The growing use of pharmacological agents means that drug interactions are of increasing interest for public health.1 Monitoring of potential drug interactions may improve the quality of drug prescribing and dispensing, and it might form a basis for education focused on appropriate prescribing.
Participants, methods, and results
In a cross sectional study, we analysed all prescriptions (n=962 013) involving two or more drugs dispensed to the Swedish population (n=7 214 509; age range 15-95) from all Swedish pharmacies (n=885) in January 1999. The data were taken from the Swedish healthcare database on pharmaceutical agents, which records all prescriptions dispensed at all of the pharmacies in Sweden. Strict registration routines and internal controls support the accuracy of the database.
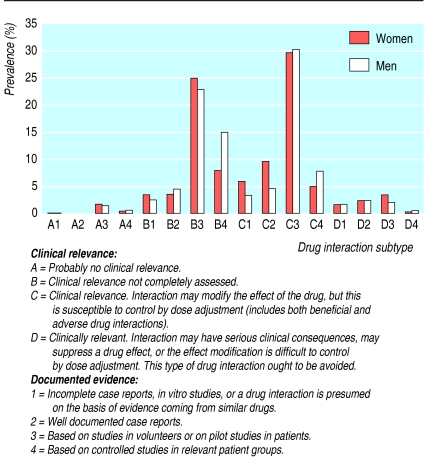
Data were stratified by age and sex, and odds ratios were calculated using multilevel logistic regression.2 Potential drug interactions were classified according to clinical relevance (types A, B, C, and D) and documented evidence (types 1, 2, 3, and 4)—for example, subtype D4 indicates an interaction with greater potential clinical relevance than that classified as subtype A1 (figure).3,4
Of the 962 013 prescriptions dispensed by pharmacies, 130 765 (13.6%) included at least one potential drug interaction. The number of potential drug interactions increased with the patient's age and with the number of drugs per prescription (data not shown). Clinically relevant potential drug interactions that could be controlled by adjusting the dose (type C) were found in 29 991/371 402 (8.1%) men and 44 754/545 857 (7.6%) women. Potential interactions that might have serious clinical consequences (type D) were found in 13 282 (1.4%) of the prescriptions (11.4% (5269/371 402) men and 1.4% (8013/590 611) women). However, 6936 (52.2%) of these potential interactions were between ipratropium and β adrenergic agonists, which result in an increased risk of acute angle closure glaucoma only when the drugs are used in nebulised form—an uncommon treatment.
After adjusting for the number of drugs dispensed, we found that combinations of drugs with potential interactions that may have serious clinical consequences (type D) were less likely to be prescribed to women than men (relative risk 0.88; 95% confidence interval 0.85 to 0.92).
Of the potential type D interactions, 2358 were between potassium supplements and potassium sparing diuretics—a combination that may result in severe and even life threatening hyperkalaemia. The combination of warfarin dispensed with a non-steroidal anti-inflammatory drug (subtype D4), which can increase the risk of gastrointestinal bleeding due to gastric mucosal damage by the non-steroidal anti-inflammatory drug and the anticoagulant effect of warfarin, was found on 644 occasions.
Dextropropoxyphene was dispensed with alprazolam on 261 occasions (this combination may increase the central depressant effects of alprazolam) and with carbamazepine on 240 (this combination may cause serious toxic effects by increasing plasma concentrations of carbamazepine). Cisapride was dispensed with erythromycin on five occasions, with clarithromycin on three, fluconazole on 24, and itraconazole on one; any of these combinations may result in torsades de pointes, syncope, cardiac arrest, and sudden death.
Comment
Although the percentage of potential drug interactions that may have serious clinical consequences (type D) was low (1.4%), serious and potentially fatal drug interactions—for example, NSAID and warfarin, potassium supplements and potassium sparing diuretics, dextropropoxyphene and carbamazepine, and cisapride and fluconazole—were detected. The risk of interactions with cisapride was known in 1996,5 and cisapride, which is still available in Sweden, is being withdrawn in many countries.
Prescribing pairs of drugs with potential interactions increases the risk of, but need not lead to, an adverse reaction. Many drug interactions are susceptible to control by dose adjustment; moreover, some are beneficial and are exploited in therapeutics.
National monitoring of potential drug interactions in Sweden is feasible. Differences in healthcare systems need to be considered when extrapolating the results of this study to other countries.
Figure.
Prevalence of potential drug-drug interaction subtypes3 4 among 962 013 prescriptions containing two or more drugs dispensed to patients aged 15-95 from Swedish pharmacies in January 1999.
Acknowledgments
Presented in part at the International Society of Pharmacoepidemiology annual meeting in Barcelona, Spain, 19-23 August 2000. We are grateful to the Swedish Centre for Epidemiology (National Board of Health and Welfare) and the National Corporation of Swedish Pharmacies (Apoteket AB, formerly Apotekesbolaget) for access to the Swedish Health Care Database on Pharmaceutical Agents, and to Frank Wollheim, Department of Rheumatology, Lund University Hospital for his support.
Footnotes
Funding: Nätverk för Läkemedelsepidemiologi (NEPI) foundation; Government grant (JM) from Avtal om Läkarutbildning och Forskning (founding document number M : E 39 390/98).
Competing interests: None declared.
References
- 1.Stockley IH. Drug interactions: a source book of adverse interactions, their mechanisms, clinical importance and management. London: Pharmaceutical Press; 1996. [DOI] [PubMed] [Google Scholar]
- 2.Rasbash J, Browne W, Goldstein H, Yang M, Plewis I, Healy M, et al. A user's guide to MLwiN. London: Institute of Education, University of London; 1999. [Google Scholar]
- 3.Sjöqvist F. The Swedish Drug Compendium “FASS”. Läkemedel i Sverige. Förtecking över humanläkemedel. Stockholm: LINFO Läkemedelsinformation AB; 1999. Drug interactions; pp. 1383–1453. [Google Scholar]
- 4.Sjöqvist F. The Swedish Drug Compendium “FASS”. Läkemedel i Sverige. Förtecking över humanläkemedel. Stockholm: LINFO Läkemedelsinformation AB; 2000. Drug interactions; pp. 1481–1556. [Google Scholar]
- 5.Biverkningsinformation: Prepulsid (cisaprid) - hjärtarytmi: Påminnelse. Information från Läkemedelsverket. 1996;7(6):27. [Google Scholar]