Abstract
The effect of aflatoxin-contaminated corn on albino mice was investigated using the sperm morphology assay. Blood parameter levels including; total white blood cells (WBC), total red blood cells (RBC), packed cell volume (PCV), serum bilirubin (SB) and fasting blood sugar (FBS) were also determined in the tested mice. Test mice were exposed to aflatoxin-contaminated corn (contamination level of 100 ppb) for 1~4 weeks while aflatoxin-free corn and cyclophosphamide were used as negative and positive controls, respectively. Sperm cells showed varieties of morphological abnormality when assessed after 5 weeks. The percentage frequencies of the negative and positive controls were 18.8% and 48.87%, respectively, while the percentage abnormalities for the 1, 2, 3 and 4 weeks exposures were 41.38%, 48.17%, 57.13% and 61.67%, respectively. PCV, WBC, total bilirubin and glucose level values of mice in all concentrations were higher and statistically significant as compared to the negative control values using Dunnett's test. Therefore, abnormal sperm cell induction is concentration-dependent such that continuous consumption of aflatoxin-contaminated corn is capable of negatively affecting spermatogenesis by inducing or increasing the frequency of morphologically abnormal sperm cells produced.
Keywords: Aflatoxin, Blood, Corn, Mice, Sperm
Aflatoxins are naturally occurring mycotoxins produced by many species of Aspergillus, most notably, A. flavus and A. parasiticus. They are toxic and carcinogenic and are produced most commonly in moist grains (corn, barley, wheat, rice, etc) and nut products (Fink-Grennels, 1999). They are also found in peanuts and cottonseed (Pitt, 2000). Corn is a staple food in most countries and probably the commodity of greatest worldwide concern because it is grown in climates that are likely to have perennial contamination with aflatoxins.
Studies have shown that aflatoxins, especially Aflatoxin B is a potential carcinogen on many animal species like rats and rainbow trouts (Smela et al., 2001). After ingestion, Aflatoxin is metabolized by cytochrome p450 group of enzymes in the liver, where it is converts to many metabolic products like aflatoxicol, Aflatoxin Q1, P1 and M1 depending on the genetic predisposition of the species. Along with these there is another metabolite called Aflatoxin 8, 9 epoxide, which is equally formed. The amount of this metabolite decides the species susceptibility, as this can induce mutations by intercalating into DNA and forming adducts with guanine moiety in the DNA (Bondy and Pestka, 2000). Moreover, species susceptibility to Aflatoxin mainly depends on its liver detoxification systems, genetic make-up, age and other nutritional factors (Fink-Grennels, 1999).
It has also been shown that no animal species is resistant to the acute toxic effects of aflatoxins, which the International Agency for Research in Cancer has grouped as Category 1 carcinogen. A wide variation in Lethal Dose 50 (LD50) value has been obtained in animal species tested with single doses of aflatoxins (Maia et al., 2002). Evidence of acute aflatoxicosis in humans has been reported from many parts of the world (Gong et al., 2002). Due to the capacity of aflatoxins to cross the placental barrier, it can also cause genetic defects at fetal stages (Maxwell et al., 1998).
Most of the works done on aflatoxins have been on somatic cells, not much work has been reported on the effects of Aflatoxin on germ cells according to the literature available to us. This study therefore was aimed at investigating the effect of Aflatoxin-contaminated corn on the sperm morphology of albino mice. Blood reactions were also analyzed in the tested mice.
Materials and Methods
Test animals
Nine week old Winster strain albino mice were obtained from Nigeria Institute of Medical Research (NIMR) Yaba, Lagos, Nigeria and acclimatized in a pathogen free, well-ventilated room in the animal house of Babcock University, Nigeria for two weeks. Supply of food and water was constant (a libido) to avoid the problem caused by malnutrition in mice spermatogenesis. The weight of the mice were taken weekly throughout the study period.
Test grains
Aflatoxin-contaminated corn was obtained from Animal Care services Konsult, Iperu, Ogun State, Nigeria. The corn had a contamination level of 100 ppb when tested using the ELISA AgraQuant total Aflatoxin assay 4/40 kit (Neogen, USA).
Assay of sperm abnormalities
Sperm-head abnormalities test was carried out according to the method of Wyrobek et al. (1983). Seven mice per group were used for the assay. The mice were fed with the aflatoxin contaminated corn after being acclimatized (fed) with aflatoxin-free corn for two weeks. One to four week exposure period was utilized in this study. Group A was exposed (fed) to aflatoxin-contaminated corn for 1 week, while Group B, C and D were fed for 2, 3 and 4 weeks, respectively. The positive control group was injected intraperitoneally with 0.5 ml cyclophosphamide (a known mutagen) alongside liberal feeding with uncontaminated corn and water. At the termination of the injection of positive control mice and mice exposed to contaminated corn (depending on exposure duration) liberal continuation of feeding with uncontaminated corn and water followed. The negative control was fed with uncontaminated corn and water all through the experimental period. Five weeks post-treatment period was considered since spermatogenesis in mice takes about 35 days to complete (Bartke et al., 1974). At 5 weeks from the first day of exposure, five mice from each group and the controls were sacrificed by cervical dislocation and surgically, their candal epididymes were removed as previously described by Wyrobek et al. (1983). For each mouse, a total of 600 sperm cells were assessed for sperm morphological abnormalities and 4 mice were analyzed per exposure group. Before sacrifice, mice were also observed for physical activity, appetite, and change in body weight with weight measurements taken at 4-day intervals (Fapohunda et al., 2007).
Blood test
Various samples of blood were obtained using sterile syringes and needles. The blood was withdrawn with minimum stasis from a suitable vein in the forelimb. The needle of the syringe was removed and the blood was slowly ejected into a sample tube. The blood was then analyzed for the following parameters: total white blood cells (WBC), total red blood cells (RBC), packed cell volume (PCV), estimation of total serum bilirubin (SB) and fasting blood sugar (FBS) according to the method of Baker et al. (2001).
Statistical analysis
The differences between the negative and positive controls and the individual dose group were analyzed by means of ANOVA test of significance at P < 0.05 level. The assay result was considered positive when the frequency of abnormal sperm-heads was at least twice the negative control, when statistically significant increases were seen at least at two consecutive dose levels and when there was evidence of a dose-related increase in abnormalities. Dunnett's t-test and student's t-test were also employed.
Results
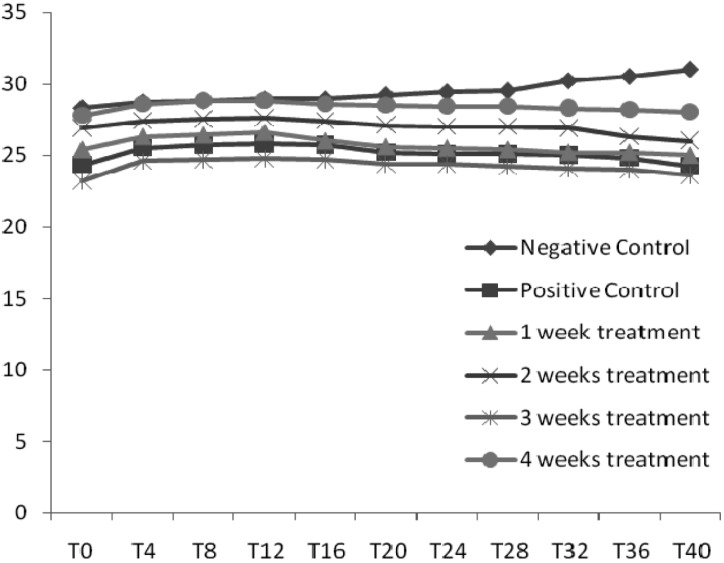
Analysis of the weight of the mice in all the groups showed that the weight of mice for the negative control treatment increased throughout the duration of the study while all other treatment groups started decreasing after acclimatization i.e. when the contaminated corn was introduced (Fig. 1). It was observed that most of the mice had swollen limbs after being fed with contaminated corn with the exception of the negative and positive controls (data not shown).
Fig. 1.
Weight changes (grams) recorded every 4 days (T) in mice during the experiment.
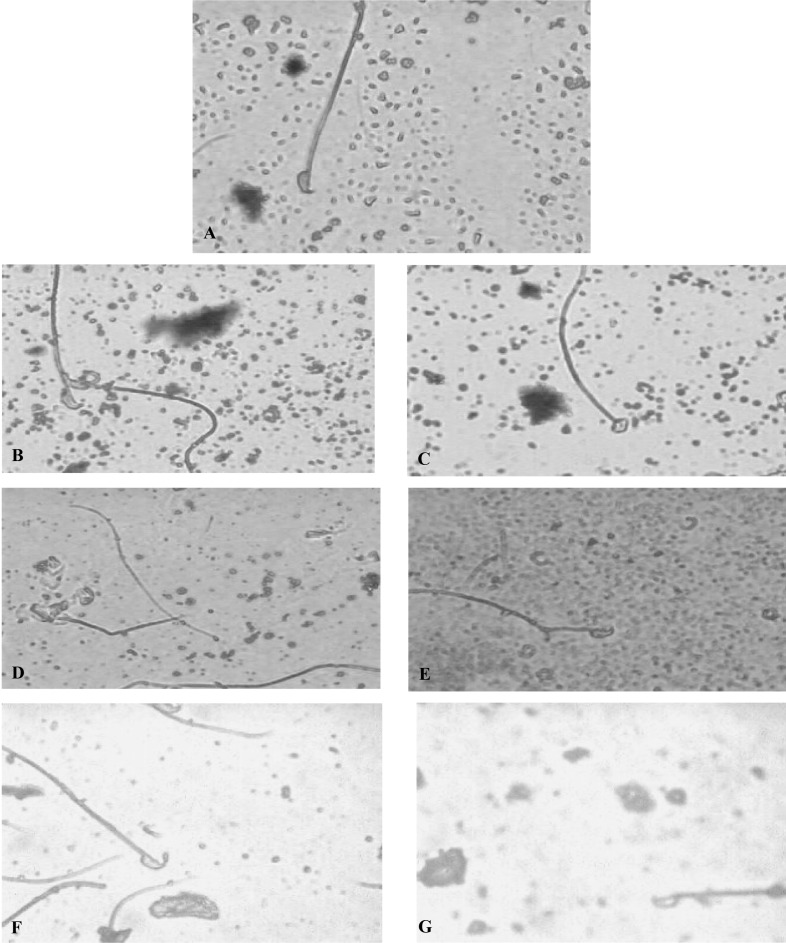
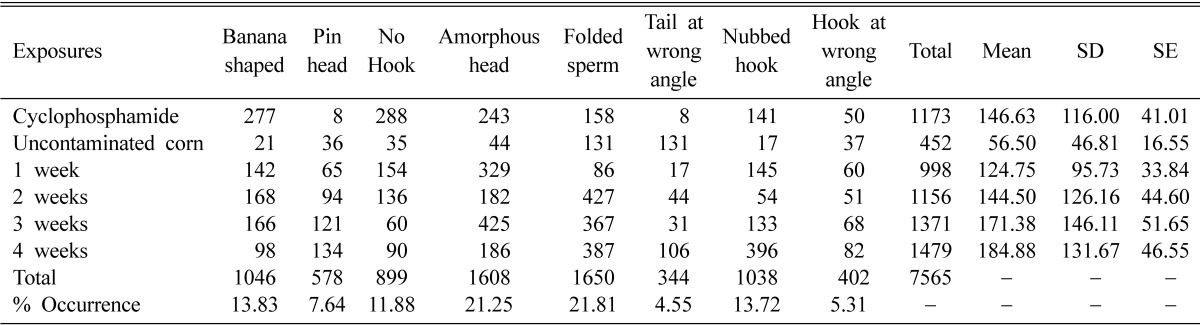
Sperm-head abnormalities were analyzed at 5 weeks after first day exposure to the aflatoxin-contaminated corn. Sperm cells observed at this time were presumably exposed to the aflatoxin-contaminated corn while they were early primary spermatocytes and spermatogonia. Table 1 shows the summary of the frequency of occurrence, standard deviation, mean and standard error of the different sperm abnormalities induced by varying concentrations of aflatoxin-contaminated corn. The negative control showed a percentage frequency of 18.8% while the positive control showed a statistically significant increase of 48.8%. The test concentrations showed different types of abnormal sperm heads (Fig. 2a~g). The percentage abnormalities for the different exposures of weeks 1, 2, 3 and 4 were 41.38%, 48.17%, 57.13% and 61.67%, respectively. This induction of abnormal sperm cells was concentration-dependant.
Table 1.
Frequency of occurrence, standard deviation, mean and standard error of the different sperm abnormalities induced by varying concentrations of aflatoxin-contaminated corn
SD, Standard Deviation; SE, Standard Error.
Fig. 2.
Photomicrograph of mice sperm cells. A, normal sperm cell; B, folded; C, amorphous head; D, pin head; E, hook at wrong angle; F, knobbed hook; G, no hook.
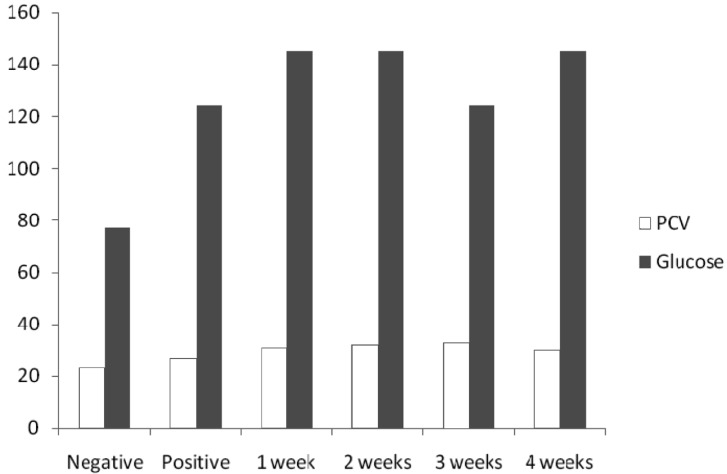
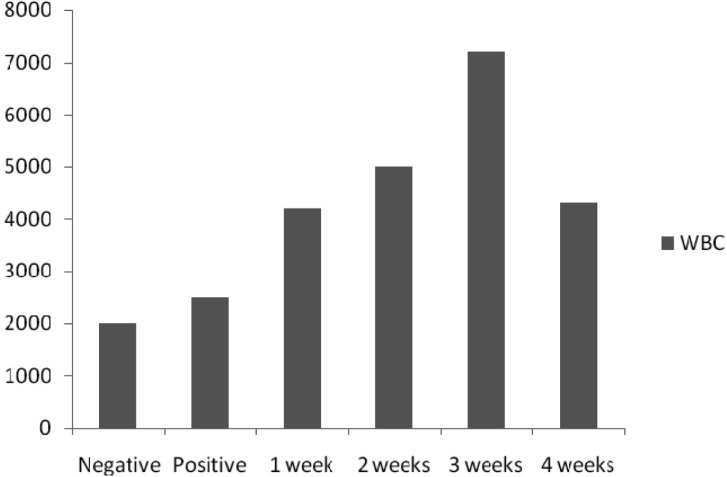
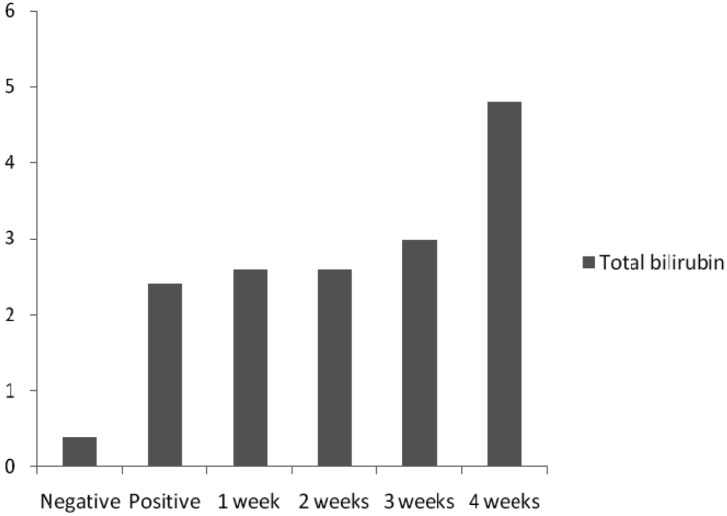
PCV and glucose (Fig. 3), WC (Fig. 4) and SB (Fig. 5) level values in mice of all concentrations were high and statistically significant as compared with the negative control values using Dunnett's test.
Fig. 3.
PCV (%) and blood glucose levels (mg/dl) in mice.
Fig. 4.
WBC levels (%) in mice.
Fig. 5.
Total SB levels (mg/dl) in mice.
Discussion
This study showed that the aflatoxin-contaminated corn induced abnormal sperm morphology and the induction was dose-dependent. Aflatoxins often occur in crops in the field prior to harvest. Post harvest contamination can also occur if crop drying is delayed and during storage of crop if water is allowed to exceed critical values for the mold growth (Pitt, 2000). The observed abnormalities in sperm morphology indicate that aflatoxin in the corn had an effect on sperm. This might have caused damage to the pre-meiotic stages of spermatogenesis since during spermatogenesis DNA synthesis occurs before the pre-meiotic stages and no further DNA synthesis occurs throughout spermatogenesis in the cell cycle (Bakare et al., 2005). It may also be as a result of chromosomal aberrations that occurred during the packaging of the genetic material in the sperm head or due to the occurrence of point mutation during spermatogenesis.
The observations reported here are in accordance with previous reports on aflatoxin carcinogenicity on many animal species like rats and rainbow trout (Smela et al., 2001). A wide variation in LD50 values have also been obtained in animal species tested with single doses of aflatoxins (Maia et al., 2002).
The PCV of mice in all treatments except negative control was high, presumably because of the intake of aflatoxin. PCV is a measure of the relative mass of erythrocytes present in a sample of whole blood. The blood glucose of mice was high in all treatment concentration except the negative control. When the blood glucose rises above the set point, more insulin is secreted from the pancreas but there cannot be a breakdown in this system like in any other system, the pancreas either cannot secrete insulin if the target cells lost their responsiveness to insulin, then blood glucose concentration reaches dangerously high levels (Kent, 2000). The serum bilirubin level was higher in all concentrations as compared to the negative control. This probably implies the adverse effects of aflatoxins on the liver. Bilirubin is formed when erythrocyte at the end of their lifespan (normally 120 days) are removed from circulation by the reticule-endothelial system (RES) and the protoporphyrin ring of haem group of haemoglobin opens up. An excessive breakdown of erythrocyte leads to increase in unconjugated bilirubin and it is present in plasma. An increase in both conjugated and unconjugated bilirubin is as a diminished function of the liver as in hepatocellular or toxic jaundice (Baker et al., 2001). The white blood cell count shows a statistically significant increase in all concentrations than the negative control. WBC are responsible for both specific and non-specific immunity. WBC increase when there is an infection, they cooperate with each other first to recognize the pathogen as an invader and then to destroy it. There is an increase in WBC during infections and allergic responses of the host (Willey et al., 2008). Aflatoxin therefore might have enhanced the invasion of pathogens in the tested animals thereby giving way to opportunistic infections and hence the subsequent increase in WBC level.
This is one of the few reports on the assessment of the effect of aflatoxin-contaminated corn on mice sperm morphology and blood parameters. This study may be significant in Nigeria where corn consumption is very high and the storage facilities are grossly inadequate. In conclusion, aflatoxin is capable of inducing genetic effects in mice and affects some blood parameter levels. This is relevant to human health because the toxicological target is DNA which exists in all cellular forms (Houk, 1992).
References
- 1.Bakare AA, Mosuro AA, Osibanjo O. An in-vivo evaluation of abnormal sperm morphology in mice by landfill leachates. Mutat Res. 2005;582:28–34. doi: 10.1016/j.mrgentox.2004.12.007. [DOI] [PubMed] [Google Scholar]
- 2.Baker FJ, Silverton RE, Pallister CJ. Introduction to Medical Laboratory Technology. 9th edition. Nigeria: Bunnty press; 2001. pp. 354–360. [Google Scholar]
- 3.Bartke A, Weir JA, Mathison P, Roberson C, Dalterio S. Testicular function in mouse strain with different age of sexual maturation. J Hered. 1974;65:204–208. doi: 10.1093/oxfordjournals.jhered.a108504. [DOI] [PubMed] [Google Scholar]
- 4.Bondy GS, Pestka JJ. Immunomodulation by fungal toxins. J Toxicol Environ Health B Crit Rev. 2000;3:109–143. doi: 10.1080/109374000281113. [DOI] [PubMed] [Google Scholar]
- 5.Fapohunda SO, Awoyinka AO, Olajuyigbe OO, Ezekiel CN, Esiaba I. Enzyme-related aflatoxin production in vital organs of rats fed with Aspergillus species-inoculated rat chow. J Biol Environ Sci. 2007;1:1–3. [Google Scholar]
- 6.Fink-Gremmels J. Mycotoxins: their implications for human and animal health. Vet Q. 1999;21:115–120. doi: 10.1080/01652176.1999.9695005. [DOI] [PubMed] [Google Scholar]
- 7.Gong YY, Cardwell K, Hounsa A, Egal S, Turner PC, Hall AJ, Wild CP. Dietary Aflatoxin exposure and impaired growth in young children from Benin and Togo: cross sectional study. Brit Med J. 2002;325:20–21. doi: 10.1136/bmj.325.7354.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Houk VS. The genotoxicity of industrial wastes and effluents: a review. Mutat Res. 1992;277:91–138. doi: 10.1016/0165-1110(92)90001-p. [DOI] [PubMed] [Google Scholar]
- 9.Kent M. Advance Microbiology. 5th edition. London: Oxford University press; 2000. p. 138. [Google Scholar]
- 10.Maia PP, Pereira B, Siqueira M. Occurrence of aflatoxins B1, B2, G2 in some Brazilian pet foods. Food Addit Contam. 2002;19:1180–1183. doi: 10.1080/0265203021000011214. [DOI] [PubMed] [Google Scholar]
- 11.Maxwell SM, Apeagyei F, de Vries HR, Mwanmut DD, Hendriekse RG. Aflatoxins in breast milk, neonatal cord blood and sera of pregnant women. J Toxicol. 1998;8:19–29. [Google Scholar]
- 12.Pitt JL. Toxigenic fungi and mycotoxins. Brit Med Bull. 2000;56:184–192. doi: 10.1258/0007142001902888. [DOI] [PubMed] [Google Scholar]
- 13.Smela ME, Sophie S, Curier E, Bailey A, John ME. The chemistry and biology of aflatoxin B1. Carcinogenesis. 2001;22:535–545. doi: 10.1093/carcin/22.4.535. [DOI] [PubMed] [Google Scholar]
- 14.Willey JM, Sherwood LM, Woolverton CJ. Microbiology. 7th edition. NY: McGrawHill; 2008. pp. 746–747. [Google Scholar]
- 15.Wyrobek AJ, Gordon LA, Burkhart JG, Francis MW, Kapp RW, Jr, Letz G, Malling HG, Topham JC, Whorton MD. An evaluation of the mouse sperm morphology test and other sperm tests in non-human mammals. A report of the United States Environmental Protection Agency Gene-Tox Programme. Mutat Res. 1983;115:1–72. doi: 10.1016/0165-1110(83)90014-3. [DOI] [PubMed] [Google Scholar]