Abstract
Inonotus obliquus is a fungus that causes white heart rot on several broad-leaved species. This fungus forms typical charcoal-black, sterile conks (chaga) or cinder conks on infected stems of the birche (Betula spp). The dark brown pulp of the sterile conk is formed by a pure mycelial mass of fungus. Chaga are a folk remedy in Russia, reflecting the circumboreal distribution of I. obliquus in boreal forest ecosystems on Betula spp. and in meridional mountain forests on beech (Fagus spp.) in Russia, Scandinavia, Central Europe, and Eastern Europe. Distribution at lower latitudes in Western and Southern Europe, Northern America, Asia, Japan, and Korea is rare. Infected trees grow for many years without several symptoms of decline. The infection can penetrate through stem injuries with exterior sterile conks developing later. In the Czech Republic, cinder conk is found on birches inhabiting peat bogs and in mountain areas with a colder and more humid climate, although it is widespread in other broad leaved species over the Czech Republic. The most common hosts are B. pendula, B. pubescens, B. carpatica, and F. sylvatica. Less frequent hosts include Acer campestre, Acer pseudoplatanus, Alnus glutinosa, Alnus incana, Fraxinus excelsior, Quercus cerris, Q. petraea, Q. robur, Q. delachampii, and Ulmus sp.
Keywords: Betula spp., Birch, Chaga, Cinder conk, Inonotus obliquus, Sterile conks
Black sterile conks formed by Inonotus obliquus (Fr.) Pilát on birches, was firstly described and named by Katayebskaya (1928) as a form of Fomes igniarius (Fr.) Gill. (syn. Phellinus igniarius (L.:Fr.) Quélet). Bourdet and Galzen (1927) had previously identified the fungus as being identical with Poria obliqua found from birches in Sweden. Findlay (1939) observed the same fungus under the name Poria obliqua (Pers.:Fr.) P. Karst. as an interesting abnormal fungal growth on birch trees during a 1938 study in England (Campbell and Davidson, 1938; True et al., 1955). The fungus typically consists of a large coalblack mass about 30 cm in approximate diameter. Culture isolates are readily obtained from fungal tissue of the growth. Such cultures grow readily on malt extract agar, and spores are produced in diffuse light medium. Common names of I. obliquus include cinder conk, clinker fungus, birch canker polypore, sterile conk, trunk rot of birch, or, in Russia, Chaga (Czaga, Tchaga).
Cinder conk in folk medicine
During the 16th and 17th centuries, the medicinal attributes of cinder conks of Inonotus obliquus became recognized and used as a folk medicine in Northern Europe (Kahlos and Hiltunen, 1983, 1985, 1986; Kahlos et al., 1984, 1986), especially in Russia, Poland, and Baltic countries. Positive health effects were observed in populations using water cushions from this fungus. It is highly prized in Siberia as a cleaning and disinfecting substance, particularly in the treatment of stomach disorders. The Khanty population of West Siberia in Russia uses Chaga to prevent and treat tuberculosis and ailments of the heart, liver, and stomach (Saar, 1991). The name Chaga is derived from the expression for mushroom in the Komi-Permyak language of the native West Uralian population in the Kama River Basin. The Norwegian name for this fungus, "kreftkjuke," translates as "cancer polypore." In the Karelia region of Northern Europe, North-Western Russia, and Finland, Chaga is used for the same medicinal purposes. Liquid extracts of Chaga are commercially available as Befungin or Chaga.
I. obliquus extracts may be beneficial in controlling cancer, infection by human immunodeficiency virus-1, and stomach ulcer (Mizuno, 1999; Lim et al., 2005), and are effective as an inhibitor of mitosis (Burczyk et al., 1996), tumor growth (Kahlos et al., 1986, 1987), and oxidative damage to DNA (Park et al., 2004). Other active compounds in I. obliquus may contain additional active compounds (Kahlos et al., 1989; Babitskaia et al., 2000, 2002; Kukulyanskaya et al., 2002).
Morphological features of I. obliquus
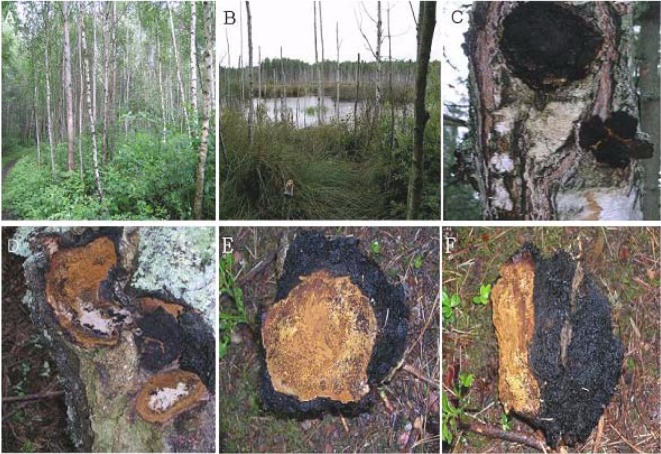
In boreal forest ecosystems, the commonly involved tree is the birch (Betula spp.) and in meridional mountain forests the common tree is the beech (Fagus spp.) (Figs. 1A and 1B). I. obliquus cause a white heart rot. When the sterile conk is cut off from the host log or trunk, a yellowish-brown margin between fungal growth and the tree is typically evident (Fig. 1C). The blackish outer conk is not a fruiting body, but a dead fungal mass (Fig. 1D). The fungus penetrates the tree through wounds, especially through poorly healed branch stubs. Decay spreads throughout heartwood, but does not penetrate sapwood in the infection cycle that occurs in living trees, except for the portion around the sterile conk. Sapwood is colonized only in limited areas around basidiocarps on dead wood. Decay will continue for 10~80+ years inside a living host tree. Within infected living trees, the fungus produces only sterile cinder conks, 1~3 per stem.
Fig. 1.
Plantation area of birch and sterile conk development of Chaga. A, Location of birch stand area in peat bog site; B, Wetland of birch stand in peat bog; C, Infected tree by I. obliquus. The black conk is the mycelia mass of the fungus; D, Infested area visualized by cutting off the cinder conk. The scar of the infected portion is evident as a yellowish-brown color; E, The front view of a sterile conk cut off from a host plant. The yellowish-brown color is typical of the inside of a sterile conk; F, Side view of a sterile conk. Part of the yellowish-brown portion was attached to the host tree, and the black color is an out-growth produced during development of the sterile conk.
I. obliquus produces two stages of fructification: an anamorphic phase and the aforementioned sterile cinder conks: the latter occur perennially and do not produce sexual chlamydospores or a sexual teleomorphic having basidicarps with hymenium, whereas the fertile fruiting body that appears while the host tree or some portion dies forms only once in the infection cycle. Basidiocarps of the teleomorphic stage occur as a layer of pores on cracks respiring on exposed wood, which are processed by special tissues of fungus-infected stem branches. In infected stems, withering occurs after production of basidiocarps. Thus, I. obliquus is connected with living trees like a real parasite (i.e., strictly connected with the host and with an adapted life cycle). The shape and structure of I. obliquus in the sexual stage is resupinate, forming layers of pores several square decimeters in area and 10~15 mm in depth. The surface is porous and whitish in the early stage, and then becomes dark brown with a brown gleam in the matured late stage. Pores are angular to oblong, 1~1.5 mm in diameter, and slant obliquely upward.
The basidiospores are hyaline or faintly yellowish, with average dimensions of 8~12 × 4~5 micrometers, having and dark-brown and thick-walled bulbous setae in the hymenium. The inconspicuous and short-lived fruiting bodies form under the bark and ultimately break through. They are flat, thin, and brown. The subiculum is barely developed. The consistency is soft and corky when fresh, and hard and brittle when dry.
The anamorphic stage of I. obliquus is more visible and can be observed as a sterile conk. Visually, it is a crispy-textured, charcoal-black fungal mass that forms on living stems of birches and alders. The shape of sterile conks is similar to buried cancers on other woody plants like the beech, contrary to the swellings that develop on birches. There are not more than 1~3 cinder conks on one tree. The black, deeply-scarred, and cracked appearance of the outer surface of the protruding growth resembles burnt charcoal (Figs. 1E and 1F).
The fungus infects host plants by spores and causes white rot in heartwood. The role of chlamydospores produced in sterile conks is not clear. It is presumed that the infection starts from basidiospores. Morphological characterization of I. obliquus has recently been reported (Park et al., 2005).
Distribution and ecology
I. obliquus has a circumboreal region within holarctic area of distribution, being found from the mountain areas of meridional zones to subarctic areas in the Northern Hemisphere. It is well-known in regions of Canada, United States, Asia (e.g., Kazachstan), Siberia, Japan, Korea, and Europe. It is a rare species in Western and Southern Europe, although it is relatively common in Central and Northern Europe.
The main Betula spp. are B. platyphlla, B. pendula, B. davurica, B. ermani, and B. costata. Fungus from alders (Alnus spp.), beech (Fagus spp.), oaks (Quercus spp.), or poplars (Populus spp.) have been reported (Ryvarden and Gilbertson, 1993). However cinder conks produce this fungus only in birches and alders. Ulmus spp. probably involve I. ulmicola Corfix (Ryvarden and Gilbertson, 1993).
Distribution in Central Europe with the Czech Republic as an example
Some 93 locales of I. obliquus distribution have been noted in former Czechoslovakia (Kotlaba, 1984). More specifically, these involved Bohemia (n = 30 locales), Moravia (n = 30), and Slovakia (n = 33). The distribution is vast, encompassing lowlands to mountain areas, and from altitude 155 m a.s.l. in Bohemian Elbe lowland to 1515 m a.s.l. in the Slovakian High Tatras (Kotlaba, 1984). Most of these localities are in uplands and highlands, with lowland distribution being restricted to thermophilic broadleaved species. In some areas it is still quite common.
The birch species B. pendula, B. pubescens, and B. carpatica accounted for 38% of the infections in one study, with F. sylvatica accounting for 37%, and less frequent involvement of Acer pseudoplatanus, Alnus glutinosa, Alnus incana, Fraxinus excelsior, Quercus cerris, Q. petraea, Q. robur, Q. delachampii and Ulmus sp. (Kotlaba, 1984). The infection on elm trees could be specifically due to I. ulmicola Corfix, however, this species has not been described in the Czech Republic. Sterile conks on birches occur only in specific areas with natural range of its distribution (Figs. 1C and 1D). Typical cinder conks are only observed in birches and alders; sterile conks in other species of woody plants produce mostly buried cancers in host stems. Sterile conks are relatively common in the Czech Republic, but they are not used in folk medicine although mushroom picking is very popular. Sterile conks on birches are present only in colder and more humid areas, which correspond to the natural distribution of host plants. Plant protection from this parasitic fungus of I. obliquus is not known.
Conclusions
Sterile conks of I. obliquus are used as one of the traditional medicinal fungi in many countries. The fungus is still common in some natural forest ecosystems in boreal zones of North America, Europe, and Asia on several host trees. Typical cinder conks are produced on birches and alders. Results from application of this fungus rank high among the polypores. It is also one of the most intensively studied species of fungi given its recognized potential as a source of pharmaceuticals and its biotechnological utility.
Acknowledgement
This study was supported by an International Cooperative Research Grant (No. C00004) from the Korean Research Foundation, Ministry of Education, Science and Technology.
References
- 1.Babitskaia VG, Shcherba VV, Ikonnikova VV. Melanin complex of the fungus Inonotus obliquus. Prikadnaya Biokhimiya Mikrobiologiya. 2000;36:439–444. [PubMed] [Google Scholar]
- 2.Babitskaya VG, Scherba VV, Ikonnikova NV, Bisko NA, Mitropolskaya NY. Melanin complex from medicinal mushroom Inonotus obliquus (Pers.:Fr.) Pilát (Chaga) (Aphyllophoromycetidae) Int J Med Mushrooms. 2002;4:139–145. [Google Scholar]
- 3.Bourdot H, Galzin A. Hymenomycete de France. Paris: 1927. p. 643. [Google Scholar]
- 4.Burczyk J, Gawron A, Slotwinska M, Smietana B, Terminsk K. Antimitotic activity of aqueous extracts of Inonotus obliquus. Boll Chim Farm. 1996;135:306–309. [PubMed] [Google Scholar]
- 5.Campbell WA, Davidson RW. A poria as the fruiting stage of the fungus causing the sterile conks on birch. Mycologia. 1938;30:553–560. [Google Scholar]
- 6.Findlay WPK. Note on an abnormal fungus on birch. Trans Bri Mycol Soc. 1939;23:169–170. [Google Scholar]
- 7.Kahlos K, Kangas L, Hiltunen R. Antitumor activity of some compounds and fractions from an n-hexane extract of Inonotus obliquus in vitro. Acta Pharm Fennica. 1987;96:33–40. [Google Scholar]
- 8.Kahlos K, Hiltunen R. Identification of some lanostane type triterpens from Inonotus obliquus. Acta Pharm Fennica. 1983;92:220. [Google Scholar]
- 9.Kahlos K, Hiltunen R. The sterols and triterpenes in Inonotus obliquus. Acta Agronomica. 1985;34:82. [Google Scholar]
- 10.Kahlos K, Hiltunen R. Two new oxygenated lanostance type triterpenes from Inonotus obliquus. Acta Pharm Fennica. 1986;95:71–76. [Google Scholar]
- 11.Kahlos K, Kahlos L, Hiltunen R. Antitumor tests of inotodiol from the fungus Inonotus obliquus. Acta Pharm Fennica. 1986;95:173–177. [Google Scholar]
- 12.Kahlos K, Kangas L, Hiltunen R, Schantz MV. The antitumor activity of some extracts and compound isolated from Inonotus obliquus. Farmaceutisch tudschrift voor Belgie. 1984;61:305–306. [Google Scholar]
- 13.Kahlos K, Kangas L, Hiltunen R. Ergosterol peroxide, an active compound from Inonotus obliquus. Planta Medica. 1989;55:389–390. doi: 10.1055/s-2006-962036. [DOI] [PubMed] [Google Scholar]
- 14.Katayevskaya NI. Tchaga (contribution to the study of tree rots) Mitt Forsl Versuchsw Omsk. 1928;1(6) (Extract in Rev. Appl. Mycol. VIII, 345) [Google Scholar]
- 15.Kotlaba F. Zeměpisné rozšíření a ekologie chorošů v Československu. [Geographically distribution and ecology of polypores in Czechoslovakia] Academia Praha; 1984. [Google Scholar]
- 16.Kukulyanskaya TA, Kurchenko NV, Kurchenko VP, Babitskaya VG. Physicochemical properties of melanins produced by the sterile form of Inonotus obliquus in natural and cultured fungus. App Biochem Microbiol. 2002;38:58–61. [Google Scholar]
- 17.Kim YO, Park HW, Kim JH, Lee JY, Moon SH, Shin CS. Anti-cancer effect and structural characterization of endo-polysaccharide from cultivate mycelia of Inonotus obliquus. Life Sci. 2006;79:72–80. doi: 10.1016/j.lfs.2005.12.047. [DOI] [PubMed] [Google Scholar]
- 18.Mizuno T, Zhuang C, Abe K, Okamoto H, Kiho T, Ukai S, Leclerec S, Meijer L. Antitumor and hypoglycemic activities of polysaccharides from the sclerotia and mycelia of Inonotus obliquus (Per.:Fr.) Pil. Intl J Med Mushrooms. 1999;1:301–316. [Google Scholar]
- 19.Park H, Lee BH, Bak WC. Cultural characteristics of Inonotus obliquus isolated from Betula costata at Mt. Jumbong in Korea. J Mushroom Sci Production. 2005;3:71–74. [Google Scholar]
- 20.Park YK, Lee HB, Jeon EJ, Jung HS, Kang MH. Chaga mushroom extract inhibit oxidative DNA damage in human lymphocytes as assessed by comet assay. Biofactors. 2004;21:109–112. doi: 10.1002/biof.552210120. [DOI] [PubMed] [Google Scholar]
- 21.Ryvarden L, Gilbertson RL. European polypores. Part 1. Oslo: Fungiflora-Fungiflora; 1993. pp. 1–387. [Google Scholar]
- 22.Saar M. Fungi in Khanty fork medicine. J Ethnopharmacol. 1991;3:175–179. doi: 10.1016/0378-8741(91)90003-v. [DOI] [PubMed] [Google Scholar]
- 23.True RP, Tyron EH, King JF. Cankers and decays of birch associated with two Poria species. J For. 1955;53:412–415. [Google Scholar]