Abstract
During a continuing search for antimicrobial substances from Korean native wild mushroom extracts, we found that the methanolic extract of the fruiting body of Clitocybe nebularis exhibited mild antifungal activity against pathogenic fungi. Therefore we evaluated the antifungal substances and other chemical components of the fruiting body of Clitocybe nebularis, which led to the isolation of nebularine, phenylacetic acid, purine, uridine, adenine, uracil, benzoic acid, and mannitol. Nebularine showed mild antifungal activity against Magnaphorthe grisea and Trichophyton mentagrophytes, and phenylacetic acid potently inhibited the growth of Pythium ultiumand displayed moderate antifungal activity against Magnaphorthe grisea, Botrytis cinerea, and Trichophyton mentagrophytes. The other isolated compounds showed no antimicrobial activity.
Keywords: Antifungal activity, Clitocybe nebularis, Nebularine, Phenylacetic acid
Mushrooms produce a large variety of secondary metabolites with unique chemical structures and interesting biological activities. Clitocybe nebularis (= Lepista nebularis), which belongs to the family Tricholomataceae and is known as the clouded agaric or cloud funnel, is a fungus that appears both in conifer-dominated forests and those that primarily consist of deciduous trees. The chemical constituents of C. nebularis were first investigated by Ehrenberg, who identified its characteristic metabolites (Ehrenberg et al., 1946). Subsequently, nebularine, 9-β-Dribofuranosyl-9H-purine, was isolated from C. nebularis as an antibiotic against various mycobacteria including Mycobacterium phlei, M. avium, M. tuberculosis, and Brucella abortus (Löfgren and Lüning, 1953; Ehrenberg et al., 1946; Gordon and Brown, 1956). In addition, clitocypin was isolated from C. nebularis as a specific cysteine protease inhibitor. Clitocypin was the first cysteine protease inhibitor isolated from fungi and has since been found to be involved in defense reactions against the invasion of pathogens and mycoviruses (Brizin et al., 2000).
During the course of screening for antimicrobial agents from Korean native wild mushroom extracts, we found that the methanolic extract of the fruiting body of C. nebularis exhibited moderate antifungal activity against several pathogenic fungi. In this study, we describe the isolation and determination of the structure of the antifungal substances, nebularine (compound 1) and phenylacetic acid (compound 6), as well as the major chemical constituents, purine (compound 2), uracil (compound 3), adenine (compound 4), uridine (compound 5), benzoic acid (compound 7), and mannitol (compound 8), from the fruiting bodies of Clitocybe nebularis.
Materials and Methods
Chemicals
All chemicals were purchased from Sigma-Aldrich Korea (Yongin, Korea). All solvents used for extraction and separation were of analytical grade, whereas HPLC solvents were of HPLC grade. The extraction, separation and HPLC solvents were obtained from SK Chemicals (Ulsan, Korea). Reversed-phase TLC plates were purchased from Merck Ltd. (Seoul, Korea). Sephadex LH-20 and an ODS sep-pak cartridge were obtained from Amersham Biosciences (Uppsala, Sweden) and Allteck (USA), respectively.
Fungal materials
The fruiting bodies of C. nebularis were collected at Dukyu Mountain, North Jeolla Province, Korea in 2006, and identified according to the taxonomic key described by Hongo (Imazeki et al., 1988).
Isolation and Purification of Compounds 1~8
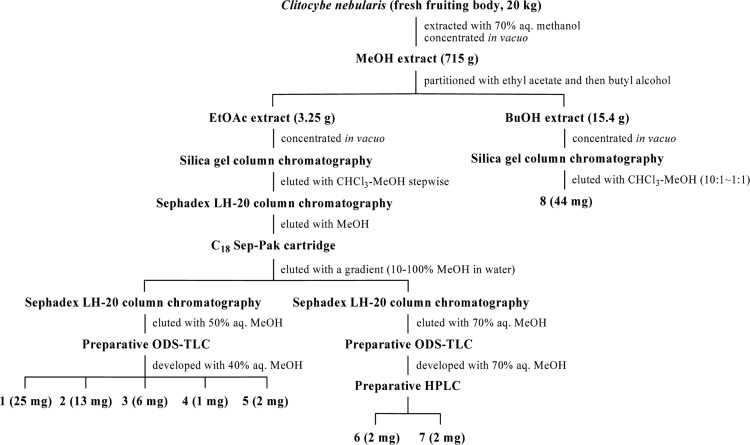
The fresh fruiting bodies (20 kg) of C. nebularis were cut into small pieces and extracted with 70% aqueous MeOH at room temperature for 2 days. Following removal of the methanol under reduced pressure, the resulting solution was partitioned between ethyl acetate and H2O and then butyl alcohol and H2O. The ethyl acetate-soluble portion was then concentrated in vacuo, after which the residue was subjected to chromatography using a silica gel column. The reside was then eluted using a gradient of increasing amounts of MeOH in CHCl3. A fraction eluted with CHCl3-MeOH (4 : 1~2 : 1, v/v) was then concentrated and subjected to Sephadex LH-20 column chromatography, after which it was eluted with MeOH. A yellow antibiotic fraction was then subjected to chromatography on an ODS sep-pak cartridge, after which it was eluted using a gradient of increasing amounts of MeOH (10~100%) in water to give two yellow fractions. One of the yellow fractions was then purified by chromatography on a Sephadex LH-20 column with 50% aqueous MeOH, followed by reversed-phase TLC with 40% aqueous MeOH to provide compound 1 (25 mg, Rf 0.62), compound 2 (13 mg, Rf 0.5), compound 3 (6 mg, Rf 0.8), compound 4 (1 mg, Rf 0.4), and compound 5 (2 mg, Rf 0.82). The other fraction was purified on a Sephadex LH-20 column and then eluted with 70% aqueous MeOH. Next, the sample was subjected to preparative reversed-phase TLC and then developed with 70% aqueous MeOH, followed by preparative HPLC (column: ODS (4.6 × 250 mm, solvent: 80% aqueous MeOH, flow rate: 1 ml/min) to afford compound 6 (2 mg) and compound 7 (2 mg). The BuOH-soluble portion was then concentrated in vacuo, after which the residue was subjected to chromatography on a column of silica gel and eluted with a gradient with increasing amounts of MeOH in CHCl3. Each fraction was incubated at room temperature for 2 days, after which compound 8 (44 mg) was obtained as a crystal (Fig. 1).
Fig. 1.
Procedures used to isolate compounds 1-8 from the fruiting bodies of Clitocybe nebularis.
Spectroscopic analysis
The EI-mass was determined using a JMS-700 JEOL mass spectrometer, and the ESI-mass was determined using a Navigator mass spectrometer in positive and negative modes. NMR spectra were obtained using a Varian UNITY Inova NMR spectrometer with 1H NMR at 400MHz and 13C NMR at 100MHz. Chemical shifts are given in ppm (δ) using TMS as an internal standard.
Antimicrobial activity
Antimicrobial activity was determined using the conventional paper disk (Advantec, 8 mm in diameter) method. Three phytopathogenic fungi, Botrytis cinerea, Magnaporthe grisea, and Pythium ultimum, one human tinea pedis fungus Trichophyton mentagrophytes, and two bacteria, Bacillus subtilis and Staphylococcus aureus, were used as test microorganisms. Paper disks containing 500 µg of each sample were placed on an agar plate that was inoculated with the test organisms. The antibiotic activity was then assessed by measuring the diameter of zone of inhibition following incubation for 24 hours at 37℃ for bacteria, and incubation for 2~7 days at 27℃ for fungi.
Results and Discussion
Structure determination and identification
The structure of compound 1 was established by 1H and 13C NMR and HMBC experiments. The 1H NMR spectrum of compound 1 in CD3OD exhibited signals that indicated the presence of three aromatic singlet protons at δ 9.10, 8.95, and 8.80, an anomeric proton at δ 6.18 (1H, d, J = 5.2 Hz), three oxygenated methine protons at δ 4.76 (1H, t, J = 5.2 Hz), 4.39 (1H, dd, J = 4.8, 3.6 Hz), and 4.18 (1H, d, J = 6.8, 3.2 Hz), and oxygenated methylene protons at δ 3.90 (1H, dd, J = 12.4, 2.8 Hz) and 3.79 (1H, d, J = 12.4, 3.6 Hz). In the 13C NMR spectrum, five aromatic carbons at δ 153.2, 152.3, 149.1, 147.2, and 135.7, four oxygenated methine carbons at δ 90.5, 87.6, 75.8, and 72.1, and a methylene carbon at δ 62.9 were evident. These spectral data suggested that compound 1 was a member of the nucleoside class. Therefore, we compared the NMR spectral data of compound 1 and nebularine, a nucleoside compound previously isolated from this mushroom. The results revealed that the structure of compound 1 was identical to that of nebularine, which is composed of purine and ribose. Nebularine is a naturally occurring cytotoxic nucleoside that has been used as an antineoplastic agent as well as a competitive inhibitor of enzymes (Aran et al., 1990; Bohr, 1978). Nebularine was first isolated as the active principle of a press-juice from C. nebularis that exhibited potent antibiotic activity against various mycobacteria and Brucella abortus (Löfgren and Lüning, 1953; Ehrenberg et al., 1946; Gordon and Brown, 1956) and very selective activity against bacteria (Biesele et al., 1955; Brown and Weliky, 1953; Brown and Konuk, 1995).
The 1H NMR spectrum of compound 2 in CD3OD showed signals that indicated the presence of three aromatic singlet protons at δ 9.12, 8.96, and 8.60, with chemical shift values that were similar to the corresponding protons of purine in compound 1. Thus, the structure of compound 2 revealed that it was purine, which is known to have various biological activities in many systems.
The chemical structures of compound 3 and compound 4 were determined by 1H NMR spectroscopic analysis and a literature survey. The 1H NMR spectrum of compound 3 in DMSO-d6 exhibited signals due to the presence of two amide protons at δ 11.0 and two methine protons at δ 7.38 (1H, d, J = 7.5 Hz) and 5.44 (1H, d, J = 7.5 Hz). These signals were well matched to uracil. The 1H NMR spectrum of compound 4 in CD3OD revealed the presence of two aromatic singlet methine protons at δ 8.04 and 8.11. These signals were consistent with those of adenine.
The structure of compound 5 was established by 1H NMR spectrum and ESI-mass spectrometry. The 1H NMR spectrum in CD3OD revealed the presence of two methine doublets at δ 8.00 (1H, d, J = 7.6 Hz) and 5.71 (1H, d, J = 7.6 Hz), which were very similar to uracil and ribose signals. The above spectral data suggested that compound 5 was a nucleoside that consisted of uracil and ribose. This finding was supported by the ESI-mass measurements, which provided quasi-molecular ion peaks at m/z 267 [M+Na]+ in positive mode and m/z 243 [M-H]- in negative mode. These findings suggested that the molecular weight of compound 5 was 244. Therefore, compound 5 was identified as uridine.
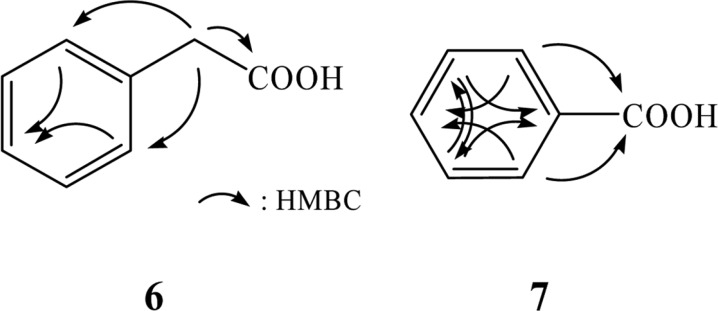
The structure of compound 6 was determined by EI-mass, 1H and 13C NMR, and HMBC experiments. The molecular weight of compound 6 was established to be 136 by EI-mass measurement. The 1H NMR spectrum of compound 6 in CD3OD showed five aromatic protons at δ 7.28 that were attributed to a phenyl group and a methylene singlet at δ 3.58. In the 13C NMR spectrum, a carbonyl carbon at δ 175.6, aromatic carbons at δ 136.1 130.4 (× 2), 129.4 (× 2), and 127.9 due to a phenyl moiety, and a methylene carbon at δ 42.0 were evident. The structure of compound 6 was determined by HMBC, which revealed long-range correlations of the methylene singlet at δ 3.58 with the carbonyl carbon at δ 175.6 and aromatic carbons at δ 136.1 and 130.4 (× 2), as shown in Fig. 3. Thus, compound 6 was identified as phenylacetic acid.
Fig. 3.
Structures of compounds 6 and 7, which were elucidated by the HMBC experiments.
The molecular weight of compound 7 was established as 122 by EI-mass measurement. The 1H NMR spectrum of compound 7 in CD3OD showed five aromatic proton signals at δ 8.01 (2H, m), 7.57 (1H, m), and 7.45 (2H, br t). A carbonyl carbon at δ 170.0 and aromatic carbons at δ 134.0, 132.0, 130.7 (× 2), and 129.4 (× 2) were observed in the 13C NMR spectrum. These spectral data suggested that compound 7 was benzoic acid. The structure of compound 7 was confirmed by HMBC experiment, which revealed long-range correlations of the aromatic protons at δ 8.01 with the carbonyl carbon at δ 170.0 and aromatic carbons at δ 134.0 and 130.7 (× 2), as well as correlations of the aromatic protons at δ 7.45 with aromatic carbons at δ 132.0 and 129.4 (× 2) (Fig. 3). Therefore, compound 7 was identified as benzoic acid.
Compound 8, which was isolated from the butanol layer, was believed to be a monosaccharide based on 1H NMR measurement and its polarity. 1H NMR peaks were observed between δ 3.6 to 3.9, and only three carbons at δ 71.3, 69.7, and 63.9 were evident in the 13C NMR spectrum. Based on an extensive literature search, this compound was believed to be mannitol. In addition, the 1H and 13C NMR spectra of compound 8 were in good agreement with those of mannitol. Therefore, compound 8 was identified as mannitol.
Antimicrobial activity
Nebularine (compound 1) exhibited moderate antifungal activity against the plant pathogenic fungus Magnaporthe grisea and against human tinea pedis fungus Trichophyton mentagrophyte (Table 1). However, it was not active against other fungi and bacteria tested, such as Botrytis cinerea, Pythium ultimum, Bacillus subtilis, and Staphylococcus aureus. Phenylacetic acid (compound 6) completely inhibited the growth of P. ultium and showed moderate activity against M. grisea, B. cinerea, T. mentagrophytes (Table 1). The antimicrobial activity of phenylacetic acid has been documented by Hwang et al. (2001), who reported that phenylacetic acid was very effective at inhibiting zoospore germination and the mycelial growth of Phytophthora capsici, as well as at controlling phytophthora blight in pepper plants.
Table 1.
Antimicrobial activity of nebularine (compound 1) and phenylacetic acid (compound 6) isolated from the fruiting body of C. nebularis
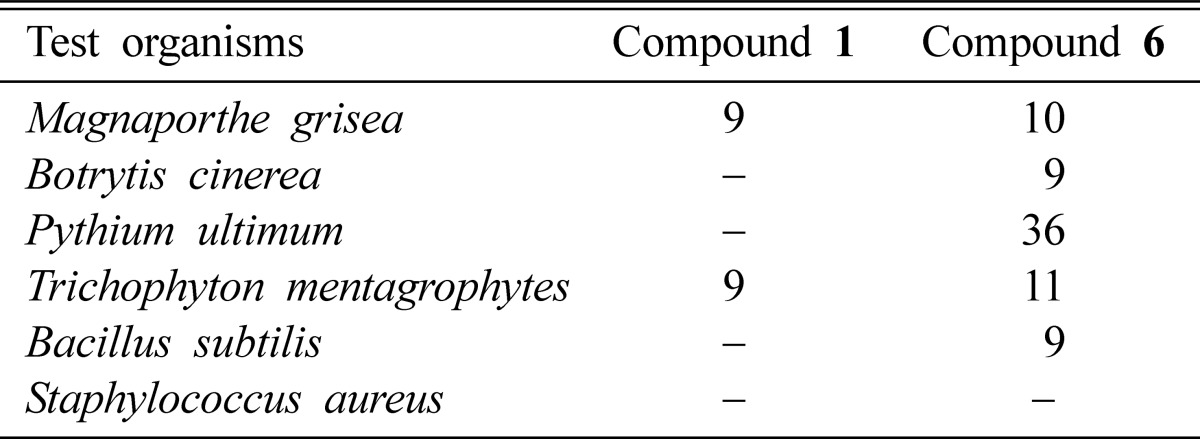
Antibiotic activity was determined by measuring the diameter of the zone of inhibition (mm, including the 8 mm diameter of the disc) following incubation for 24 hours at 37℃ for bacteria and 2~5 days at 27℃ for fungi.
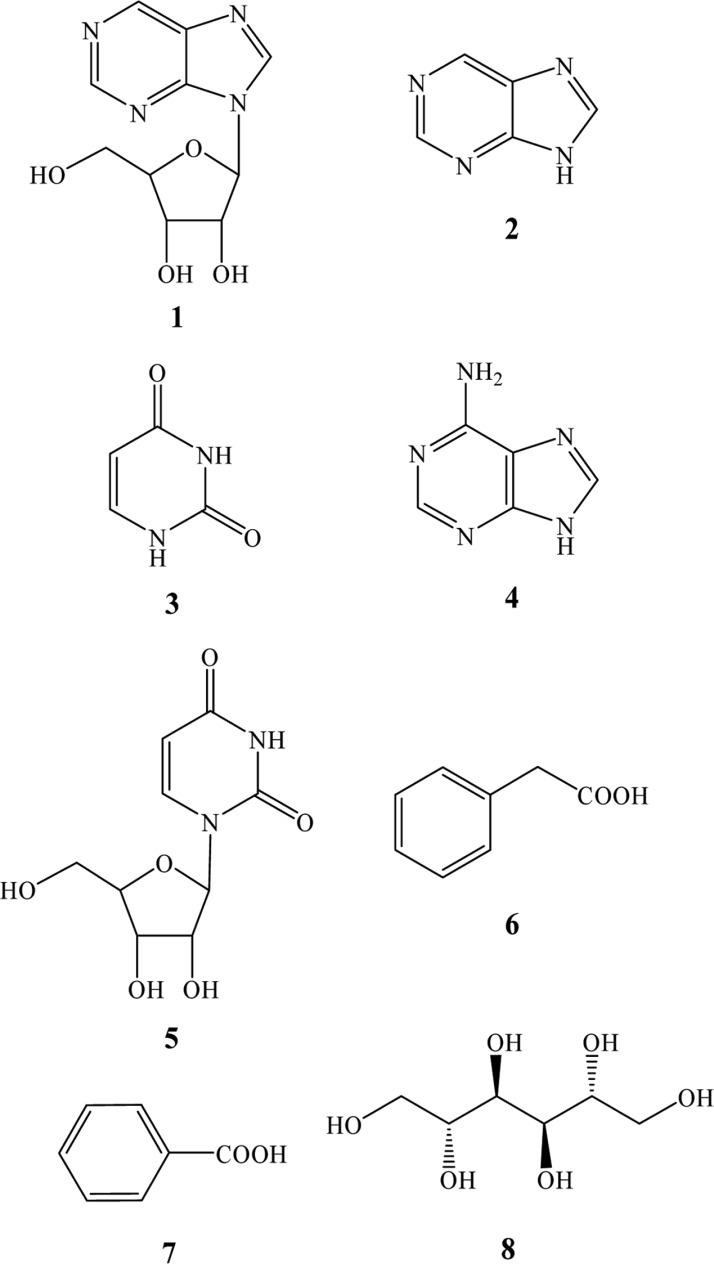
Fig. 2.
Structures of compounds 1~8.
Acknowledgements
This work was supported by a Korea Research Foundation Grant funded by the Korean Government (MOEHRD) (KRF-2006-532-F00001) and grant from the BioGreen 21 (20080401-034-069) Program and the On-Site Cooperative Agriculture Research Project of Rural Development Administration, Republic of Korea.
References
- 1.Aran JM, Canela EI, Franco R. Preparative purification of adenosine deaminase from human erythrocytes by affinity chromatography. J Chromatogr. 1990;532:75–85. doi: 10.1016/s0378-4347(00)83753-6. [DOI] [PubMed] [Google Scholar]
- 2.Bohr V. Effects of purine riboside on nucleic acid synthesis in ascites cells. Biochem Biophys Acta. 1978;519:125–137. doi: 10.1016/0005-2787(78)90067-9. [DOI] [PubMed] [Google Scholar]
- 3.Brown GB, Weliky VS. The synthesis of 9-β-D-ribofuranosylpurine and the identity of nebularine. J Biol Chem. 1953;204:1019–1024. [PubMed] [Google Scholar]
- 4.Brown EG, Konuk M. Biosynthesis of nebularine (purine-9-β-d-ribofuranoside) involves enzymic release of hydroxylamine from adenosine. Phytochemistry. 1995;38:61–71. [Google Scholar]
- 5.Biesele JJ, Slautterback MC, Margolis M. Unsubstituted purine and its riboside as toxic antimetabolites in mouse tissue cultures. Cancer. 1955;8:87–96. doi: 10.1002/1097-0142(1955)8:1<87::aid-cncr2820080112>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 6.Brzin J, Rogelj B, Popovic T, Strukelj B, Ritonja A. Clitocypin, a new type of cysteine proteinase inhibitor from fruit bodies of mushroom Clitocybe nebularis. J Biol Chem. 2000;275:20104–20109. doi: 10.1074/jbc.M001392200. [DOI] [PubMed] [Google Scholar]
- 7.Ehrenberg L, Hedström H, Löfgren N, Takman B. Antibiotic effect of Agarics on Tubercle Bacilli. Svensk Kem Tid. 1946;58:269–270. [Google Scholar]
- 8.Gordon MP, Brown GB. A study of the metabolism of purine riboside. J Biol Chem. 1956;220:927–937. [PubMed] [Google Scholar]
- 9.Hwang BK, Lim SW, Kim BS, Lee JY, Moon SS. Isolation and in vivo and in vitro antifungal activity of phenylacetic acid and sodium phenylacetate from Streptomyces humidus. Appl Environ Microbiol. 2001;67:3739–3745. doi: 10.1128/AEM.67.8.3739-3745.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Imazeki R, Otani Y, Hongo T. in Fungi of Japan. Tokyo: Yama-kei publishers; 1988. p. 66. [Google Scholar]
- 11.Löfgren N, Lüning B. On the structure of nebularine. Acta them Scand. 1953;7:225–229. [Google Scholar]