass cuvette for 5mins at 37℃. For the assay, we added HMG-CoA to one reaction mixture, and we added HMG-CoA with cell-free extract to the other reaction mixture. The mixtures were assayed at 37℃ in a recording spectrophotometer. The initial velocity of the reaction was measured, and the net rate of the NADPH oxidation was determined by subtracting the rate of oxidation in the absence of HMG-CoA from the rate observed with both substrates present. Thus, we calculated the HMG-CoA reductase inhibitory activity as follows:
HMG-CoA reducatse inhibitory activity (%)
{1 - (A340 of sample - A340 sample of blank/A340 of control - A340 sample of control)} × 100
The nitrite-scavenging activity was assayed by the method of Kato et al. (1987) with NaNO2 and Griess reagents.
The antioxidant activity was also assayed by the method of Blois (Blois, 1958) with 1,1-diphenyl-2-picryl hydrazyl (DPPH). A 0.8 ml DPPH solution (12.5 mg of DPPH dissolved in 100 ml of ethanol) was added to 0.2 ml of the sample, shaken for 10 s, and left for 10 mins. The absorbance at 525 nm was then determined and calculated as follows:
[(A525 of reaction mixture - A525 of sample alone)/A525 of blank] × 100.
The tyrosinase inhibitory activity was assayed by the method of Kim et al. (1997). The conversion of L-DOPA to a red-colored oxidation product, dopachrome, was measured spectrophotometrically. A 0.1 ml sample was incubated for 5 min at 30℃. At time zero, 1 ml of the L-DOPA (4 mg/ml) solution was rapidly added while stirring, and the absorbance was measured at 475 nm. After incubation for an additional 5 mins, the mixture was shaken again, and a second reading was determined.
[1 - (A475 of sample reaction mixture)/A475 of blank] × 100.
The glutathione S-transferase-activating activity of the cell-free extracts was measured by the method of Habig et al. (1974). The reaction system comprised 10 µl of 1.0 mM 1-chloro-2,4-dinitrobenzene, 10 µl glutathione, and 100 µl of 0.1 mM phosphate buffer (pH 6.5). The mixture was preincubated for 5 min at 25℃. After the addition of the 10 µl sample and 15 µl of GST (1.5 unit) to the system, the change of absorbance at 314 nm for 5 min was measured. Distilled water was used as a blank, and the GST-activating activity was calculated as follows.
GST-activating activity (%) = (A314 of reactants A314 of sample itself)/(A314 of control(blank)) × 100
Assay of enzyme activity
The activities of acidic, neutral, and alkaline proteases were spectrophotometrically determined by using different buffer solutions (pH 3.0, 7.0, 10.0) containing 0.6% skim milk and Folin reagents (Lee et al., 1997). α-Amylase and glucoamylase activity were determined using 1% soluble starch, and β-galactosidase and invertase activity were also determined using 4 mg/ml of ONPG and 2% sucrose as a substrate, respectively (Lee et al., 1997). Inulinase activity was measured by determining the amount of the released reducing sugar from inulin (Kwon et al., 1999).
Results and Discussion
Production of Pichia anomala biomass
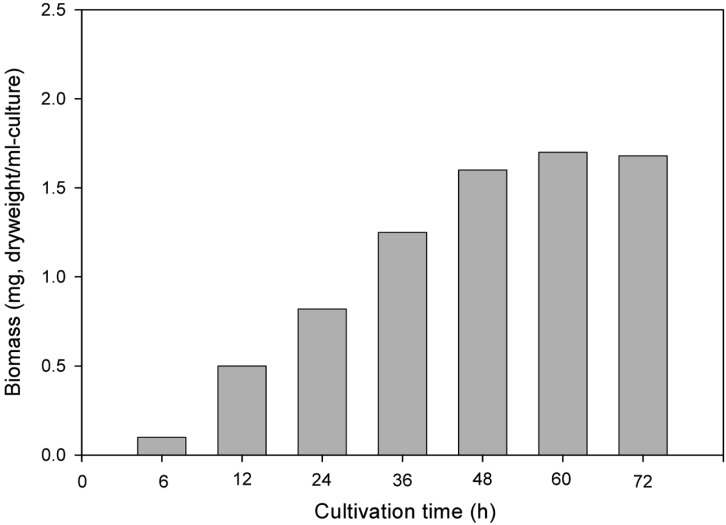
Fig. 1 shows the biomass content of Pichia anomala during fermentation. The biomass content increased as the cultural time increased, and the maximum biomass content (1.6 mg/ml of cultures) was obtained at 48 h of cultivation.
Fig. 1.
Time course of biomass production from Pichia anomala KCCM 11473 in the ginseng-steaming effluent.
Physiological functionality of the biomass
The functionality of the biomass was investigated (Table 1). The antihypertensive ACE inhibitory activity of the biomass was 72.0%, which is about 20% higher than that of the biomass from Pichia anomala grown on the yeast extractpeptone-dextrose (YEPD) medium containing 1% yeast extract, 2% peptone and 2% dextrose (51.2%). These results were also higher than those of the biomass from Pichia anomala (31.0%) and its mutant HA-2 (16.0%) grown on the yeast extract-malt extract (YM) medium containing 0.3% yeast extract, 0.3% malt extract, 0.5% peptone and 1% dextrose (Kim et al., 2002), and the biomass from Saccharomyces cerevisiae (42.1%) (Kim et al., 2004). From reports on antihypertensive ACE inhibitory activity of ginseng extracts (Lee et al., 2003) and adventitious root extracts of wild ginseng (Hong et al., 2008), we guessed that ACE inhibitory activity of the biomass is caused by transferation of antihypertensive agents in GSE into Pichia anomala during fermentation. ACE is known to regulate blood pressure by converting inactive decapeptide angiotensin I to the potent vasoconstrictor octapeptide angiotensinand inactivating the vasodilating nonapeptide bradykinin to raise blood pressure (Kim et al., 2004). Recently, various ACE inhibitors with antihypertensive effects have been isolated via the enzymatic digestion of food protein, sake and its by-products, Korean traditional rice wines, cereals and legumes, and microbes such as yeast and mushrooms (Kim et al., 2004).
Table 1.
Physiological functionalities of the biomass from Pichia anomala KCCM 11473 grown on ginseng-steaming effluent (GSE) (Unit: %)
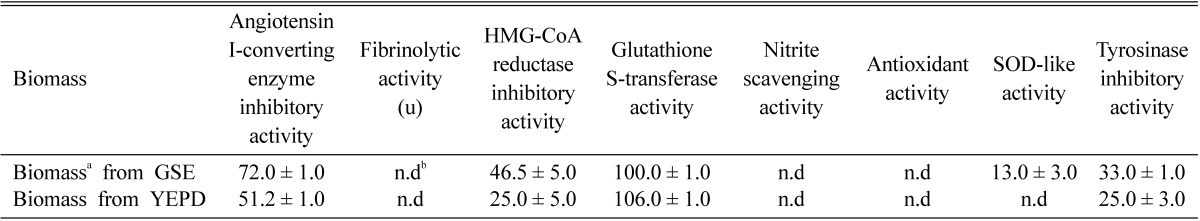
aBiomass which were obtained from cultivation in GSE and YEPD medium, respectively.
bn.d : not detected.
To investigate industrial application for ACE inhibitor of the biomass, the ACE inhibitor of cell-free extract was partially purified by ultrafiltration and systematic solvent extraction. It was ultrafiltrated with 5000Da cut-off filter (Labscale TFF System, Millipore Co., USA) and then ACE inhibitory activity of the filtrate was determined. Its ACE inhibitory activity was 61.6% (IC50 0.18 mg). The 5000 Da cut-off filtrates was performed systematic solvent extraction. D.W extracts of final step and butanol extracts showed high ACE inhibitory activity of 79.5% and 52.1%, respectively (Table 2). It suggest that the ACE inhibitor should be hydrophilic compound such as peptides. It is necessary further purification to characterize and elucidate structure-function relationship of the ACE inhibitior.
Table 2.
Angiotensin I-converting enzyme inhibitory activity of various extracts by ultrafiltration and systematic solvent extraction of the biomass from Pichia anomala KCCM 11473 (Unit: %)

an.d : not detected.
The HMG-CoA reductase inhibitory activity and tyrosinase inhibitory activity were 8% to 20% higher than those grown on the YEPD medium and YM medium (Kim et al., 2002). However, the other functionalities were not determined or were lower than those of fibrinolytic activity (9.4%) and SOD-like activity (16.8%) of Pichia anomala and nitrite scavenging activity (47%) of the mutant, P. anomala HA-2 grown on YM medium (Kim et al., 2002).
Overall, these results suggest that Pichia anomala grown on GSE may be beneficial in the production of a high-grade functional yeast biomass, which in turn could serve to eliminate pollution problems arising from GSE waste.
Enzyme activity of the biomass
Some industrial enzyme activities of the cell-free extract from the biomass were determined (Table 3). Neutral protease and alkaline protease activities were showed 13.0 U per ml and 8.5 U per ml, respectively. It suggest the GSE is valuable for application into enzyme industry such as detergent or medical industry. Meanwhile, invertase and inulinase activities were below 1 U per ml. The other enzyme activities were not detected.
Table 3.
Enzyme activities of the biomass from Pichia anomala KCCM 11473 grown on ginseng-steaming effluents (Unit: U/ml)

an.d : not detected.
References
- 1.Blois MS. Antioxidant determination by the use of stable free radical. Nature. 1958;191:1199–1200. [Google Scholar]
- 2.Cushman DW, Cheung HS. Spectrophotometric assay and properties of the angiotensin-converting enzyme of rabbit lung. Biochem Pharmacol. 1971;20:1637–1648. doi: 10.1016/0006-2952(71)90292-9. [DOI] [PubMed] [Google Scholar]
- 3.Fayek KI, El-Sayed ST. Purification and properties of fibrinolytic enzyme from Bacillus subtilis. Zeit fur Allgem Mikrobiol. 1980;20:375–382. doi: 10.1002/jobm.3630200603. [DOI] [PubMed] [Google Scholar]
- 4.Habig WH, Pabst MJ, Fleischner G, Gatmaitan Z, Arias IM, Jakoby WB. The identity of glutathione S-transferase B with ligandin, a major binding protein of liver. Proc Natl Acad Sci USA. 1974;71:3879–3882. doi: 10.1073/pnas.71.10.3879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hong MH, Lim HK, Park JE, Jun NJ, Lee YJ, Cho MJ, Cho SM. The antihypertensive and vasodilating effects of adventitiousroot extracts of wild ginseng. J Korean Soc Appl Biol Chem. 2008;51:102–107. [Google Scholar]
- 6.Jeong SC, Lee DH, Lee JS. Production and characterization of an anti-angiogenic agent from Saccharomyces cerevisiae K-7. J Microbiol Biotechnol. 2006;16:1904–1911. [Google Scholar]
- 7.Kato H, Lee IE, Chuyen NV, Kim SB, Hayase F. Inhibition of nitrosamine formation by nondialyzable melanoidins. Agric Biol Chem. 1987;51:1333–1338. [Google Scholar]
- 8.Kim NM, Lee JS, Lee BH. Enzymatic hydrolysis of Korean ginseng starch and characteristics of produced maltooligosaccharides. J Ginseng Res. 2000;24:41–45. [Google Scholar]
- 9.Kim HJ, Lee DH, Hwang YY, Lee KS, Lee JS. Characterization of β-hydroxy-β-methylglutaryl coenzyme A reductase inhibitor from Pueraria thunbergiana. J Agric Food Chem. 2005;53:5882–5888. doi: 10.1021/jf0505978. [DOI] [PubMed] [Google Scholar]
- 10.Kim JH, Lee KS, Kim NM, Lee JS. Production and characterization of chitosan from ginseng-steaming effluents by Mucor miehei. J Microbiol Biotechnol. 2002;12:760–765. [Google Scholar]
- 11.Kim JH, Lee BH, Lee JS. Production of ribonucleotides by autolysis of Hansenula anomala grown on Korean ginseng steaming effluent. J Biosci Bioeng. 2002;93:318–321. doi: 10.1016/s1389-1723(02)80035-4. [DOI] [PubMed] [Google Scholar]
- 12.Kim JH, Lee DH, Jeong SC, Chung KS, Lee JS. Characterization of antihypertensive angiotensin 1-converting enzyme inhibitor from Saccharomyces cerevisiae. J Microbiol Biotechnol. 2004;14:1318–1323. [Google Scholar]
- 13.Kim JK, Cha WS, Park JH, Oh SL, Cho YJ, Chun SS, Choi CC. Inhibition effect against tyrosinase of condensed tannins from Korean green tea. Korean J Food Sci Technol. 1997;29:173–177. [Google Scholar]
- 14.Kwon SJ, Yoo JY, Lee JS. Isolation of intracellular inulinase-producing bacterium and culture condition for inulinase production. J Food Sci Biotechnol. 1999;8:24–29. [Google Scholar]
- 15.Lee DH, Lee DH, Lee JS. Production and characterization of antidementia β-secretase inhibitor from Saccharomyces cerevisiae; 2006 Aunnual meeting and international symposium; The Korean Society for Microbiology and Biotechnology; 2006. [Google Scholar]
- 16.Lee JS, Hyun KW, Jeung SC, Kim JH, Choi YJ, Miguez CB. Production of ribonuclotides by autolysis of Pichia anomala mutant and some physiologica activities. Can J Microbiol. 2004;50:489–492. doi: 10.1139/w04-032. [DOI] [PubMed] [Google Scholar]
- 17.Lee JS, Yi SH, Kown SJ, Ahn C, Yoo JY. Enzyme activities and physiological functionality of yeasts from traditional Meju. Korean J Appl Microbiol Bioeng. 1997;25:448–453. [Google Scholar]
- 18.Lee SE, Seong NS, Bang JK, Kang SW, Lee SW, Cheng TY. Inhibitory effect against angiotensin converting enzyme and antioxidant activity of Panax ginseng C. A. Meyer extracts. Korean J Med Crop Sci. 2003;11:236–245. [Google Scholar]