Abstract
Twenty isolates of Bacillus species obtained from livestock manure composts and cotton-waste composts were tested for their antagonistic effects in vitro against three green mold pathogens of mushrooms (Trichoderma harzianum, T. koningii, and T. viridescens). However, there exists a possibility Bacillus species may have antagonistic effects against mushrooms themselves, and thus the same 20 isolates were tested in vitro against three species of mushrooms (Flammulina velutipes, Lentinus edodes, and Pleurotus ostreatus). Of the 20 Bacillus species isolates tested, two inhibited mycelial growth of T. harzianum, seven that of T. koningii, and eight that of T. viridescens. Importantly, the bacterial isolates M27 and RM29 strongly inhibited mycelial growth of all the Trichoderma spp. isolates tested. The isolate M27 was subsequently identified as the most effective in inhibiting mycelial growth of all the Trichoderma species. Interesting results of the effect Bacillus isolates had upon the mushroom species followed. It was found that most Bacillus isolates except 5T33 at least somewhat inhibited mycelial growth of the three mushroom species or some of the mushrooms. Furhermore, the antagonistic effects of the bacterial isolates against the three species of mushrooms varied depending on the mushroom species, suggesting a role for mushroom type in the mechanism of inhibition. The bacterial isolates M27 and RM29 were identified as having the most antagonistic activity, inhibiting mycelial growth of all the Trichoderma spp. as well as mycelial growth of the three species of mushrooms. These results suggest that the bacterial isolates and their antagonistic effects on green mold pathogens should be further studied for their practical use for biological control of green mold in the growing room of the mushrooms.
Keywords: Antagonistic effect, Bacillus species, Inhibition zone, Mushrooms, Mycelial growth, Trichoderma spp.
Edible mushrooms are a widely cultivated food source throughout the world and are integral in the cuisines of numerous cultures. The genera of commonly cultivated mushrooms for edibility include Agaricus, Auricularia, Flammulina, Lentinus, Pleurotus, and Volvariella (Chang et al., 1993; Chang and Miles, 1989). In Korea, oyster mushroom [Pleurotus ostreatus (Jacq.:Fr.) Kummer], winter mushroom [Flammulina velutipes (Curt.:Fr.) Sing.], oak mushroom [Lentinus edodes (Berk.) Sing.], and button mushroom [Agaricus bisporus (Lange) Imbach] are the most widely cultivated mushroom species. Mushrooms comprise the spore-forming, fruiting body of a fungus and therefore are commonly found on compost in wooden trays, in bags, in plastic bottles, on shelves, or on beds in growing rooms at low temperatures with high humidity (Hall et al., 2003). However, an emerging problem particularly relevant to agriculture is the development of green mold that frequently occurs on composts or the beds of mushroom populations. It has been reported that several Trichoderma spp. are responsible for the green mold therefore can potentially cause substantial economic losses in mushroom production worldwide (Jaklitsch et al., 2006; Samuels et al., 2002; Seaby, 1998).
Both mushrooms and the green mold made of Trichoderma spp. that grows on them belong to the Kingdom Fungi and thus it is reasonable to assume their growth conditions may be similar. This implies preventing the growth of green mold while simultaneously promoting, or at least maintaining, mushroom growth could be difficult. For example, the growth of mushroom green mold is very difficult to control using chemicals because growth of the mushrooms themselves is affected by chemicals as well. This is even excluding that food safety considerations for cultivated mushrooms demand alternatives to chemical control of diseases, including that of green mold.
Biological control using antagonistic bacteria has been applied as one of alternative methods to chemical control of plant diseases (Baker and Cook, 1974; Cook and Baker, 1983; Hornby, 1990; Gnanamanickam, 2002; Kheten, 2001; Parker et al., 1985). Although Kim et al. (2008) recently tested prospective antagonistic bacteria against four soilborne phytopathogenic fungi, there have been no reports on using microorganisms for the biological control of mushroom green mold. It is considered that effective biological control of green mold relies both on the selection of antagonists that act specifically against the pathogens which cause the green mold, and that these same antagonists have no inhibition on the mycelial growth of mushrooms. This condition considered, this study was thus conducted to select prospective antagonists for the biological control of green mold of mushrooms caused by Trichoderma spp.
Materials and Methods
Isolates of Bacillus species
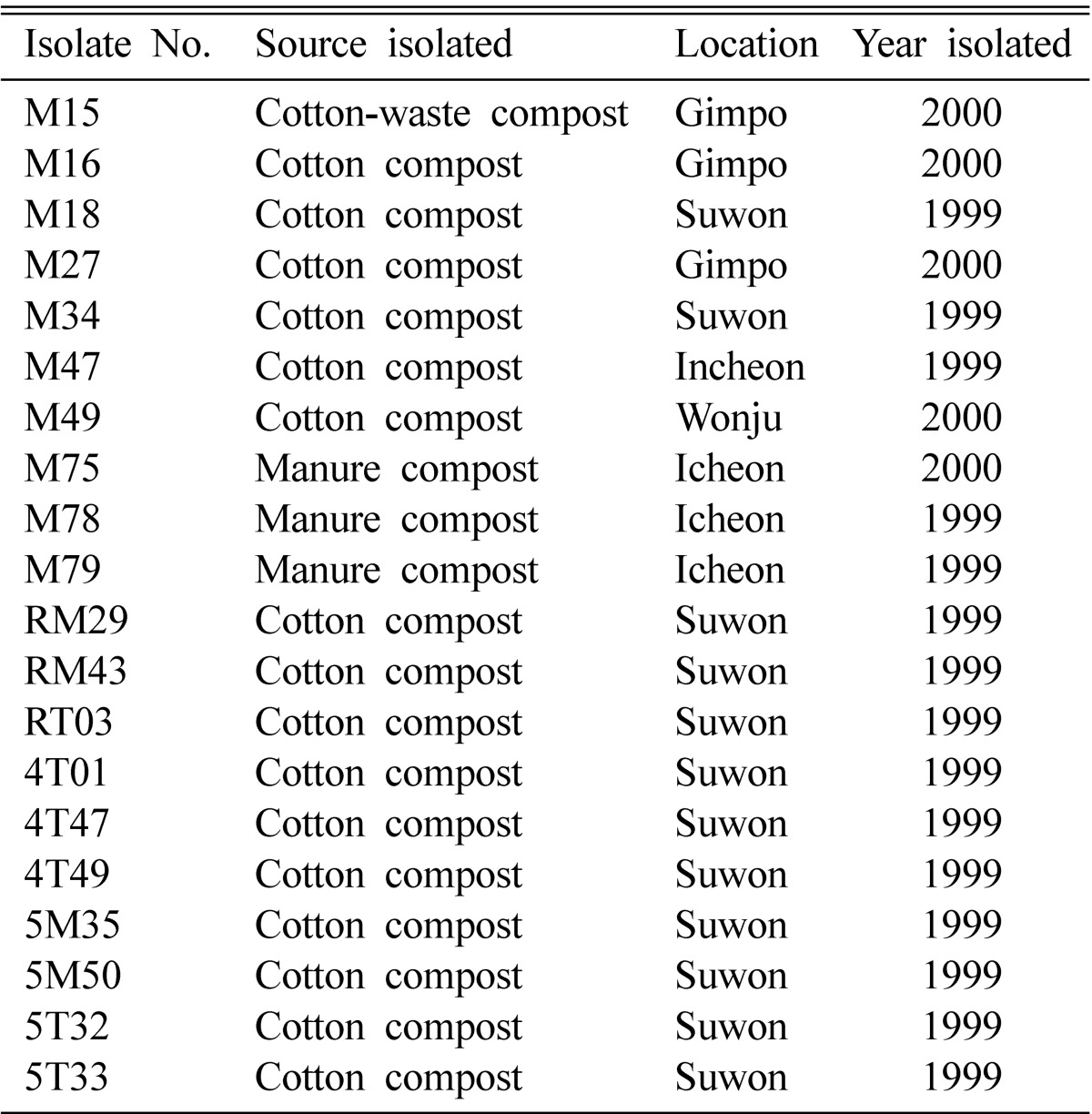
Twenty isolates of Bacillus species obtained from livestock manure composts and cotton-waste composts (Table 1) were used for antagonistic tests in vitro against three species of Trichoderma, which cause green mold of mushrooms. The bacterial isolates used in this study were identified in the previous report (Kim et al., 2008).
Table 1.
List of Bacillus species isolates from livestock manure composts and cotton-waste composts used in this study
Isolates of Trichoderma species and mushrooms
Isolates of Trichoderma harzianum Rifai, T. koningii Oudem. and T. viridescens (A.S. Horne & H.S. Williamson) Jaklitsch & Samuels were obtained from samples of green mold found on the mycelia and beds of oyster mushrooms and button mushrooms. After these isolates were confirmed as causing green mold on three species of mushrooms (F. velutipes, L. edodes, and P. ostreatus), subsequent antagonistic tests were performed with the selected Bacillus species isolates. Additional isolates of F. velutipes, L. edodes, and P. ostreatus obtained from the National Agrobiodiversity Center, National Academy of Agricultural Science were also used for antagonistic tests with the bacterial isolates.
Test of antagonistic effects
Twenty isolates of the Bacillus species were tested in vitro for antagonistic effects against the three species of Trichoderma previously confirmed to cause green mold on mushrooms. A 5 mm-mycelial mat taken from the isolate of each Trichoderma species was placed on one side of potato dextrose agar (PDA). All 20 bacterial isolates were streaked on the other side of the medium, each individually paired to the three Trichoderma species. The paired PDA plate was cultured at 25℃ for 6 to 12 days and the dual culture test was performed in triplicate. During cultivation, the plates were examined for any possible antagonistic effect between the bacterial isolates and the Trichoderma isolates by measuring the inhibition zone formed between them to the nearest width. The bacterial isolates were also tested with the same method in vitro for possible antagonistic effects against the three species of mushrooms.
Results and Discussion
Formation of inhibition zones
Of the twenty Bacillus species isolates analyzed in the dual culture experiments, several isolates demonstrated antagonistic effects against the mycelial growth of Trichoderma species and mushrooms. The width of the inhibition zone produced in dual culture tests shows the degree of antagonism bacterial isolates have against target organisms, and has been used extensively as an in vitro test for preliminary screening of biological control agents (Desai et al., 2002). Therefore, the width of the inhibition zones between the Bacillus isolates and the Trichoderma isolates was a reliable measure and was used to rate the antagonistic effects of the Bacillus species (Fig. 1). The mode of antagonism generally observed with Bacillus spp. is antibiosis (Edwards et al., 1994). This is supported by reports that most Bacillus spp. produce many antibiotics such as bacillomycin, fengycin, mycosubtilin and zwittermicin, which are all effective at suppressing growth of target pathogens in vitro and/or in situ (Pal and Gardener, 2006). This evidence allows the assumption that antibiotics are related to the formation of inhibition zones between the bacterial and the fungal isolates shown in this study.
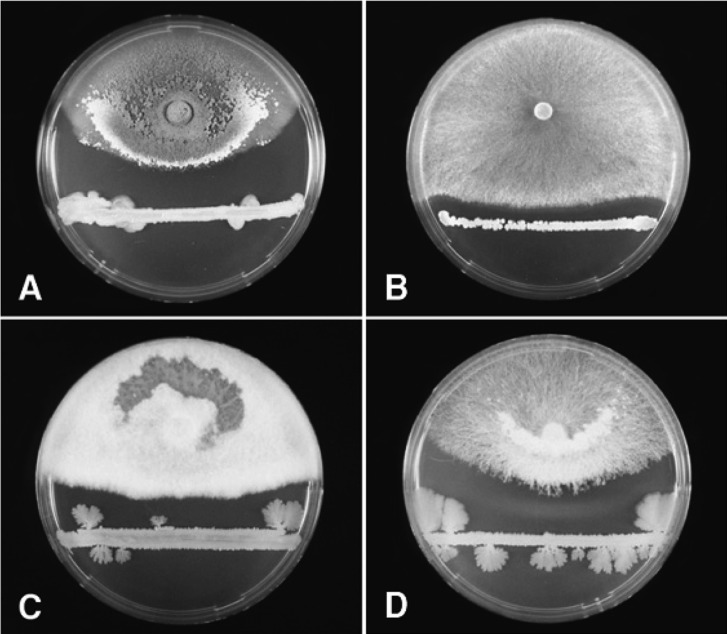
Fig. 1.
Dual culture tests for the antagonistic evaluation of Bacillus species isolates against mycelial growth of Trichoderma spp. (A and B) and mushrooms (C and D) on PDA medium. A, an inhibition zone of mycelial growth of T. harzianum by Bacillus sp. M27; B, an inhibition zone of mycelial growth of T. viridescens by Bacillus sp. RM29; C, an inhibition zone of mycelial growth of Flammulina velutipes by Bacillus sp. M27; D, an inhibition zone of mycelial growth of Lentinus edodes by Bacillus sp. M27.
Antagonistic effects
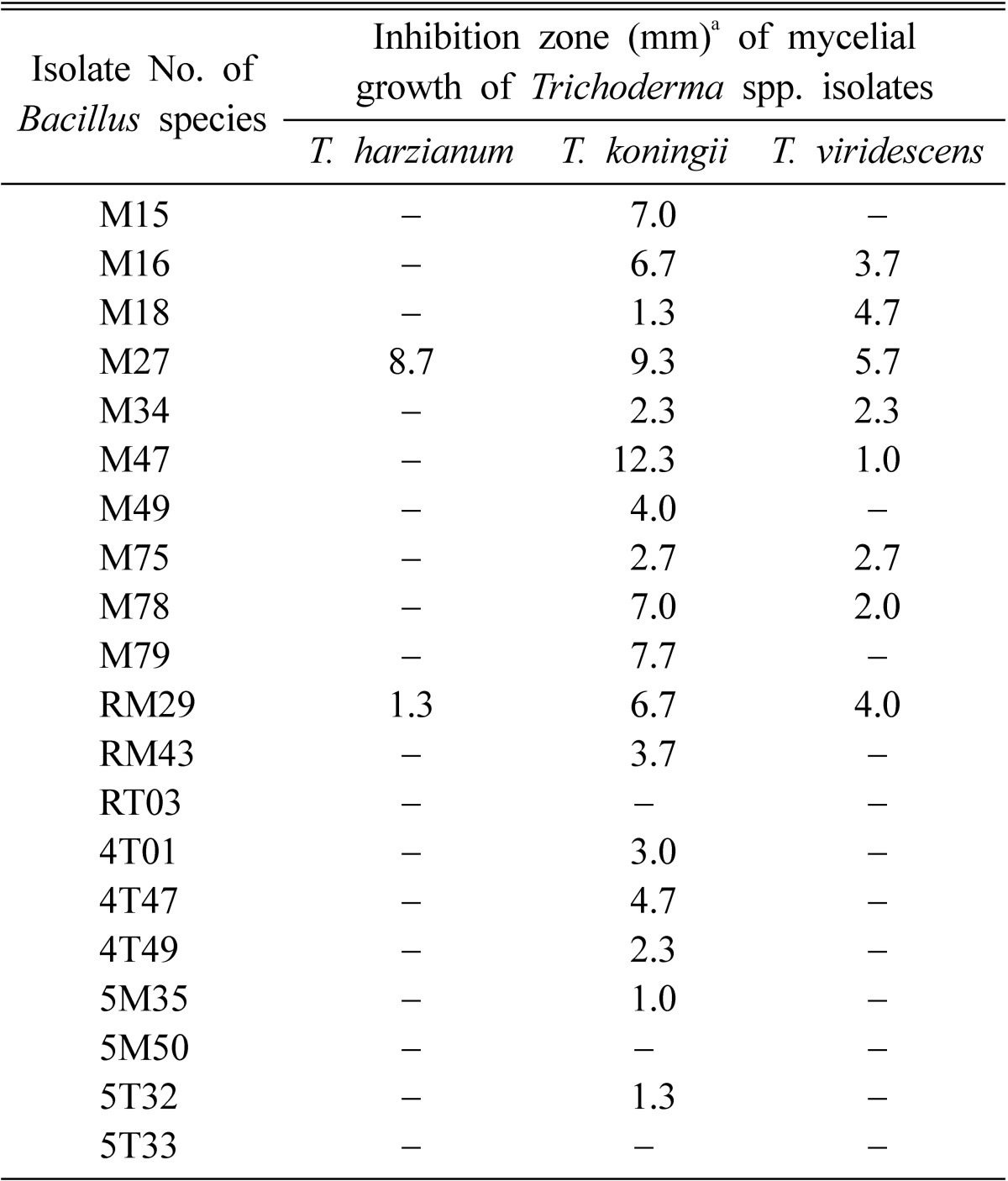
Of the 20 Bacillus species isolates tested, two inhibited mycelial growth of T. harzianum, seven that of T. koningii, and eight that of T. viridescens (Table 2). The bacterial isolates M27 and RM29 provided the most noteworthy result as both strongly inhibited mycelial growth of all Trichoderma spp. isolates tested. The isolate M27 was the most effective in the formation of inhibition zones against the Trichoderma spp. isolates.
Table 2.
Antagonistic effect of Bacillus species isolates against three species of Trichoderma
aMeasurement was made after six days of cultivation. The data represents the average of three replicates. -, no inhibition.
Bacillus species have previously been reported as effective biological control agents against plant diseases (Boland and Kuykendall, 1998; Gnanamanickam, 2002; Jacobsen et al., 2004; Tjamos et al., 1991). Supporting this claim is a study where Kim et al. (2008) identified several prospective antagonistic isolates for the biological control of diseases caused by soilborne phytopathogenic fungi by screening 20 Bacillus species isolates for antagonistic effects in vitro. It has further been reported that T. harzianum, T. koningii, T. viridescens, etc. cause green mold of mushrooms (Jaklitsch et al., 2006; Samuels et al., 2002; Seaby, 1998). Our study therefore attempted to examine the antagonistic effects of Bacillus on Trichoderma spp. and indeed two Bacillus species isolates were identified as prospective antagonists against Trichoderma spp. Accordingly, these selected antagonists could be used for control of green mold caused by the Trichoderma spp.
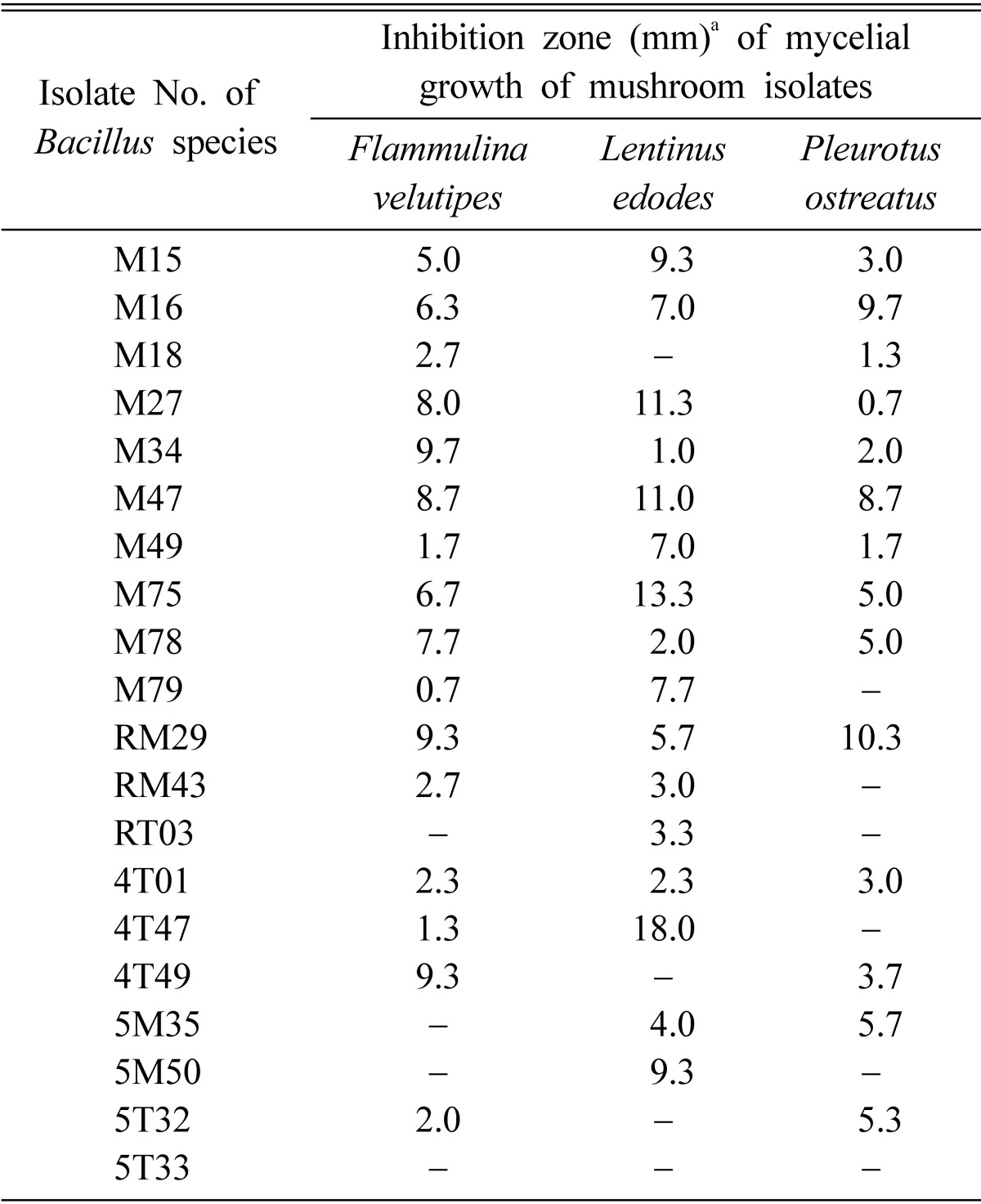
Except 5T33, most isolates of Bacillus species inhibited mycelial growth of the three experimental mushroom species, Flammulina velutipes, Lentinus edodes, and Pleurotus ostreatus (Table 3). The antagonistic effects of the bacterial isolates against the three species of mushrooms varied depending on the mushroom species. The bacterial isolates M27 and RM29, which had antagonistic effects against all the Trichoderma spp., also inhibited mycelial growth of the three species of mushrooms. Therefore, although the Bacillus isolates may be effective antagonists against green mold pathogens, Trichoderma spp., it must be recognized that the additional inhibitive effect on the mycelial growth of mushrooms is not useful as prospective antagonists for the biological control of green mold in the growing room of the mushrooms. Further study is needed in order to explore any possible practical use of the antagonistic bacterial isolates for the biological control of green mold in the growing room of the mushrooms.
Table 3.
Antagonistic effect of Bacillus species isolates against three species of mushrooms
aMeasurement was made after 12 days of cultivation. The data represents the average of three replicates. -, no inhibition.
References
- 1.Baker KF, Cook RJ. Biological Control of Plant Pathogens. St. Paul, MN, USA: Amer. Phytopathol. Soc.; 1974. [Google Scholar]
- 2.Boland GJ, Kuykendall LD. Plant-Microbe Interactions and Biological Control. New York, USA: Marcel Dekker, Inc.; 1998. [Google Scholar]
- 3.Chang ST, Buswell JA, Chiu SW. Mushroom Biology and Mushroom Products. Hong Kong: The Chinese University Press; 1993. [Google Scholar]
- 4.Chang ST, Miles PG. Edible Mushrooms and Their Cultivation. Florida, USA.: CRC Press, Inc.; 1989. [Google Scholar]
- 5.Cook RJ, Baker KF. The Nature and Practice of Biological Control of Plant Pathogens. St. Paul, MN, USA: Amer. Phytopathol. Soc.; 1983. [Google Scholar]
- 6.Desai S, Reddy MS, Kloepper JW. Comprehensive testing of biocontrol agents. In: Gnanamanickam SS, editor. Biological Control of Crop Diseases. New York·Basel, USA: Marcel Dekker, Inc.; 2002. pp. 387–420. [Google Scholar]
- 7.Edwards SG, McKay T, Seddon B. Interaction of Bacillus species with phytopathogenic fungi - Methods of analysis and manipulation for bicontrol purposes. In: Blakeman JP, Williamson B, editors. Ecology of Plant Pathogens. Wallingford, Oxon, UK: CAB International; 1994. pp. 101–118. [Google Scholar]
- 8.Gnanamanickam SS. Biological Control of Crop Diseases. New York·Basel., USA: Marcel Dekker, Inc.; 2002. [Google Scholar]
- 9.Hall IR, Stephenson SL, Buchanan PK, Yun W, Cole ALJ. Edible and Poisonous Mushrooms of the World. Portland·Cambridge: Timber Press; 2003. [Google Scholar]
- 10.Hornby D. Biological Control of Soil-borne Plant Pathogens. Wallingford, Oxon, UK: C.A.B. International; 1990. [Google Scholar]
- 11.Jacobsen BJ, Zidack NK, Larson BJ. The role of Bacillus-based biological control agents in integrated pest management systems: Plant diseases. Phytopathology. 2004;94:1272–1275. doi: 10.1094/PHYTO.2004.94.11.1272. [DOI] [PubMed] [Google Scholar]
- 12.Jaklitsch WM, Samuels GJ, Dodd SL, Lu BS, Druzhinina IS. Hypocrea rufa/Trichoderma viride: a reassessment, and description of five closely related species with and without warted conidia. Stud Mycol. 2006;56:135–177. doi: 10.3114/sim.2006.56.04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Khetan SK. Microbial Pest Control. New York·Basel, USA: Marcel Dekker, Inc.; 2001. [Google Scholar]
- 14.Kim WG, Weon HY, Lee SY. In vitro antagonistic effects of Bacilli isolates against four soilborne plant pathogenic fungi. Plant Pathol J. 2008;24:52–57. [Google Scholar]
- 15.Pal KK, Gardener BM. Biological control of plant pathogens. The Plant Health Instructor. 2006 DOI: 10.1094/PHI-A-2006-1117-02. [Google Scholar]
- 16.Parker CA, Rovira AD, Moore KJ, Wong PTW, Kollmorgen JF. Ecology and Management of Soilborne Plant Pathogens. St. Paul, Minnesota, USA: The American Phytopathological Society; 1985. [Google Scholar]
- 17.Samuels GJ, Dodd SL, Gams W, Castlebury LA, Petrini O. Trichoderma species associated with the green mold epidemic of commercially grown Agaricus bisporus. Mycologia. 2002;94:146–170. [PubMed] [Google Scholar]
- 18.Seaby D. Trichoderma as a weed mould or pathogen in mushroom cultivation. In: Harman GE, Kubicek CP, editors. Trichoderma and Gliocladium Vol. 2. Enzymes, biological control and commercial applications. London, UK: Taylor & Francis Ltd; 1998. pp. 267–287. [Google Scholar]
- 19.Tjamos EC, Papavizas GC, Cook RC. Biological Control of Plant Diseases. New York and London: Plenum Press; 1991. [Google Scholar]