Abstract
Medicinal mushrooms, including Cordyceps militaris, have received attention in Korea because of their biological activities. In the fruiting body and in corpus of C. militaris, the total free amino acid content was 69.32 mg/g and 14.03 mg/g, respectively. In the fruiting body, the most abundant amino acids were lysine, glutamic acid, proline and threonine. The fruiting body was rich in unsaturated fatty acids, which comprised about 70% of the total fatty acids. The most abundant unsaturated acid was linoleic acid. There were differences in adenosine and cordycepin contents between the fruiting body and the corpus. The adenosine concentration was 0.18% in the fruiting body and 0.06% in the corpus, and the cordycepin concentration was 0.97% in the fruiting body and 0.36% in the corpus.
Keywords: Adenosine, Amino acid, Cordycepin, Cordyceps militaris, Fatty acid
"Winter Worm Summer Grass" (Cordyceps), known as "Dong Chung Ha Cao" in Korea and "Dong Chong Xia-Cao" in China, has been used as a traditional folk medicine in Asia for hundreds of years. Cordyceps is in the family Clavicipitaceae in the class Pyrenomycetes of the order Hypocreales of the ascomycetous fungi. It parasitizes insects (Kobayashi, 1982; Spatafora and Blackwell, 1993) and colonizes dead or living Hepialus (Lepidoptera) caterpillars. Spores germinate inside the caterpillars, hyphae fill the caterpillar body, and a stalked fruiting body is produced (Li et al., 1998). The fruiting body of Cordyceps is collected from infected pupae or larvae.
Various bioactive compounds are found in Cordyceps spp. Compounds from C. sinensis can modulate immune responses (Kuo et al., 1996), inhibit the growth of tumor cells (Bok et al., 1999), enhance hepatic energy (Manabe et al., 1996) and promote the secretion of adrenal hormones (Wang et al., 1998), and it posseses hypotensive and vasorelaxant activities (Chiou et al., 2000). Cordycepin from C. militaris has several biological activities, including inhibition of RNA and DNA synthesis and suppression of viral replication (Kuo et al., 1994). Galactomannan from C. cicadae prevents the growth of sarcoma in mice (Huang et al., 1997). Polysaccharides purified from C. ophioglossoides have antitumor properties (Wu et al., 2001). N6-(2-hydroxyethyl) adenosine (HEA), a nucleoside derivative isolated from C. pruinosa, has a Ca2+ antagonistic effect and negative inotropic response (Furuya et al., 1983). Compounds from C. pruinosa suppress inflammation through the suppression of NF-κB-dependent inflammatory gene expression (Kim et al., 2003).
Naturally grown fruiting bodies of Cordyceps are expensive and scarce but the demand for Cordyceps has increased. The Cordyceps species, C.militaris and C. pruinosa are cultivated mainly in Korea. The aim of this study was to find and to compare the chemical ingredients of the fruiting body and corpus of Cordyceps militaris
Materials and Methods
Samples
Cordyceps militaris was purchased at the Kyong-dong market in Seoul. Specimens were divided into fruiting body and corpus, then milled.
Amino acid assay
The amino acid composition was determined by hydrolyzing Cordyceps militaris samples with 6 N HCl for 24 h at 105℃, then deriving the amino acids in a Waters Pico-Tag work station (Pico-Tag System, Waters Co.). The derivative amino acids were analyzed by liquid chromatograph consisting of Waters 515 pumps, Waters 486 UV detector, and Reodyne injector (Waters Co.), equipped with Waters Pico-Tag column (3.9 × 150 mm, Waters Co.). Amino acids were identified by comparing retention times and areas to an authentic standard mixture.
Fatty acid assay
Fatty acids were extracted from dried samples using the method of Hamilton & Hamilton (1992). Fatty acids were determined as fatty acid methyl esters (FAMEs) by gas chromatography using Hewlett-Packard, Model 5890 Series II gas chromatograph (Agilent Co.) equipped with a fused silica capillary column (SP-2560, with a 0.25 mm diameters, 100 m length, and 0.20 µm film thickness; Supelco Ltd.). The sample was injected into the GC using a Hewlett-Packard 7673 autoinjector (Agilent Co.). The temperature program was: 140℃ for 5 min; increase to 240℃ at 4℃/min; maintain at 240℃ for 15 min. Helium was used as the carrier gas and was maintained at a flow rate of 20 cm/s. The injection port and the flame ionization detector oven temperatures were 260℃. FAMEs were identified by comparing retention times to an authentic standard mixture (Supelco 37 Component FAME Mix, Supelco Co.).
Adenosine and cordycepin
Known amounts of adenosine and cordycepin were dissolved in mobile phase solution to give various concentrations for calibration. Samples were extracted in hot water at 100℃ for 2 h, and then filtered through a 0.45 µm filter membrane. HPLC analysis was performed using a HITACHI L-6200 pump with a RHEODYNE M-4250 detector and D-2500 integrator. A pre-packed RP column Cosmosil 5C18 (4.6 × 250 mm, 5 µm particle size) from Nacalai Tesque (Kyoto, Japan) was used. The mobile phase was a mixture of methanol and 0.02M potassium dihydrogenphosphate (15 : 85). Elution was performed at a solvent flow rate of 1 ml/min starting with 30% methanol for 15 min. A gradient was then used to obtain 40% methanol at 20 min, 45% methanol at 30 min, 60% methanol at 50 min, and 80% methanol at 52 min. Elution then remained isocratic at 80% methanol for another 60 min. Detection was performed with a variable-wavelength UV detector (L-4250) at 260 nm.
Results
Amino acids
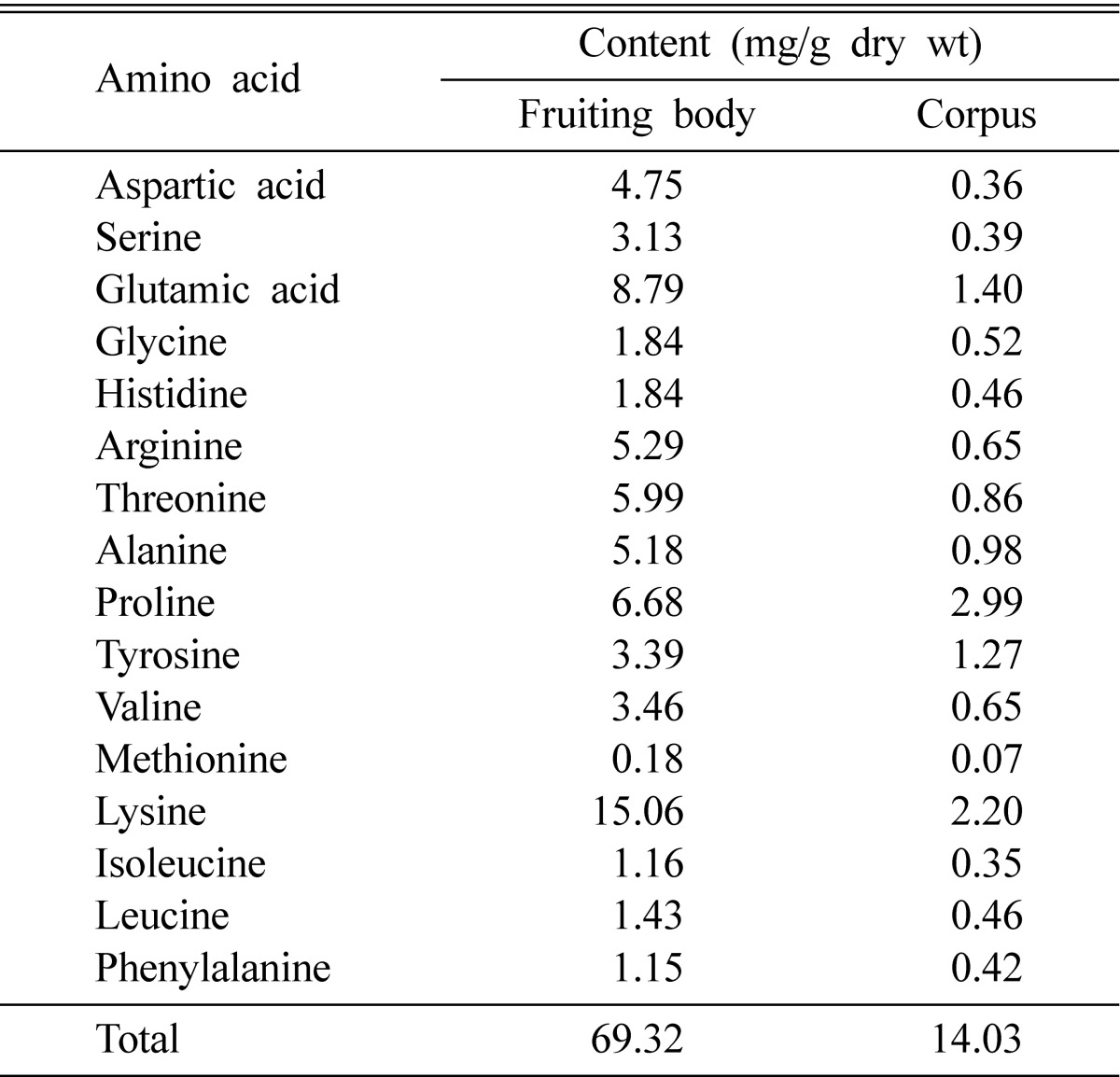
Amino acid compositions of C. militaris are presented in Table 1. The total amino acid content (dry weight) was higher in the fruiting body (69.32 mg/g) than in the corpus (14.03 mg/g). The content of individual amino acids in the fruiting body and corpus of C. militaris ranged from 1.15 to 15.06 mg/g and from 0.36 to 2.99 mg/g, respectively. Amino acids present at concentrations of more than 5.00 mg/g were lysine (15.06 mg/g), glutamic acid (8.79 mg/g), prolin (6.68 mg/g), threonine (5.99 mg/g), arginine (5.29 mg/g), and alanine (5.18 mg/g) in the fruiting body. Chang et al. (2001) reported that the most abundant amino acids in C. militaris mycelia were aspartic acid (2.66 mg/g), valine (2.21 mg/g) and tyrosine (1.57 mg/g).
Table 1.
Contents of free amino acids in Cordyceps militaris
Fatty acids
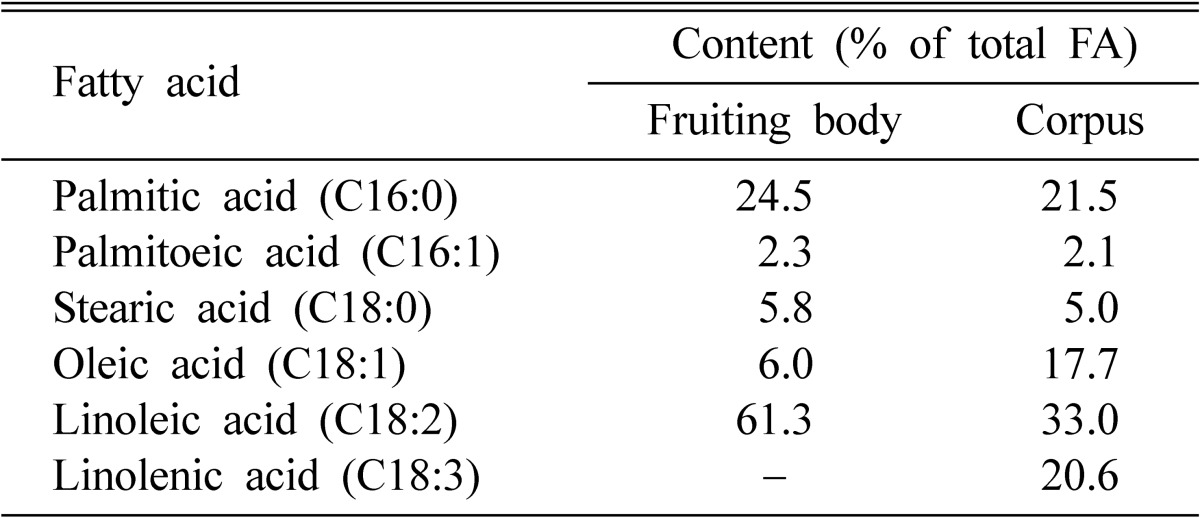
Fatty acid compositions of C. militaris are presented in Table 2. The fruiting body of C. militaris was rich in unsaturated fatty acids (about 70% of the total fatty acids). The most abundant saturated acid was palmitic acid. Its levels were 24.5% in fruiting body and 21.5% in the corpus. The most abundant unsaturated acid was linoleic acid. Its levels were 61.3% in fruiting body and 33.0% in the corpus.
Table 2.
Contents of fatty acids of Cordyceps militaris
Adenosine and cordycepin
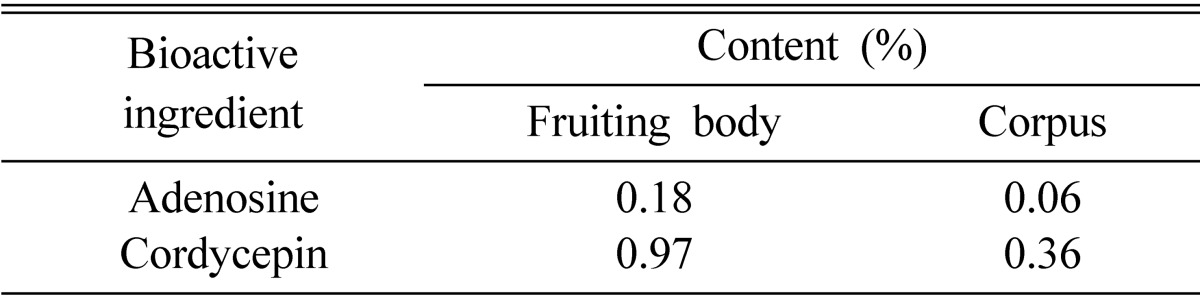
Adenosine and cordycepin concentrations in the Cordyceps militaris are presented in Table 3. The adenosine concentration was 0.18% in the fruiting body and 0.06% in the corpus. The cordycepin concentration was 0.97% in the fruiting body and 0.36% in the corpus There were differences in adenosine and cordycepin contents between the fruiting body and the corpus of C. militaris. The adenosine and cordycepin concentration in the fruiting body was approximately 3 fold higher than in the corpus. The adenosine concentration was lower than the concentration of cordycepin.
Table 3.
Contents of adenosine and cordycepin in Cordyceps militaris
Discussion
The use of Dong Chong Xia Cao as a health or functional food has been appreciated for hundreds of years in Asia. Recently, an artificial cultivation method was developed in Korea. It uses living silkworm larvae and pupae as growth substrates for Cordyceps militaris.The amino acid and fatty acid composition of Cordyceps militaris was obtained. The total content of amino acids in the fruiting body was much higher than in the corpus. The most abundant amino acids of C. militaris were lysine, glutamic acid, proline and threonine in the fruiting body, and proline and lysine in the corpus. Chang et al. (2001) reported that the most abundant amino acids in C. militaris mycelia were aspartic acid (2.66 mg/g), valine (2.21 mg/g) and tyrosine (1.57 mg/g). These results show that the most abundant amino acids of C. militaris are not similar to the fermented mycelia and the fruiting body. Chen (1986) found that alanine, glycine and threonine (sweet), and aspartic and glutamic acids (MSG-like) were taste-active amino acids in common mushrooms.
The fruiting body of C. militaris was rich in unsaturated fatty acids.The fruiting body of C. militaris is a better source of essential fatty acids, such as linoleic acid (C18:2).
There were differences in adenosine and cordycepin contents between the fruiting body and the corpus of C. militaris. This cordycepin concentration in the fruiting body was relatively high compared with previous reports of 0.46% (Yun et al., 2003).
It was believed that the fruiting body and the corpus of C. militaris had different functions, due to the former growing above ground and the latter existing underground (Hong et al., 2007). This study has clarified the differences with regard to amino and fatty acid profiles, and the cordycepin and adenosine concentrations of C. militaris.
References
- 1.Bok JW, Lermer L, Chilton J, Klingeman HG, Towers GH. Antitumor sterols from the mycelia of Cordyceps sinensis. Phytochemistry. 1999;51:891–898. doi: 10.1016/s0031-9422(99)00128-4. [DOI] [PubMed] [Google Scholar]
- 2.Chang HL, Chao GR, Chen CC, Mau JL. Non-volatile taste components of Agaricus blazei, Antrodia camphorata and Cordyceps militaris mtcelia. Food Chem. 2001;74:203–207. [Google Scholar]
- 3.Chen HK. Studies on the characteristics of taste-active components in mushroom concentrate and its powderization. Taichung, Taiwan: National Chung-Hsing University; 1986. Master's Thesis. [Google Scholar]
- 4.Chiou WF, Chang PC, Chou CJ, Chen CF. Protein constituent contributes to the hypotensive and vasorelaxant activities of Cordyceps sinensis. Life Sci. 2000;66:1369–1376. doi: 10.1016/s0024-3205(00)00445-8. [DOI] [PubMed] [Google Scholar]
- 5.Furuya T, Hirotani M, Matsuzawa M. N6-(2-hydro-xyethyl) adenosine, a biologically active compound from cultured mycelia of Cordyceps and Isaria species. Phytochemistry. 1983;22:2509–2512. [Google Scholar]
- 6.Hong IP, Nam SH, Sung GB, Chung IM, Hur H, Lee MW, Kim MK, Guo SH. Chemical components of Paecilomyces tenupes (Peck) Samson. Mycobiology. 2007;35:215–218. doi: 10.4489/MYCO.2007.35.4.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Huang BM, Stocco DM, Norman RL. The cellular mechanism of corticotropin-releasing hormone (CRH) stimulated steroidogenesis in mouse Leydig cells are similar to those for LH. J Androl. 1997;18:528–534. [PubMed] [Google Scholar]
- 8.Kim KM, Kwon YG, Chung HT, Yun YG, Pae HO, Han JA, Ha KS, Kim TW, Kim YM. Methanol extract of Cordyceps pruinosa inhibits in vitro and in vivo inflammatory mediators by suppressing NF-κB activation. Toxicol Appl Pharm. 2003;190:1–8. doi: 10.1016/s0041-008x(03)00152-2. [DOI] [PubMed] [Google Scholar]
- 9.Kobayashi Y. Key to the taxa of the genera Cordyceps and Torrubiella. Trans Mycol Soc Jpn. 1982;23:29–364. [Google Scholar]
- 10.Kuo YC, Lin CY, Tsai WJ, Wu CL, Chen CF, Shiao MS. Growth inhibitors against tumor cells in Cordyceps sinensis other than cordycepin and polysaccharides. Cancer Invest. 1994;12:611–615. doi: 10.3109/07357909409023046. [DOI] [PubMed] [Google Scholar]
- 11.Kuo YC, Tsai WJ, Shiao MS, Chen CF, Lin CY. Cordyceps sinensis as an immunomodulatory agent. Am J Chin Med. 1996;24:111–125. doi: 10.1142/S0192415X96000165. [DOI] [PubMed] [Google Scholar]
- 12.Li QS, Zeng W, Yi DH, Huang TF. Studies on the alternation of generations in Cordyceps sinensis. Chung Kuo Yao Tsa Chil. 1998;23:210–212. [PubMed] [Google Scholar]
- 13.Manabe N, Sugimoto M, Azuma Y, Taketomo N, Yamashita A, Tsuboi H, Tsunoo A, Kinjo N, Nian-Lai H, Miyamoto H. Effect of the mycelial extract of cultured Cordyceps sinensis on in vivo hepatic energy metabolism in the mouse. Jpn J Pharmacol. 1996;70:23–29. doi: 10.1254/jjp.70.85. [DOI] [PubMed] [Google Scholar]
- 14.Spatafora JW, Blackwell M. Molecular systematics of unitunicate perithecia ascomycetes; the Clavicipitales-Hypocreales connection. Mycologia. 1993;85:912–922. [Google Scholar]
- 15.Wang SM, Lee LJ, Lin WW, Chang CM. Effect of a water-soluble extract of Cordyceps sinensis on steroidogenesis and capsular morphology of lipid droplet in cultured rat adrenocortical cell. J Cell Biochem. 1998;69:483–489. doi: 10.1002/(sici)1097-4644(19980615)69:4<483::aid-jcb9>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 16.Wu CS, Leu SF, Yang HY, Huang BM. Melatonin inhibits the expression of steroidogenic acute regulatory protein and steroidogenesis in MA-10 cells. J Androl. 2001;22:245–254. [PubMed] [Google Scholar]
- 17.Yun YH, Han SH, Lee SJ, Ko SK, Lee CK, Ha NJ, Kim KJ. Anti-diabetic effects of CCCA, CMESS and cordycepin from C. militaris and the immune responses in streptozotocin-induced diabetic mice. Nat Prod Sci. 2003;9:291–298. [Google Scholar]


