Abstract
The optimal conditions for mycelial growth of Phellinus linteus ATCC 26710 were determined to be a log length of 20 cm, temperature of 30℃ and pH of 6.0. Mycelial growth was excellent on the mushroom complete medium, and was optimal when sucrose, mannose and glucose were supplied as carbon sources. Potassium nitrate and sodium nitrate as nitrogen sources supported good mycelial growth. To evaluate P. linteus mycelial colonization on logs, sterilized short log inoculation, drilling inoculation and log-end sandwich inoculation techniques were used. Only sterilized short log inoculation produced good mycelial colonization. Initial mycelial growth and full mycelial colonization were best on 20 cm logs having 42% moisture content. The initial mycelial growth of P. linteus was accelerated over 12 hr of sterilization. Basidiocarp formation was optimal using a burying method of logs after 5~6 months, and fruiting body formation was superior in cultivation house conditions of 31~35℃ and in excess of 96% relative humidity.
Keywords: Fruiting body formation, Inoculation method, Mycelial growth, Phellinus linteus
Increasingly, mushrooms are being recognized for their potential as functional foods and as a source of physiologically beneficial medicines. Ganoderma spp. and species of Phellinus are currently popular in Korea. The genus Phellinus belongs to the Hymenochaetaceae in the basidiomycetes, and has been recognized for over a century (Quelet, 1886). P. linteus, which was first designated Polyporus linteus (Berkeley and Curtis, 1860) is an example of a Phellinus species that exhibits antitumor and immunomodulating activities. Remarkable host-mediatory antitumor activity against grafted cancer in animals was first described (as Sarcoma 180) 40 years ago (Ikekawa et al., 1968). This fungus, which mainly inhabits the mulberry tree and which has perennial forms in Korea, has been known as "Sanghuang (yellow polyporus)" for hundreds of years in traditional Chinese medicine (Chen and Chen, 2000). Numerous studies conducted using P. linteus have isolated essential substances capable of stimulating the human immune system (Chung and Kim, 1994; Lee et al., 1996; Song et al., 1998).
Physiological characterization, chemical composition, and development of cultivation methods pertaining to P. linteus and other members of this genus have been reported (Chi et al., 1996; Jung et al., 1997). Basidiocarps of P. linteus (yellow medicinal polyporus), which occur naturally with rarity, are highly prized for the antitumor activity of the bioactive protein-polysaccharide complex (Oh and Han, 1993). Despite such a great medicinal value and their rare occurrence in nature, the artificial cultivation of P. linteus has essentially remained unstudied.
The present study was carried out to investigate the physiological characteristics required for mycelial growth and especially to explore the artificial production of P. linteus using Mulberry (Morus alba) logs.
Materials and Methods
Culture media, inoculation, and determination of optimal growth
Phellinus linteus ATCC 26710 was obtained from the American Type Culture Collection (Manassas, VA, USA) and was cultured at 25℃ on yeast-malt agar (YMA) slants. Each version of the medium consisted of 0.5% peptone, 0.3% yeast extract, 0.3% malt extract, 1% dextrose, and 2% agar in 1 l. The medium was sterilized by autoclaving (121℃, 15 psi, 15 min, here and hereafter unless otherwise noted) and volumes of the sterile medium were poured into a Petri dish. A piece of mycelia from the slant was inoculated on the cooled agar, providing an inoculum for the next step. Subcultures were made routinely every 30 days. Four different culture media (Table 1) were used to determine the medium favoring optimal growth of P. linteus. Following 14 days growth on YMA, fungal mycelium of the fungus were inoculated at the center of five agar-containing Petri dish using a sterile 5 mm diameter cork borer. After 14 days of incubation at 25℃, the extent of mycelial growth was observed.
Table 1.
Composition of media used

*MCM (Mushroom Complete Medium), MEA (Malt Extract Agar), PDA (Potato Dextrose Agar), YMA (Yeast Malt Agar)
Effect of temperature on mycelial growth
The effect of temperature on mycelial growth was carried out using the medium determined to be optimal. After autoclaving the medium was aseptically poured into five Petri dish and inoculation conducted as described above. Incubation was carried out for 14 days at 20℃, 25℃, 30℃, 35℃, and 40℃, prior to observation for mycelial growth.
Effect of pH on mycelial growth
The effect of pH on mycelial growth was studied by measuring mycelial dryweight. A liquid medium was employed and the temperature used was the previously determined optimum temperature. The medium was adjusted to pH 4~8 with 1 N NaOH or HCl before being dispensed into 250 ml culture flasks at the rate of 50 ml per flask and then autoclaved. The inoculum was obtained by growing the fungus for 14 days in a Petri dish containing YMA medium. Following inoculation with mycelia, each flask was incubated at 25℃ for 14 days. The mycelia were filtered through 9 cm diameter Whatman No. 2 filter paper, dried in an oven at 85℃ for 24 h, cooled in a desiccator, and weighed.
Effect of carbon source on mycelial growth
Carbon source utilization by P. linteus was tested as previously described (Lilly & Barnett's medium) using modified versions (with respect to carbon source) of the described medium (10 g glucose, 2 g asparagine, 1 g KH2PO4, 0.5 g MgSO4·7H2O, 20 g agar in 1 l distilled water). Various carbon sources were substituted for glucose. Based on the molecular weight of 13 different carbon sources, each was added to the basal medium to achieve a concentration of 0.1M. The basal medium was adjusted to pH 6.0 and then autoclaved. Cellobiose and xylose were sterilized by Millipore™ filtration to avoid their heat-stimulated breakdown to glucose and furfural, respectively. The inoculum was obtained by growing fungus for 14 days in Petri dishes containing the basal medium. Five Petri dishes of each carbon source were inoculated with a 5 mm diameter disk of the fungus. Five Petri dishes of carbon-free basal medium were also inoculated as the control. Following incubation at 25℃ for 14 days the colony diameter of mycelia was measured as described above.
Effect of nitrogen source on mycelial growth
The ability of P. linteus to utilize different nitrogen sources for growth was studied. Except for the substitution of 1% fructose (per L) as the carbon source, the basal medium retained the composition used for the carbon source determinations. Twelve nitrogen sources were substituted for asparagine; based on their individual molecular weights, each was dispensed into the basal medium to achieve a concentration of 0.02 M. The basal medium was adjusted to pH 6.0 and autoclaved. All the other processes including inoculation, incubation, and measurement of mycelial growth were performed as described for the carbon source determinations.
Spawn preparation and cultivation
The process for basidiocarp production of P. linteus constituted two major stages. The first stage included the preparation of the stock culture, mother spawn and planting spawn, and the second entailed the preparation of the growth substrates for mushroom cultivation. To prepare mother spawn, Mulberry tree sawdust was mixed with rice bran (4 : 1 v/v) and the moisture content was adjusted to about 65% with water. The mixed medium was put into a 250 ml flask and sterilized at 121℃ for 45 min. After cooling to 20℃, a piece of mycelia from the agar plate was inoculated on this sawdust medium as an inoculum for the planting spawn. The planting spawn medium was prepared by adding the sawdust- and rice bran-containing medium prepared as described above to an 850 ml polyethylene bottle. Following sterilization at 121℃ for 90 min and cooling to 20℃, two or three spoonfuls of an inoculum of the precultured sawdust medium (mother spawn) retrieved from a 250 ml flask were added. Each inoculated sawdust medium was incubated at 25℃ for about 45 days until the mycelia were observed to have spread completely over the media.
Retrieved mycelia provided the inoculum for the Mulberry log cultivation procedure. The cultivation method for P. linteus involved, in order, selection and felling of the tree, sawing/cutting the log into short segments, transfer of segments to polyethylene bags, sterilization, inoculation, spawn preparation and use, burial of logs in soil, and tending the fruiting bodies during development from the pinhead stage to maturity.
To select natural logs suitable for cultivation of P. linteus mushrooms, the carbon/nitrogen (C/N) reduction rate of sawdust was measured using a Model CHN-1000 elemental analyzer (Leco, St. Joseph, MI, USA). The media were prepared with sawdust of Mulberry (M. alba), Oak (Quercus acutissima), Suwon-Populus (Populus euamericana) and Chestnut (Castanea crenata). The moisture of each sawdust medium was adjusted to about 65% with water. Fifty grams of each medium was put into a column (Ø 3.0 × 20.0 cm) and sterilized at 121℃ for 60 min. After cooling to 20℃, one spoonful of precultured sawdust medium was inoculated in the column of the sawdust media. Each inoculated medium was incubated in a darkened room under controlled growth parameters. After 40 days of incubation, the C/N ratio of the sawdust was measured.
To inoculate the sterilized short log sections, selected natural logs cut to a length of approximately about 60 cm were put in a heat-resistant polyethylene bag and covered with a cotton plug. The contents of each bag were sterilized by autoclaving (121℃, 15 psi, 2 hr) and then cooled to 20℃. Inoculation was made by the top spawning method, in which 0.5 cm (20~30 g) thickness of an inoculum of sawdust mycelia (planting spawn) was left on the top of a cut log and sealed with a cotton plug. The inoculated logs were incubated in a darkened room under controlled growth parameters. After one month of incubation, mycelial growth was observed.
Influence of log size and moisture content
Logs were cut to lengths of 20 cm, 30 cm, 40 cm, 50 cm, and 60 cm and were individually added to polyethylene bags. Sterilization and inoculation were accomplished as described above. After 2 months of incubation, the mycelial growth was observed. The moisture content of the various sized logs was adjusted to 35%, 40%, or 42%. Logs having moisture content of 35% prepared by drying for 3~4 months after felling of the tree were used as a control. Logs having moisture content of 40% were made by submerging dried logs in water for one day, whereas moisture content of 42% was generated by adding water to the submerged logs for one day. Inoculation and incubation were accomplished as described above. After 4 months of incubation, mycelial growth was observed.
Sterilization time of substrates (short log)
Short logs having an approximate diameter of 15 cm and approximate length of 20 cm long were placed in polyethylene bags, fitted with ring necks, and plugged with cotton to allow for air exchange. The logs were sterilized by autoclaving (121℃ and 15 psi), for 8, 10, 12, or 14 h. The colonized sawdust spawns (planting spawns) having a thickness 0.5 cm thick were inoculated on top of the sterilized short logs by the top spawning inoculation method described above. After 6 months of incubation, mycelial growth was observed.
Influence of supplementation
Short logs obtained as described above that had been placed in polyethylene bags received supplements added on top of the logs prior to plugging. These consisted of Mulberry tree sawdust, sawdust-rice bran (4 : 1 v/v), Mulberry tree leaves, and Mulberry tree leaves-rice bran (4 : 1 v/v). Following sterilization (121℃, 15 psi, 14 h) inoculation was via top spawning. After 3 months of incubation, mycelial growth of the short logs was observed.
Influences of incubation time, cultivation temperature, relative humidity, and burial depth on mushroom development
The inoculated logs were placed in a darkened room at a temperature of 22~25℃ and 65~70% relative humidity, and were incubated for 3, 4, 5, or 6 months. The colonized logs were buried in soil in the mushroom cultivation house, and the formation of fruiting bodies was assessed following the various incubation periods. Logs that were extensively colonized with mycelium were retrieved from the polyethylene bags and buried vertically in soil in the mushroom cultivation house. The cultivation house temperature was controlled at 20~25℃, 26~30℃, or 31~35℃. The pinhead formation of the mushrooms was assessed at each temperature range. The mycelium-colonized logs were buried in the soil of a mushroom cultivation house whose temperature was maintained at 31~35℃. Relative humidity of the cultivation house was maintained at 80~90%, 91~95%, or 96~99%. Formation of fruiting bodies was assessed under each condition of relative humidity. Well-colonized logs were buried vertically one-quarter length, one-half length, or three-quarters in cultivation house soil at 31~35℃ with relative humidity of 96~99%. Pinhead formation of the fungus was assessed at each burial depth.
Results and Discussion
Selection of optimal media
Four different culture media were used to assess growth of P. linteus. Mycelial growth was excellent on MCM, with a colony diameter of 70.2 mm achieved after 14 days incubation (Fig. 1). Colony diameters in the range of 40.2~45.7 mm were evident on malt extract agar, yeast malt agar, and potato dextrose agar.
Fig. 1.
Mycelial growth of P. linteus on different culture media.
Effect of culture temperature on mycelial growth
Mycelial growth of P. linteus cultured on MCM for 14 days at five different temperature levels was optimal at 25~30℃ (Fig. 2). When the temperature exceeded 30℃, mycelial growth was less, consistent with previous observations (Chi et al., 1996).
Fig. 2.
Effect of temperature on the mycelial growth of P. linteus.
Effect of pH on mycelial growth
As assessed by mycelial dry weight, pH 6.0~7.0 was most favorable for growth of P. linteus, consistent with previous observations (Chi et al., 1996). Mycelial growth was optimal at pH 6.0, with a dry weight of 74.2 mg, while growth was most unfavorable at pH 4.0 (Fig. 3). Decreasing pH of the medium is a feature of fungal growth and, thus, mycelial growth can be expected to decrease over time, consequent with fungal development.
Fig. 3.
Effect of pH on the mycelial growth of P. linteus in MCM broth medium.
Effect of carbon source on mycelial growth
Ten of the 13 tested carbon sources were favorable to mycelial growth of P. linteus as compared with the control (Table 2). Mycelial growth was best in the presence of sucrose, with a colony diameter of 57.3 mm, followed in order by mannose, glucose and fructose. Sucrose and maltose were the only oligosaccharides that supported good growth, with sucrose being superior. Arabinose, galactose and lactose were unable to support mycelial growth compared with the control. The three polysaccharides that were tested (dextrin, inulin and soluble starch) afforded good mycelial growth.
Table 2.
Effect of carbon source on the mycelial growth of P. linteus in the basal medium

aEach carbon source was added to the basal medium to a concentration of 0.1M.
bThe colony diameter was measured after 14 days of incubation.
cMycelial density: C; compact, SC; somewhat compact, ST; somewhat thin, T; thin.
Effect of nitrogen source on mycelial growth
Among the nine tested nitrogen sources, mycelial growth of P. linteus was best on media supplemented with potassium nitrate, with a recorded colony diameter of 82.2 mm, followed by sodium nitrate and ammonium tartrate (Table 3). No mycelial growth was evident in the presence of sodium nitrite, while growth was barely detectable in the presence of ammonium sulfate and urea.
Table 3.
Effect of nitrogen source on the mycelial growth of P. linteus in the basal medium

aEach nitrogen source was added to the basal medium to a concentration of 0.1M.
bThe colony diameter was measured after 14 days of incubation.
cMycelial density: C; compact, SC; somewhat compact, ST; somewhat thin, T; thin.
Selection of suitable logs
To select the optimal tree for P. linteus mushroom cultivation, the C/N ratio of seven different kinds of sawdust was measured after 40 days of incubation. The C/N ratio was the highest in the sawdust of Poplus tomentiglandulosa (Suwon-poplus), followed in order by Pinus sp., Alnus japonica, and Castanea ctrenata (Chestnut) before inoculation (Table 4). Table 4 summarizes the C/N ratio reduction after 40 days incubation. The ratio of the Mulberry (Morus alba) sawdust was slightly reduced, while those of A. japonica and P. tomentiglandulosa were markedly reduced. Therefore, the log of the Mulberry tree was chosen for P. linteus mushroom cultivation. Additionally, P. linteus is a perennial mushroom that naturally inhabits the Mulberry tree, with a modest reduction of the C/N ratio being naturally evident.
Table 4.
C/N ratio of various sawdust media after cultivation of P. linteus

aC/N ratio was measured after 40 days of incubation of P. linteus.
Effect of spawn inoculation methods on mycelial colonization of logs
To select an effective inoculation method for the artificial cultivation of P. linteus mushroom, drilling, log-end sandwich, and sterilized short log inoculation methods were compared. The results Sterilized short logs were best for mycelial running, while the drilling inoculation and log-end sandwich inoculation methods were not suitable for P. linteus mushroom cultivation because the rate of initial colonization was low (< 7%) (Table 5). Many inoculation models have been used in Korea to cultivate mushrooms. The drilling inoculation and log-end sandwich inoculation methods have traditionally been used for Lentinus edodes and Ganoderma spp. cultivation, respectively, and the sterilized short log inoculation was recently conducted as a modified procedure for mushroom cultivation.
Table 5.
Effect of various inoculation methods on the mycelial colonization in logs

aRate of initial colonization was measured after 1 month of incubation.
Effect of log size on mycelial colonization of logs
To determine the favorable log size for the mycelial colonization, P. linteus was cultivated on different lengths of logs as described in Materials and Methods. The rate of initial mycelial colonization was 30~40% after 2 months of incubation, but that of full mycelial colonization was 5~17%. The rate of mycelial colonization tended to increase with shorter length logs. Therefore, logs approximately 20 cm long proved suitable for the cultivation of P. linteus mushrooms. However, the rate of full mycelial colonization on these logs did not exceed 17% (Table 6).
Table 6.
Effect of log size on the mycelial colonization of P. linteus

aRate of initial mycelial colonization was measured after 2 months of incubation.
bRate of full mycelial colonization was measured after 4 months of incubation.
Effect of moisture content on mycelial colonization of logs
To ascertain the log moisture content that was suitable for mycelial colonization, Mulberry tree moisture contents of 35%, 40%, and 42% were established. After 4 months of incubation, mycelial growth was evident. Full mycelial colonization rates of 20% and 25% were apparent for moisture contents of 35% and 40%, respectively. A superior colonization rate was evident at 42% moisture content (Table 7). With increasing moisture content, the rate of full mycelial colonization tended to increase and mycelia tended to become more compact. Still, the rate of full mycelial colonization was below 27%, similar to that of P. pini (Rew et al., 2000).
Table 7.
Effect of moisture content in logs on mycelial growth
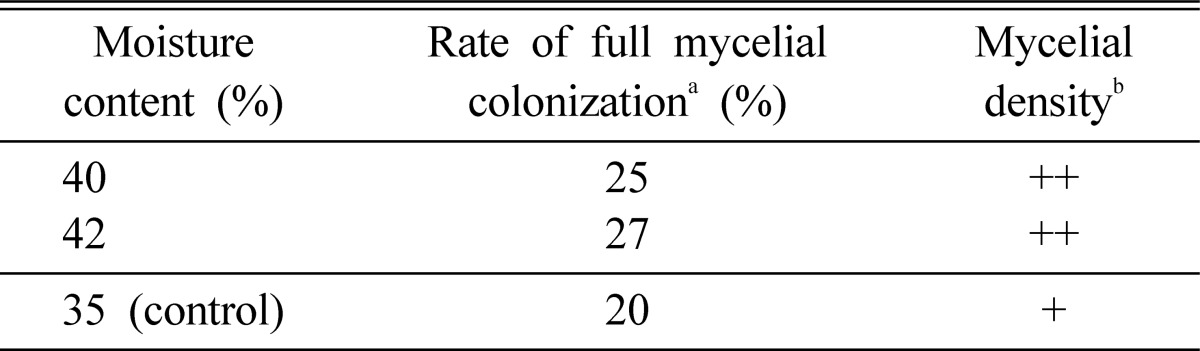
aRate of full mycelial colonization was measured after 4 months of incubation.
bMycelial density: +; poor, ++; good.
Sterilization time of logs
Using the sterilized short log inoculation, mycelial colonization was observed on logs after 6 months of incubation. The rates of initial mycelial colonization and full mycelial colonization were the best when sterilization was for 14 hr (98% and 85%, respectively) (Table 8). In general, liquid media are autoclaved by standardized sterilization at 121℃ (15 psi) for 15 min and sawdust substrates for 90 min. On the other hand, these results showed that polyethylene bags filled with logs for P. linteus cultivation needed to be sterilized at 121℃ for over 12 hr. Sterilization parameters needed to be adjusted depending on the nature and the bulk of the substrate, and fungus activity.
Table 8.
Effect of sterilization time on mycelial colonization of P. linteus

aRate of initial mycelial colonization was measured after 2 months of incubation.
bRate of full mycelial colonization was measured after 6 months of incubation.
Effect of supplements and incubation time on mycelial colonization of logs
Addition of supplements to short logs resulted in a high rate of full mycelial colonization and accelerated mycelial density compaction compared with control (Table 9). Among of supplements used, the rate of full mycelial colonization and the mycelial density were the best when supplementation was comprised of Mulberry tree leaves-rice bran (4 : 1; v/v). To investigate the most favorable incubation period of logs for the formation of fruiting body, logs were incubated for 3~6 months. Logs buried in soil prior to 4 months of incubation were infected with undesirable fungi. P. linteus pinhead did not form. These results show that the inoculated logs for P. linteus mushroom cultivation need to be incubated for at least 5 months (Table 10).
Table 9.
Effect of different supplements to mulberry logs on the mycelial growth

aRate of full mycelial colonization was measured after 3 months of incubation.
bMycelial density: +; poor, +++; compact.
Table 10.
Effect of the incubation time of logs on pinhead formation

aPinhead formation: -; non-formation, +; some formation.
Effect of cultivation temperature, relative humidity and burial depth on fruit body development
The influence of cultivation house temperature for the formation of fruiting bodies was investigated. The pinhead of fruiting bodies on logs which were transferred to mushroom cultivation house controlled at 31~35℃ formed well, while not forming at 21~25℃ (Table 11). The colonized logs buried in soil in the mushroom cultivation house at 96~99% relative humidity produced fruiting bodies well, while fruiting bodies did not form at a relative humidity of 81~90% (Table 12). Logs that were well colonized and buried vertically at one-quarter or one-half-their length in soil proved superior for the formation of pinheads (Table 13), whereas fruiting bodies formation depth in soil. The burial depth of logs was closely related with relative humidity in mushroom cultivation house conditions. If the relative humidity of mushroom condition. If the relative humidity of mushroom cultivation house was insufficient, the pinhead of fruiting body formed on nearby soil, otherwise, if sufficient, the pinhead of fruiting body formed on upper parts of logs.
Table 11.
Effect of temperature in the cultivation house on the fruiting body formation

aFruiting body formation: -; non-formation, +; some formation, ++; good formation.
Table 12.
Effect of relative humidity in the cultivation house on the fruiting body formation of P. linteus

aFruiting body formation: -; non-formation, +; some formation, ++; good formation.
Table 13.
Effect of burial depth of logs on fruiting body pinhead formation

aPinhead formation: -; non-formation, +; some formation, ++; good formation.
P. linteus basidiocarps
P. linteus fruting bodies formed after one year of inoculation. Basidiocarps grown for 2 years on logs were ungulate, sessile, 144 × 71 mm and hard woody. The upper surface of the basidiocarps was concentrically zonate and shallowly sulcate, and dark chestnut in color (Fig. 1).
Fig. 4.
Fruiting-bodies of P. linteus ATCC 26710.
References
- 1.Berkeley MJ, Curtis MA. Characteristics of new fungi collected in the North Pacific exploring expedition by charles wright. Proc Amer Acad Arts (Boston) 1860;4:122. [Google Scholar]
- 2.Chen SY, Chen HY. Collection of mushroom prescriptions. Shanghai Science and Technology Press; 2000. pp. 133–138. [Google Scholar]
- 3.Chi JH, Ha TM, Kim YH, Rho TD. Studies on the main factors affecting the mycelial growth of Phellinus linteus. Korean J Mycol. 1996;24:214–222. [Google Scholar]
- 4.Chung KS, Kim SS. Effect of Kp, an antitumor protein-polysaccharide from mycelial culture of Phellinus linteus, on the humoral immune response of tumor-bearing ICR mice to sheep fed blood cells. Arch Phar Res. 1994;16:336–338. [Google Scholar]
- 5.Ikekawa J, Nakamishi M, Uehara N, Chihara G, Fukuoka F. Antitumor action of some basidiomycetes, esp. Phellinus linteus. Gann. 1968;59:155–157. [PubMed] [Google Scholar]
- 6.Jung IC, Kim SH, Kwon YI, Kim SY, Lee JK, Park S, Park KS, Lee JS. Cultural condition for the mycelial growth of Phellinus igniarius on chemically defined medium and grains. Korean J Mycol. 1997;25:133–142. [Google Scholar]
- 7.Lee JH, Cho SM, Song KS, Han SB, Kim HM, Hong ND, Yoo ID. Immuno-stimulating activity and characterization of polysaccharide from mycelium of Phellinus linteus. J Microbiol Biotechnol. 1996;6:213–218. [Google Scholar]
- 8.Oh GT, Han SB. Immuno-stimulating activity of Phellinus linteus extracts to B-lymphocyte. Arch Phar Res. 1993;15:379–381. [Google Scholar]
- 9.Quelet L. Enchiridition fungroum in Europa media et praesertim Gallaria Vigentium. Lutetiae. 1886 [Google Scholar]
- 10.Rew YH, Jo WS, Jeong KC, Yoon JT, Choi BS. Cultural chracteristics and fruitbody formation of Phellinus pini. Korean J Mycol. 2000;28:11–15. [Google Scholar]
- 11.Song CH, Ra KS, Yang BK, Jeon YJ. Immono-stimulating activity of Phellinus linteus. Korean J Mycol. 1998;26:86–90. [Google Scholar]






