Abstract
Mushroom cultivation has been started recently in Bangladesh. Awareness of the nutritional and medicinal importance of mushrooms is not extensive. In this study, the nutritional values of dietary mushrooms- Pleurotus ostreatus, Pleurotus sajorcaju, Pleurotus florida and Calocybe indica that are very popular among the cultivated mushrooms in Bangladesh have been determined. These mushrooms were rich in proteins (20~25%) and fibers (13~24% in dry samples) and contained a lower amount of lipid (4 to 5%). The carbohydrate contents ranged from 37 to 48% (on the basis of dry weight). These were also rich in mineral contents (total ash content is 8~13%). The pileus and gills were protein and lipid rich and stripe was carbohydrate and fiber-rich. The moisture content of mushrooms ranged from 86 to 87.5%. Data of this study suggest that mushrooms are rich in nutritional value.
Keywords: Carbohydrate, Lipid, Minerals, Oyster and milky mushroom, Protein
Mushroom have been a widely used as food and food supplements for millennia. It is an important food item concerning human health, nutrition and disease prevention (Chang, 1996). There is a common saying that "medicines and foods have a common origin" (Kaul, 2001). Dietary mushrooms provide a wide variety of medicinal properties and they are effective against certain lifethreatening diseases. Major medicinal properties attributed to mushrooms include anticancer, antibiotic, antiviral activities, immunity and blood lipid lowering effects. Pleurotus spp. are also rich in medicinal values. Pleurotus florida has antioxidant and antitumor activities (Nayana and Janardhanan, 2000; Manpreet et al., 2004), Pleurotus sajor-caju has hypertensive effects through its active ingredients which affect the renin-angiotensin system (Chang, 1996), P. ostreatus possesses antitumor activity (Yoshioka et al., 1985) and hypoglycaemic effects in experimentally diabetic induced rats (Chorvathova et al., 1993). Oyster mushrooms are very effective in reducing the total plasma cholesterol and triglyceride level (Nuhu Alam et al., 2007) and thus reduce the chance of atherosclerosis and other cardiovascular and artery related disorders. These medicinal properties might be due to the presence of some important substance in dietary mushrooms. Mushrooms are rich in protein, minerals and vitamins and they contain an abundance of essential amino acids (Sadler, 2003). Nutritional analysis of several mushroom species of different origins had been carried out in many laboratories in the world. But nutritional values of locally cultivated mushrooms remain speculative. Moreover, nutritional composition is affected by many factors; these include differences among strains, the composition of growth substrate, the method of cultivation, stage of harvesting, specific portion of the fruiting bodies used for analysis (Benjamin, 1995).
Generally, people in Bangladesh are still not very aware of nutritional and medicinal importance of mushrooms. The history of mushroom cultivation is very recent in Bangladesh. Only some species of mushrooms are now cultivated in this country and among these Pleurotus ostreatus, P. sajor-caju, P. florida and Calocybe indica are popular and widely accepted (Ruhul Amin et al., 2007). The aim of this investigation was to analyze the nutritional values of these mushrooms cultivated in Bangladesh, with a goal of increasing awareness of the beneficial effects of edible mushrooms among the consumers.
Materials and Methods
This study was carried out 'Quality Control and Quality Assurance' laboratory of National Mushroom Development and Extension Centre (NAMDEC), Savar, Dhaka, Bangladesh. Pleurotus ostreatus, Pleurotus sajor-caju, Pleurotus florida and Calocybe indica mushrooms were cultivated and harvested in the laboratory of NAMDEC.
Moisture analysis
Twenty gram of fresh mushroom was weighed into a weighed moisture box (A&D company ltd. N 92; P1011656; Japan) and dried in an oven at 100~105℃ and cooled in a dessicator. The process of heating and cooling was repeated till a constant weight was achieved.
The moisture content was calculated as following equation:
Moisture (%) = (initial weight - final weight) × 100/weight of sample (Raghuramulu et al., 2003).
Determination of total protein
Five gram of grinded mushroom was taken with 50 ml of 0.1 N NaOH and boiled for 30 min. The solution was cooled in room temperature and centrifuged at 1000 × g by a DSC-200T tabletop centrifuge (Digisystem Laboratory Instruments, Taipei, Taiwan). The supernatant was collected and total protein content was measured according to the method of Lowry et al. (1951). For the determination of protein content from fresh mushroom, 5 g was taken with 50 ml phosphate buffer and homogenized with a tissue homogenizer (Polytron, Lucerne, Switzerland). Five milliliter of homogenized was taken with 50 ml of 0.1 N NaOH and protein content was determined as mentioned above.
Determination of total lipid
Total lipid was determined by slight modified method of Folch et al. (1957). Five gram of grinded mushroom was suspended in 50 ml of chloroform : methanol (2 : 1 v/v) mixture then mixed thoroughly and let stand for 3 days. The solution was filtrated and centrifuged at 1000 g by a table centrifuge machine. The upper layer of methanol was removed by Pasteur pipette and chloroform was evaporated by heating. The remaining was the crude lipid. For the determination of total lipid from fresh mushroom, 5 g was taken with 50 ml phosphate buffer and homogenized with a tissue homogenizer. Five ml of homogenized was taken with 50 ml of chloroform : methanol (2 : 1 v/v) mixture and lipid content was determined as mentioned above.
Determination of crude fiber
Ten grams of moisture and fat-free sample was taken in a beaker and 200 ml of boiling 0.255 N H2SO4 was added. The mixture was boiled for 30 minutes keeping the volume constant by the addition of water at frequent intervals. The mixture was then filtered through a muslin cloth and the residue washed with hot water till free from acid. The material was then transferred to the same beaker, and 200 ml of boiling 0.313 N NaOH added. After boiling for 30 minutes (keeping the volume constant as before) the mixture was filtered through a muslin cloth and the residue washed with hot water till free from alkali, followed by washing with some alcohol and ether. It was then transferred to a crucible, dried overnight at 80~100℃ and weighed (We) in an electric balance (Keyi: JY-2003; China). The crucible was heated in a muffle furnace (Nebertherm: Mod-L9/11/c6; Germany) at 600℃ for 5~6 hours, cooled and weighed again (Wa). The difference in the weights (We-Wa) represents the weight of crude fiber.
Crude fiber (g/100 g sample) = [100 - (moisture + fat)] × (We-Wa)/Wt of sample (Raghuramulu et al., 2003).
Determination of total ash
One gram of the sample was weighed accurately into a crucible. The crucible was placed on a clay pipe triangle and heated first over a low flame till all the material was completely charred, followed by heating in a muffle furnace for about 5~6 hours at 600℃. It was then cooled in a dessicator and weighed. To ensure completion of ashing, the crucible was then heated in the muffle furnace for 1 h, cooled and weighed. This was repeated till two consecutive weights were the same and the ash was almost white or grayish white in color. Then total ash was calculated as:
Ash content (g/100 g sample) = weight of ash × 100/weight of sample taken (Raghuramulu et al., 2003).
Total carbohydrate estimation
The content of the available carbohydrate was determined by the following equation:
Carbohydrate (g/100 g sample) = 100 - [(moisture + fat + protein + ash + crude fiber) g/100 g] (Raghuramulu et al., 2003).
Mineral analysis
Total ash was taken for the analysis of mineral contents. Two ml of conc. HNO3 was added to the ash and heated for 2 minutes. One drop of hydrogen peroxide was added into the solution. The solution was then transferred into a volumetric flask and total volume was made 50 ml by adding deionized distilled water. This was then used to analyze the contents of calcium (Ca), iron (Fe), manganese (Mn), magnesium (mg), zinc (Zn), Selenium (Se) and arsenic (As) by flame and graphite method with atomic absorption spectrophotometer (Perkin Elmer: AS 80).
Results and Discussion
Several nutritional parameters were measured for both fresh mushrooms (Table 1) and dry mushrooms (Table 2). The moisture contents of P. ostreatus, P. sajor-caju, P. florida and C. indica were found about 86, 87, 87.5 and 87.4% respectively. Hundred grams of fresh P. ostreatus contains 3~3.8 g of proteins, 0.63~0.73 g of lipids, 3.2~3.6 g of fiber and 5.0~5.4 g of carbohydrates. One hundred grams of fresh P. sajor-caju contained 3~3.6 g of proteins, 0.52~0.62 g of lipids, 2.8~3.1 g of fiber and 5.0~5.3 g of carbohydrates. In case of fresh P. florida these were as follows: 2.5~2.75 g of proteins, 0.5~0.6 g of lipids, 2.9~3.1 g of fiber and 5.0~5.6 g of carbohydrates and 100 g of C. indica contained 2.6~2.9 g of proteins, 0.6~0.7 g of lipids, 1.5~1.8 g of fiber and 6.3~7.3 g of carbohydrates (Table 1). The protein, lipid, fiber and carbohydrate contents in 100 g of dried P. ostreatus were found as 22~26 g, 4.4~4.8 g, 23~26 g and 35~40 g respectively. These were found as 23~26 g, 4.2~4.6 g, 22~23.6 g and 38~41.5 g respectively in P. sajor-caju. 100 g of dried P. florida contained 19~22 g of proteins, 4~4.6 g of lipids, 22~24.6 g of fiber and 40~45 g of carbohydrates and 100 g of dried C. indica contained 20~23 g of proteins, 4.6~5.3 g of lipids, 11~15 g of fiber and 46~51 g of carbohydrates (Table 2). The protein content of P. ostreatus and P. sajor-caju were found 15~20% greater than that of P. florida and C. indica. The total fat content was greater in C. indica which is significant to P. sajor-caju and P. florida. C. indica was also significantly richer in carbohydrates than the three species of Pleurotus. On the other hand the fiber content in C. indica is significantly lower (about 40~50%) than that in Pleurotus spp.
Table 1.
Nutrient contents of fresh mushrooms (g/100 g)
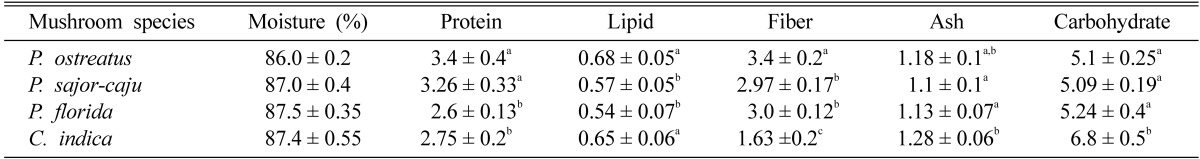
Results show mean ± SEM of 5 trials. Values in the same column that do not share a common superscript are significantly different at P < 0.05 (one way ANOVA then LSD post hoc comparison).
Table 2.
Nutrient contents of dried mushrooms (g/100 g)
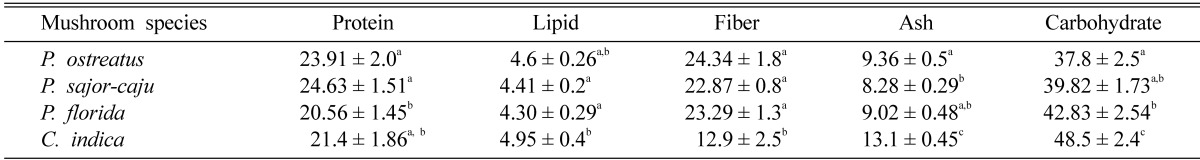
Results show mean ± SEM of 5 trials. Values in the same column that do not share a common superscript are significantly different at P < 0.05 (one way ANOVA then LSD post hoc comparison).
Mushrooms are also rich in mineral contents. The total ash content found in Pleurotus ostreatus, P. sajor-caju, P. florida and C. indica were 1.1~1.3 g, 1~1.2 g, 1.1~1.2 g and 1.2~1.4 respectively. In case of dry mushrooms these were 9~10 g, 8~8.6 g, 8.6~9.5 g and 12.5~13.5 g respectively (Tables 1, 2).
According to Breene (1990) and Ço_kuner and Özdemir (2000), protein contents of mushrooms range from 19 to 39 g in 100 g dried matter. In our study we found the protein values (g/100 g dried matter) as 23.91 g in P. ostreatus, 24.63 g in P. sajor-caju, 20.56 g in P. florida and 21.4 g in C. indica. Lipid value was 4.3~4.9 g in 100 g in dry matter of four cultivated mushroom. These results are in conformity with Shin et al. (2007). 34.8% dietary fibre value was found in oyster mushrooms by Justo et al. (1999), which are higher than the values, we obtained in our study. Watanabe et al. (1994) found the carbohydrate value as 47.9 g in 100 g dry matter. Our carbohydrate values are almost similar the study made by Watanabe et al. (1994).
Table 3 shows the contents of some important minerals. Hundred grams of dried P. ostreatus contained Ca (35.9 mg), Fe (55.5 mg), Mg (16.4 mg), Mn (2.9 mg), Zn (26.6 mg), Se (11 µg) and As (100 µg). 100 g of dried P. sajor-caju contained Ca (22.15 mg), Fe (33.45 mg), Mg (20.22 mg), Mn (2.87 mg), Zn (20.9 mg), Se (25 µg) and As (95 µg). In case of 100 g P. florida these were as follows: Ca (33.7 mg), Fe (43.2 mg), Mg (13.4 mg), Mn (2.7 mg), Zn (16 mg), Se (13.2 µg) and As (83 µg) and 100 g C. indica contained Ca (20.7 mg), Fe (56.2 mg), Mg (12.8 mg), Mn (1.65 mg), Zn (12.9 mg), Se (13.2 µg) and As (54 µg). The difference of total mineral (ash) content among the C. indica and Pleurotus spp is significant. The findings in this study are comparable to previous studies (Crisan and Sands, 1978; Chang, 1980; Bano and Rajarathnam, 1982; Hong et al., 2007; Shin et al., 2007; Dundar et al., 2008).
Table 3.
Mineral contents of dried mushrooms (mg/100 g)
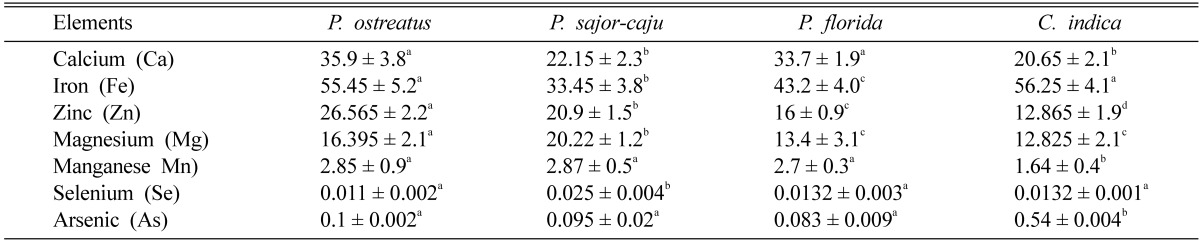
Results show mean ± SEM of 3 trials. Values in the same row that do not share a common superscript are significantly different at P < 0.05 (one way ANOVA then LSD post hoc comparison).
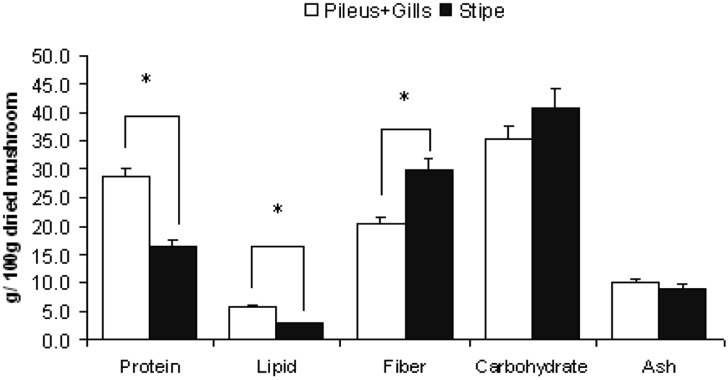
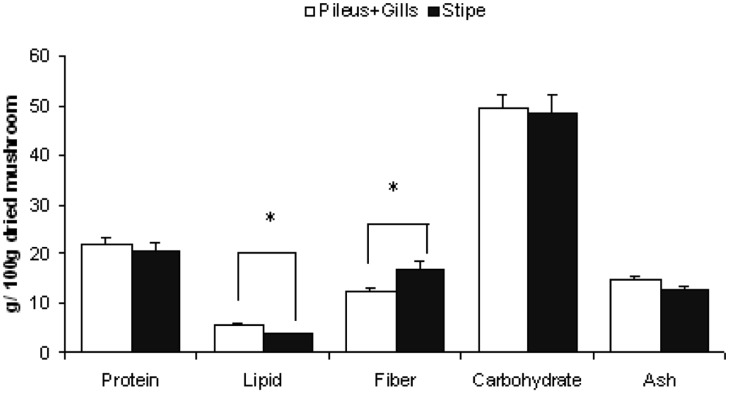
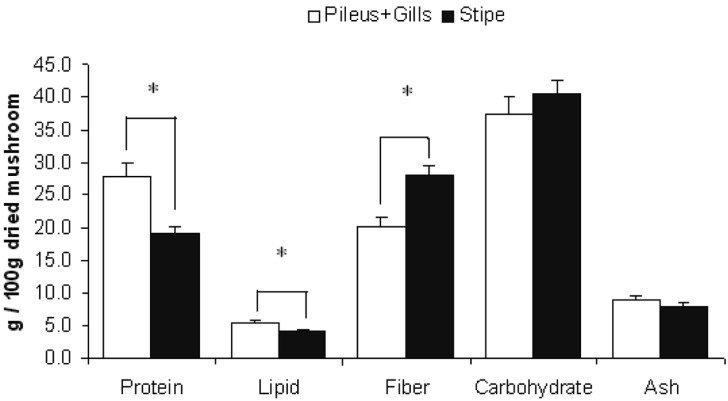
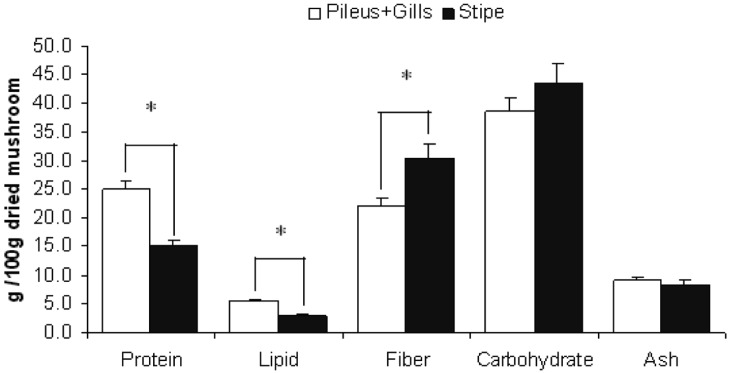
Figures 1~4 show the variation of nutritional parameters among the different parts of mushrooms. The pileus and gills are richer in protein (about 40~60%), lipid (30~60%) and ash content (5~10%) than stipe. On the other hand the stipe is richer in fiber (40~50%) and carbohydrate content (10~15%). The variation in protein, fat and fiber content is significant with the exception of C. indica in which protein variation is not significant. This result is agreeable with data collected from the previous study (Watanabe et al., 1994; Justo et al., 1999; Shin et al., 2007; Dundar et al., 2008).
Fig. 1.
Nutritional variation in different parts of Pleurotus ostreatus. Bars show mean + SEM of 5 trials. Data was analyzed by one way ANOVA and then post hoc LSD test. * indicates that difference is significant at P ≤ 0.05 level.
Fig. 4.
Nutritional variation in different parts of Calocybe indica. Bars show mean + SEM of 5 trials. Data was analyzed by one way ANOVA and then post hoc LSD test. * indicates that difference is significant at P ≤ 0.05 level.
In conclusion, the chemical composition of edible mushrooms determines their nutritional value and sensory properties as also mentioned by other authors (Shah et al., 1997; Manzi et al., 2001). They differ according to species but this difference also depends on the substratum, atmospheric conditions, age and part of the fructification. We found different nutritional values in the different part of cultivated mushrooms. These data suggest that dietary mushrooms cultivated in Bangladesh are good source of nutrients specially protein and fiber. Mushrooms are rich in protein, edible fiber and minerals but lipid content is low. These results also indicate that the studied mushrooms have good nutritive value for human. Protein is an important nutritional component and protein deficiency is the world's most serious human nutritional problem, especially in third world countries like Bangladesh. So mushroom is a promising food that may overcome protein-energy malnutrition problem in the third world.
Fig. 2.
Nutritional variation in different parts of Pleurotus sajor-caju. Bars show mean + SEM of 5 trials. Data was analyzed by one way ANOVA and then post hoc LSD test. * indicates that difference is significant at P ≤ 0.05 level.
Fig. 3.
Nutritional variation in different parts of Pleurotus florida. Bars show mean + SEM of 5 trials. Data was analyzed by one way ANOVA and then post hoc LSD test. * indicates that difference is significant at P ≤ 0.05 level.
References
- 1.Bano Z, Rajarathnam S. Pleurotus mushroom as a nutritious food. Tropical Mushrooms-Biological Nature and Cultivated Methods. Hong Kong: The Chinese University Press; 1982. pp. 362–363. [Google Scholar]
- 2.Benjamin DR. Mushroom, Poisons and Panaceas. New York, USA: W. H. Freeman & Company; 1995. pp. 151–165. [Google Scholar]
- 3.Breene WM. Nutritional and medicinal value of specialty mushrooms. J Food Protect. 1990;53:883–894. doi: 10.4315/0362-028X-53.10.883. [DOI] [PubMed] [Google Scholar]
- 4.Chang R. Functional properties of mushrooms. Nutr Rev. 1996;54:91–93. doi: 10.1111/j.1753-4887.1996.tb03825.x. [DOI] [PubMed] [Google Scholar]
- 5.Chang ST. Mushrooms as human food. Bioscience. 1980;30:399–400. [Google Scholar]
- 6.Chorvathoba V, Bobek P, Ginter E, Klavanova J. Effect of the oyster fungus on glycemia and cholesterolemia in rats with insulin depended diabetes. Physol Res. 1993;42:175–179. [PubMed] [Google Scholar]
- 7.Ço_kuner Y, Özdemir Y. Acid and EDTA blanching effects on the essential element content of mushrooms (Agaricus bisporus) J Sci Food Agric. 2000;80:2074–2076. [Google Scholar]
- 8.Crisan EV, Sands A. Nutritional value. In: Chang ST, Hayes WA, editors. The biology and cultivation of edible mushrooms. U.S.A.: New York Academic Press; 1978. pp. 137–165. [Google Scholar]
- 9.Dundar A, Acy H, Yildiz A. Yield performance and nutritional contents of three oyster mushroom species cultivated on wheat stalk. Afr J Biotechy. 2008;7:3497–3501. [Google Scholar]
- 10.Folch J, Lees M, Sloane-Stanely GH. A simple method for the isolation and purification of total lipids from animal tissues. J Biol Chem. 1957;226:497–509. [PubMed] [Google Scholar]
- 11.Hong IP, Nam SH, Sung GB, Chung IM, Hur H, Lee MW, Kim MK, Guo SX. Chemical components of Paecilomyces tenuipes (Peck) Samson. Mycobiology. 2007;35:215–218. doi: 10.4489/MYCO.2007.35.4.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Justo MB, Guzman GA, Mejia EG, Diaz CL, Martinez G, Corona EB. Calidad proteínica de tres cepas mexicanas de setas (Pleurotus ostreatus) Archivos Latinoamericanos de Nutricion. 1999;49:81–85. [PubMed] [Google Scholar]
- 13.Kaul TN. Biology and Conservation of Mushrooms. New Delhi, India: Oxford & IBH Publishing Co. Pvt. Ltd; 2001. pp. 117–145. [Google Scholar]
- 14.Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the folin phenol reagent. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- 15.Manpreet K, Giridhar S, Khanna PK. In vitro and in vivo antioxidant potentials of Pleurotus florida in experimental animals. Mushroom Res. 2004;13:21–26. [Google Scholar]
- 16.Manzi P, Aguzzi A, Pizzoferrato L. Nutritional value of mushrooms widely consumed in Italy. Food Chem. 2001;73:321–325. [Google Scholar]
- 17.Nayana J, Janardhanan KK. Antioxidant and antitumour activity of Pleurotus florida. Curr Sci. 2000;79:941–943. [Google Scholar]
- 18.Alam Nuhu, Hossain Shahdat, Khair Abul, Amin Ruhul, Asaduzzaman K. Comparative effects of oyster mushrooms on plasma lipid profile of hypercholesterolaemic rats. Bangladesh J Mushroom. 2007;1:15–22. [Google Scholar]
- 19.Raghuramulu N, Madhavan NK, Kalyanasundaram S. A Manual of Laboratory Techniques. Hyderabad, India: National Institute of Nutrition. Indian Council of Medical Research; 2003. pp. 56–58. [Google Scholar]
- 20.Amin Ruhul, Nirod CS, Mahbuba M, Jebunnahar K, Rahman Mahfuzur. Officer's Training Manual. Savar, Dhaka, Bangladesh: National Mushroom Development and Extension Centre; 2007. pp. 13–17. [Google Scholar]
- 21.Sadler M. Nutritional properties of edible fungi. Br Nutr Found Nutr Bull. 2003;28:305–308. [Google Scholar]
- 22.Shah H, Iqtidar AK, Shagufta J. Nutritional composition and protein quality of Pleurotus mushroom. Sarhad J Agric. 1997;13:621–626. [Google Scholar]
- 23.Shin CK, Yee CF, Lee JS, Atong M. Nutritional properties of some edible wild mushrooms in Sabah. J Appl Sci. 2007;7:2216–2221. [Google Scholar]
- 24.Watanabe T, Tsuehihasi N, Takai Y, Tanaka K, Suziki A. Effects of ozone exposure during cultivation of oyster mushroom (Pleurotus ostreatus) on chemical components of the fruit bodies. J Jpn Soc Food Sci Technol. 1994;41:705–708. [Google Scholar]
- 25.Yoshioka Y, Tabeta R, Saito H, Uehara N, Fukoaka F. Antitumor polysaccharides from P. ostreatus (Fr.) Quel isolation and structure of a β-glucan. Carbohydrate Res. 1985;140:93–100. doi: 10.1016/0008-6215(85)85052-7. [DOI] [PubMed] [Google Scholar]