Abstract
The retrotransposon marY1 is a gypsy family retroelement, which is detected ubiquitously within the fungal taxonomic groups in which mushrooms are included. To utilize marY1 as a molecular marker for the DNA fingerprinting of mushrooms, oligonucleotides marY1-LTR-L and marY1-LTR-R were designed on the basis of highly conserved regions from the multiple sequence alignment of 30 marY1 sequences retrieved from a nucleotide sequence database. In accordance with Retrotransposon Microsatellite Amplified Polymorphism (REMAP) fingerprinting methodology, the two oligonucleotides were utilized together with the short sequence repeat primers UBC807 and UBC818 for polymerase chain reaction using templates from different mushroom genomic DNAs. Among the tested oligonucleotides, the marY1-LTR-L and UBC807 primer set yielded the greatest amount of abundance and variation in terms of DNA band numbers and patterns. This method was successfully applied to 10 mushroom species, and the primer set successfully discriminated between different commercial mushroom cultivars of the same strains of 14 Pleurotus ostreatus and 16 P. eryngii. REMAP reproducibility was superior to other popular DNA fingerprinting methodologies including the random amplified polymorphic DNA method.
Keywords: Fingerprinting, marY1 retrotransposon, Mushroom, REMAP
Molecular approaches have proven to be potent tools in the classification of complex fungal taxonomic groups including mushrooms. The direct comparison of the DNA sequences of certain molecular markers, for example, internal transcribed spacer (ITS), intergenic spacer (IGS), and small and large ribosomal DNA can be used to confirm genetic differences between diverse fungal species. However, currently the applicability of this technique is limited principally to the classification of fungi at the species level (Garnica et al., 2003; Peintner et al., 2004). On the other hand, DNA fingerprinting techniques that recognize DNA fragmentation patterns from a specific DNA sample by randomly primed polymerase chain reaction (PCR) restriction endonuclease treatment, or a combination thereof, have proven their capacity to verify closely related species or strains belonging to the same species (Zervakis et al., 2001; Lopandic et al., 2005). Our previous study concerning the genotyping of various cultivars of the edible mushroom, Pleurotus eryngii, demonstrated the efficacy of DNA fingerprinting using a random amplified polymorphic DNA (RAPD) as compared to ITS1-5.8S rDNA-ITS2 sequence comparison (Ro et al., 2007). Despite the potential of this technique with regard to genotyping, RAPD has a critical weak point in terms of reproducibility, largely because its performance depends heavily on the PCR reaction conditions. Slight changes in the annealing temperature, changes in thermostable DNA polymerase, or even changes occurring in different batches of DNA polymerase from the same supplier often result in misleading outputs. This lack of robustness has compelled us to seek an alternative method on the basis of the variability of DNA sequences around the retrotransposon. Retrotransposon microsatellite amplified polymorphism (REMAP) is such a technique, and is premised on the amplification of the DNA region between a long terminal repeat (LTR) of the retrotransposon and simple sequence repeat (SSR) using an outward-facing LTR primer and an SSR specific primer (Kalendar et al., 1999; Kalendar and Schulman, 2006). The LTR-SSR regions are recurring sequences along the genomic DNA. The design of primers that anneal these regions enables the primers to amplify DNA fragments of different lengths, and the recurring patterns of these regions differ among various species or even among strains of the same species. Therefore, REMAP is an appealing method for study and application in the classification of mushrooms.
The gypsy-type retroelement marY1 was first identified in Tricholoma matsutake (Murata and Yamada, 2000) and later in other basidiomycetes species (Murata and Miyazaki, 2001; Murata et al., 2001, 2005). This retroelement spans approximately 6kb and harbors 5'-LTR, gag, prt, pol, and 3'-LTR regions in consecutive order (Murata and Yamada, 2000). The intact unit of the marY1 LTR retrotransposon may be contained in the genomic DNA in several to over 1,000 copies, which are equivalent to 0.05~5.5% of the approximately 34Mb genomic sequence of each different Tricholoma species (Murata and Babasaki, 2005). Interestingly, the LTR region alone has been shown to consistently evidence markedly higher copy numbers than those observed in the coding region of marY1 (Murata and Babasaki, 2005). It was also determined that this LTR region is highly conserved in a variety of higher fungi (Murata et al., 2001). These properties of marY1, and in particular the LTR region of marY1, render marY1 as an excellent candidate for fungal fingerprinting techniques.
In this study, we conducted an alignment of the marY1 sequences of multiple mushroom species and selected the highly conserved region for the cross-species amplification of DNA regions of interest. We assessed the feasibility of utilizing these primers in the fingerprinting of mushroom strains.
Materials and Methods
Strains and culture conditions
Forty mushroom species and strains including Phellinus linteus, Cordyceps spp., Ganoderma lucidum, Coprinus comatus, Hericium erinaceus, Flammulina velutipes, Lentinula edodes, Agaricus bisporus, 16 cultivars of Pleurotus eryngii, and 14 cultivars of P. ostreatus were acquired from the Culture Collection of Wild Mushroom (CCWM, Incheon University, Korea) and from GyeongNam Agricultural Research and Extension Services (GNARES, Korea). To extract total cellular DNA from each mushroom, the mushroom mycelia were grown in Potato dDextrose broth containing potato starch (4 g/l) and glucose (20 g/l) (Difco, Detroit, MI, USA) for 2 weeks at 25℃.
Multiple sequence alignment and design of oligonucleotides
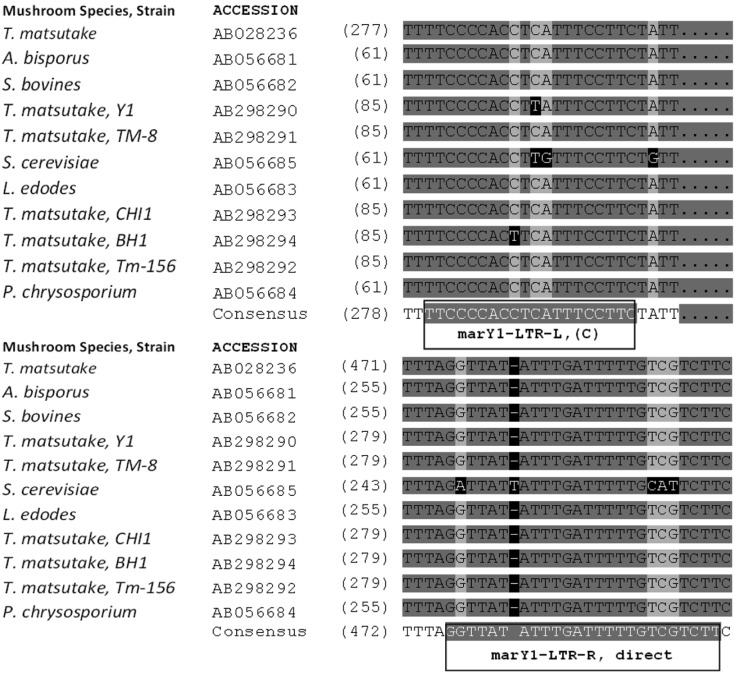
The nucleotide sequences of the marY1-LTR regions of various fungal species were retrieved from the NCBI database and aligned with the AlignX utility (Vector NTI, Invitrogen, Carlsbad, CA, USA) using standard parameters (Fig. 1). Highly conserved regions were utilized as primary sequences, upon which the outward-facing oligonucleotides were designed according to PCR parameters of selected SSR primers including melting temperatures (Tm) between 50℃ and 57℃. The resulting oligonucleotides (Fig. 1) were: 5'-GAA GGA AAT GAG GTG GGG AA-3' (marY1-LTR-L, Tm = 57℃) and 5'-TTT TTA GGT TAT ATT TGA TTT TTG TCG TCT T-3' (marY1-LTR-R, Tm = 53℃).
Fig. 1.
Highly conserved regions of the marY1 sequences from various fungi retrieved from the NCBI database. Newly designed primers are shown in boxes. (C) denotes complementary sequence.
Mushroom total cellular DNA extraction and PCR conditions
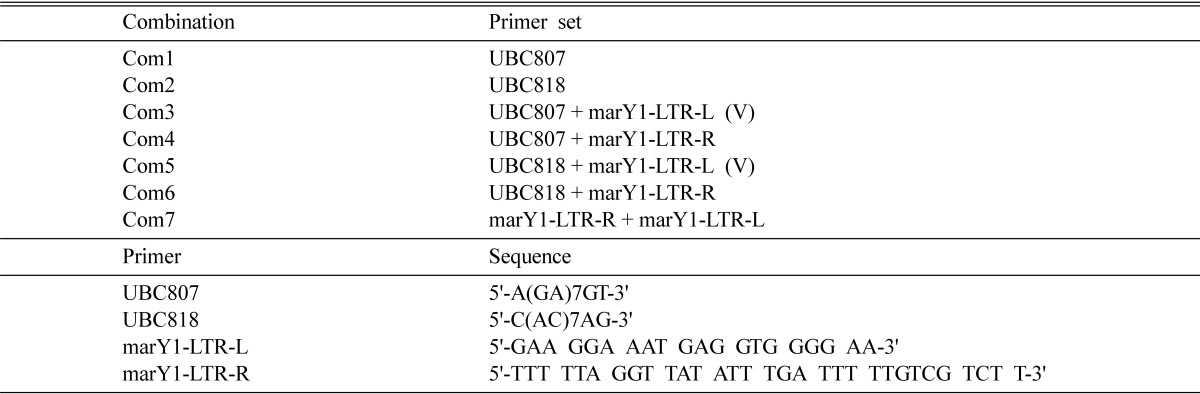
The mushroom mycelia were collected and frozen with liquid nitrogen. Total cellular DNA was extracted from the ground powder of the frozen mycelia using a DNA prep kit for plants (Solgent, Daejeon, Korea). The concentration of extracted DNA was determined using a Qubit fluorescence photometer (Invitrogen). For REMAP analysis, PCR was conducted using Taq DNA polymerase (Solgent) with a Px2 thermal cycler (Thermo Electron, Pittsburgh, PN, USA) at 94℃ for 5 min; 35 cycles at 94℃ for 30 s, 50℃ for 30 s, and 72℃ for 2 min; and 72℃ for 10 min. The primer sets were various combinations of marY1-LTR-L, marY1-LTR-R, and SSR primers (Han et al., 2002) 5'-A(GA)7GT-3' (UBC807) and 5'-C(AC)7AG-3' (UBC818), as shown in Table 1.
Table 1.
Primer combinations and sequences.
Long agarose gel electrophoresis and image analysis
Long agarose gel electrophoresis (20 cm) was conducted as previously described (Kalendar and Schulman, 2006; Branco et al., 2007). The 1.7% gel was constructed with Seakem LE agarose (Cambrex, East Rutherford, NJ, USA) in STBE buffer (20 mM Tris-borate, 5 mM Na2B4O7, and 1 mM EDTA, pH 8.6). The gel image was acquired with a Bio-Vision model 3000 gel documentation system (Vilber-Lourmat, Cedex, France) and the pattern of the DNA fragments was analyzed using Bio-profil Bio1D++ software (Vilber-Lourmat). The cluster analyses of the REMAP and the RAPD pattern data were performed as described previously (Ro et al., 2007).
Results and Discussion
REMAP analysis of mushroom genomic DNA with various combinations of primer sets
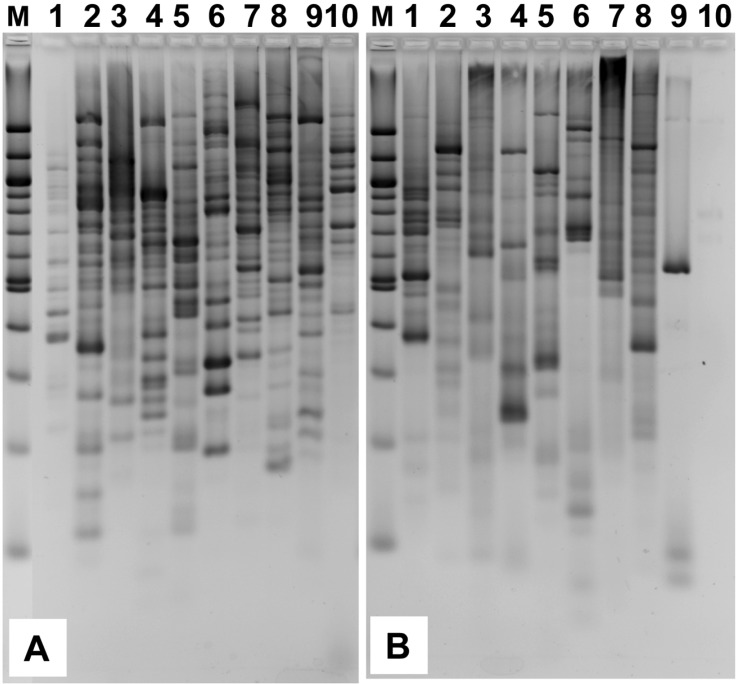
REMAP analysis was conducted using different combinations of primer sets (Table 1) with genomic DNA templates from 10 distinct mushroom species (Fig. 2). Among the tested samples, the Com3 primer set yielded the best results by showing the most abundance and variation in terms of DNA band numbers and patterns (Fig. 2A). The Com5 set generated significant numbers of bands, but not to the same degree as observed with Com3 (Fig. 2B). Remarkably, although Com4 and Com6 followed the same procedure as Com3 and Com5 did, they generated lower band numbers and less band pattern variation (data not shown). Com1 and Com2, which used only the SSR primer, yielded simple band patterns consisting of one or two bands for each lane (data not shown). Com7, which harbors only outward-facing LTR-based primers, did not lead to many bands for each of the samples (data not shown) as had been expected with the inter-retrotransposon amplified polymorphism (IRAP) technique (Kalendar et al., 1999; Kalendar and Schulman, 2006). So it may be inferred from this result that the combinations of SSR primers with marY1-LTR-L rather than with marY1-LTR-R were more successful in facilitating the REMAP analysis. Thus, the Com3 primer set proved useful for the fingerprinting of mushroom strains. In addition, because the marY1-LTR-L primer was designed on the basis of the reported marY1 retrotransposon sequences from a few other mushroom species, rather than those from the 10 species tested in this study, it strongly suggests that marY1-LTR-L can likely be utilized as a universal primer for the genotyping of various mushroom species. It is useful to point out, however, that even though Com3 markers work well on multiple mushroom species, they do not meaningfully correlate REMPAP fingerprinting of different mushroom species. Nonetheless, the fact that 10 species used in this study comprised different mushroom groups including medicinal and edible mushrooms confirms that marY1, or at least the LTR regions of marY1, are widespread among higher fungi.
Fig. 2.
REMAP analyses of 10 mushroom species. Genomic DNA of the mushrooms were subjected to REMAP analysis using the Com3 (A) and Com5 (B) primer sets. Lanes: 1, Phellinus linteus; 2, Cordyceps spp.; 3, Ganoderma lucidum; 4, Coprinus comatus; 5, Hericium erinaceus; 6, Flammulina velutipes; 7, Pleurotus eryngii; 8, Pleurotus ostreatus; 9, Lentinula edodes; 10, Agaricus bisporus.
Reproducibility of the markers
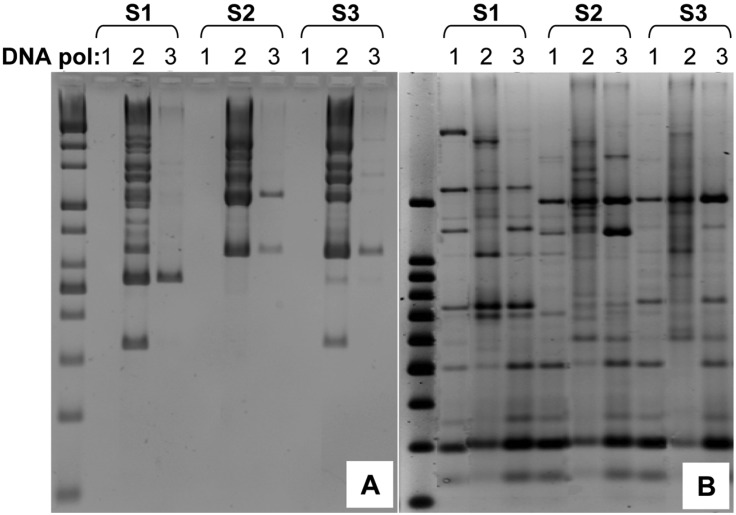
The genetic analysis conducted on the fungal genus Fellomyces previously showed that RAPD and amplified fragment length polymorphism (AFLP) demonstrate a higher potential in distinguishing strains within species than do other DNA sequence-based methods (Lopandic et al., 2005). In particular, the RAPD method was applied successfully in the verification of P. eryngii strains from various hosts from a wide range of geographical origins (Zervakis et al., 2001). However, there are concerns about the reproducibility of this PCR-based technique that have been discussed elsewhere (Skroch and Nienhuis, 1995; Jones et al., 1997). It has been widely recognized that PCR mixture and reaction conditions are factors upon which the reproducibility of this PCR-based technique is solely based. To assess the reproducibility of our Com3-based REMAP, we evaluated the DNA band patterns from both the RAPD and REMAP analyses using different thermostable DNA polymerases. Three genomic DNA samples of P. ostreatus were employed as templates for the RAPD and REMAP analyses with three different commercial DNA polymerases (Fig. 3).
Fig. 3.
Comparison of the reproducibility of REMAP to RAPD. Three mushroom genomic DNA samples (S1, S2, S3) and three commercial thermostable DNA polymerases (1: i-starTaq; 2: Taq polymerase; 3: Ex Taq) were used to assess the reproducibility of RAPD (A) and REMAP (B). PCR mixtures and conditions were optimal for each enzyme, according to the manufacturers' instructions.
Reproducibility of each method was assessed by comparing lanes of the same DNA sample (i.e Fig. 3A, group S1) with three different DNA polymerases (i.e lanes 1, 2, 3 under S1 of Fig. 3A). Reproducibility of RAPD and REMAP was compared by considering how each method performed using different DNA polymerases while using the same genomic DNA sample. The comparision was done three times with three genomic DNA samples (S1, S2, S3).
Even though variations were observed in some major bands, particularly in the case of DNA polymerase 1, REMAP was markedly less affected by differences in DNA polymerases (Fig. 3B) than RAPD, which resulted in a dramatic loss of bands (Fig. 3A). This reveals that the Com3 markers allow more reproducible results than the RAPD markers, and prompted an experiment to test Com3 markers with strains of the same species.
Intra-species REMAP analysis
Previous studies regarding the conventional methods have demonstrated the inherent difficulty in differentiation of variations within the same species or within the same strains. These include restriction fragment length polymorphism (RFLP), RAPD (Ro et al., 2007), small subunit (SSU) rDNA, and ITS sequence analyses (Gonzalez and Labarere, 2000; Zhang et al., 2006). On the other hand, REMAP has proven to be a simple yet powerful tool for the discrimination of strains of barley (Kalendar et al., 1999) and rice (Branco et al., 2007). These observations strongly advocate for the use of REMAP in the genotyping of mushroom species at the sub-species level.
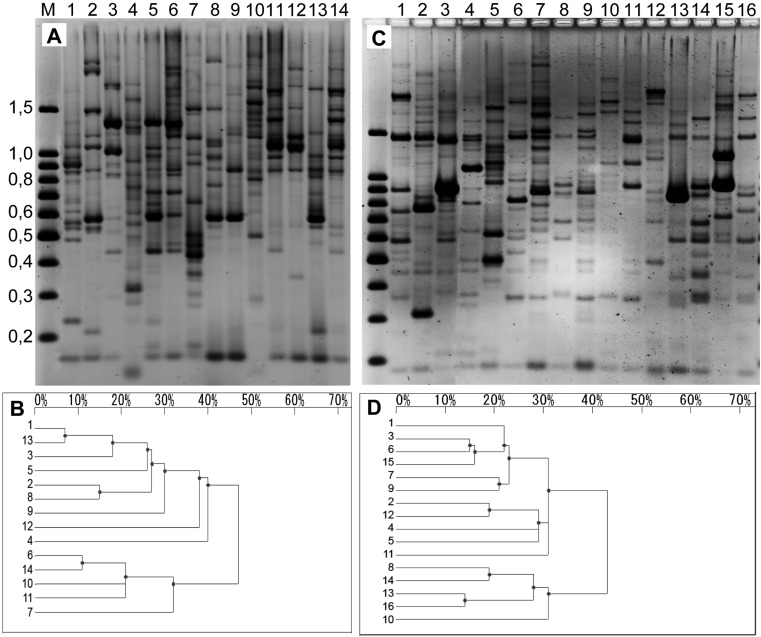
As shown in Fig. 3, the Com3 primer set verified their feasibility in generating gel patterns for the genotyping of mushroom strains. Fourteen cultivars of P. ostreatus (Fig. 4A) and 16 cultivars of P. eryngii (Fig. 4C) were selected and subjected to REMAP. Variations in the band patterns are more distinctively evident in adjacent dendrograms (Fig. 4B and 4D).
Fig. 4.
REMAP analyses on the mushroom strains within the same species. Band patterns and dendrograms of 14 P. ostreatus strains (A and B, respectively) and 16 P. eryngii strains (C and D, respectively) are displayed. The 14 P. ostreatus strains identified by lane are: 1, IUM1051; 2, IUM1313; 3, IUM1367; 4, IUM1490; 5, IUM1556; 6, IUM1491; 7, IUM557; 8, IUM1677; 9, IUM165; 10, IUM778; 11, IUM1520; 12, IUM1373; 13, IUM1341; 14, IUM1346. Similarly, the 16 P. eryngii strains are: 1, IUM2501; 2, IUM2502; 3, IUM2503; 4, IUM2510; 5, IUM1511; 6, IUM2512; 7, IUM2513; 8, IUM2514; 9, IUM2515; 10, IUM2518; 11, IUM2519; 12, IUM2520; 13, IUM2521; 14, IUM2523; 15, IUM2524; 16, IUM2322. The patterns of DNA bands were analyzed and dendograms were created with Bio-1D++.
It is necessary to emphasize that if there were the same confidence level between the two, it would be tempting to take REMAP markers as a replacement of RAPD or other markers. However, the correlation between RAPD and REMAP result is not necessarily high because the two methods are surveying different areas in the genomic DNA template. In other words, reoccurrence patterns of RAPD markers do not need to follow reoccurrence patterns of REMAP markers.
Conclusion
The REMAP markers developed herein have demonstrated their feasibility in the fingerprinting of strains of various mushroom species. Their superior reproducibility over the RAPD technique, the clear band patterns they generate, and fewer numbers of bands due to higher specificity because of longer primer length suggest that these markers are good alternatives for RAPD in the fingerprinting of mushroom strains, although it might take some time to select suitable markers. For mushroom species containing LTR regions of marY1 retroelement (including the 10 species used in this study), the Com3 markers can be utilized for fingerprinting of their strains.
Acknowledgements
This study was carried out with the support of "On-Site Cooperative Agriculture Research Project (Project No. 20070201080016)", RDA and Technology Development Program for Agriculture and Forestry, Ministry of Agriculture and Forestry, Republic of Korea.
References
- 1.Branco CJ, Vieira EA, Malone G, Kopp MM, Malone E, Bernardes A, Mistura CC, Carvalho FI, Oliveira CA. IRAP and REMAP assessments of genetic similarity in rice. J Appl Genet. 2007;48:107–113. doi: 10.1007/BF03194667. [DOI] [PubMed] [Google Scholar]
- 2.Garnica S, Weiss M, Oertel B, Oberwinkler F. Phylogenetic relationships of European Phlegmacium species (Cortinarius, Agaricales) Mycologia. 2003;95:1155–1170. doi: 10.1080/15572536.2004.11833025. [DOI] [PubMed] [Google Scholar]
- 3.Gonzalez P, Labarere J. Phylogenetic relationships of Pleurotus species according to the sequence and secondary structure of the mitochondrial small-subunit rRNA V4, V6 and V9 domains. Microbiology. 2000;146:209–221. doi: 10.1099/00221287-146-1-209. [DOI] [PubMed] [Google Scholar]
- 4.Han Q, Inglis GD, Hausner G. Phylogenetic relationships among strains of the entomopathogenic fungus, Nomuraea rileyi, as revealed by partial β-tubulin sequences and inter-simple sequence repeat (ISSR) analysis. Lett Appl Microbiol. 2002;34:376–383. doi: 10.1046/j.1472-765x.2002.01103.x. [DOI] [PubMed] [Google Scholar]
- 5.Jones CJ, Edwards KJ, Castaglione S, Winfield MO, Sala F, van de Wiel C, Bredemeijer G, Vosman B, Matthes M, Daly A, Brettschneider R, Bettini P, Buiatti M, Maestri E, Malcevschi A, Marmiroli N, Aert R, Volckaert G, Rueda J, Linacero R, Vazquez A, Karp A. Reproducibility testing of RAPD, AFLP and SSR markers in plants by a network of European laboratories. Mol Breeding. 1997;3:381–390. [Google Scholar]
- 6.Kalendar R, Grob T, Regina M, Suoniemi A, Schulman A. IRAP and REMAP: two new retrotransposon-based DNA fingerprinting techniques. TAG Theor Appl Genet. 1999;98:704–711. [Google Scholar]
- 7.Kalendar R, Schulman AH. IRAP and REMAP for retrotransposon-based fingerprinting and fingerprinting. Nature Protoc. 2006;1:2478–2484. doi: 10.1038/nprot.2006.377. [DOI] [PubMed] [Google Scholar]
- 8.Lopandic K, Molnar O, Prillinger H. Application of ITS sequence analysis, RAPD and AFLP fingerprinting in characterising the yeast genus Fellomyces. Microbiol Res. 2005;160:13–26. doi: 10.1016/j.micres.2004.09.005. [DOI] [PubMed] [Google Scholar]
- 9.Murata H, Babasaki K. Intra- and inter-specific variations in the copy number of two types of retrotransposons from the ectomycorrhizal basidiomycete Tricholoma matsutake. Mycorrhiza. 2005;15:381–386. doi: 10.1007/s00572-005-0369-y. [DOI] [PubMed] [Google Scholar]
- 10.Murata H, Babasaki K, Yamada A. Highly polymorphic DNA markers to specify strains of the ectomycorrhizal basidiomycete Tricholoma matsutake based on sigmamarY1, the long terminal repeat of gypsy-type retroelement marY1. Mycorrhiza. 2005;15:179–186. doi: 10.1007/s00572-004-0319-0. [DOI] [PubMed] [Google Scholar]
- 11.Murata H, Miyazaki Y. Expression of marY1, a gypsy-type LTR-retroelement from the ectomycorrhizal homobasidiomycete Tricholoma matsutake, in the budding yeast Saccharomyces cerevisiae. Biosci Biotechnol Biochem. 2001;65:993–995. doi: 10.1271/bbb.65.993. [DOI] [PubMed] [Google Scholar]
- 12.Murata H, Miyazaki Y, Babasaki K. The long terminal repeat (LTR) sequence of marY1, a retroelement from the ectomycorrhizal homobasidiomycete Tricholoma matsutake, is highly conserved in various higher fungi. Biosci Biotechnol Biochem. 2001;65:2297–2300. doi: 10.1271/bbb.65.2297. [DOI] [PubMed] [Google Scholar]
- 13.Murata H, Yamada A. marY1, a member of the gypsy group of long terminal repeat retroelements from the ectomycorrhizal basidiomycete Tricholoma matsutake. Appl Environ Microbiol. 2000;66:3642–3645. doi: 10.1128/aem.66.8.3642-3645.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Peintner U, Moncalvo J-M, Vilgalys R. Toward a better understanding of the infrageneric relationships in Cortinarius (Agaricales, Basidiomycota) Mycologia. 2004;96:1042–1058. [PubMed] [Google Scholar]
- 15.Ro HS, Kim SS, Ryu JS, Jeon CO, Lee TS, Lee HS. Comparative studies on the diversity of the edible mushroom Pleurotus eryngii: ITS sequence analysis, RAPD fingerprinting, and physiological characteristics. Mycol Res. 2007;111:710–715. doi: 10.1016/j.mycres.2007.03.016. [DOI] [PubMed] [Google Scholar]
- 16.Skroch P, Nienhuis J. Impact of scoring error and reproducibility RAPD data on RAPD based estimates of genetic distance. TAG Theor Appl Genet. 1995;91:1086–1091. doi: 10.1007/BF00223923. [DOI] [PubMed] [Google Scholar]
- 17.Zervakis GI, Venturella G, Papadopoulou K. Genetic polymorphism and taxonomic infrastructure of the Pleurotus eryngii species-complex as determined by RAPD analysis, isozyme profiles and ecomorphological characters. Microbiology. 2001;147:3183–3194. doi: 10.1099/00221287-147-11-3183. [DOI] [PubMed] [Google Scholar]
- 18.Zhang J, Huang C, Ng T, Wang H. Genetic polymorphism of ferula mushroom growing on Ferula sinkiangensis. Appl Microbiol Biotechnol. 2006;71:304–309. doi: 10.1007/s00253-005-0139-y. [DOI] [PubMed] [Google Scholar]