Abstract
Eight distinct bacteria were isolated form diseased mycelia of the edible mushroom, Pleurotus eryngii. 16S rDNA sequence analysis showed that the isolates belonged to a variety of bacterial genera including Bacillus (LBS5), Enterobacter (LBS1), Sphingomonas (LBS8 and LBS10), Staphylococcus (LBS3, LBS4 and LBS9) and Moraxella (LBS6). Among them, 4 bacterial isolates including LBS1, LBS4, LBS5, and LBS9 evidenced growth inhibitory activity on the mushroom mycelia. The inhibitory activity on the growth of the mushroom fruiting bodies was evaluated by the treatment of the bacterial culture broth or the heat-treated cell-free supernatant of the broth. The treatment of the culture broths or the cell-free supernatants of LBS4 or LBS9 completely inhibited the formation of the fruiting body, thereby suggesting that the inhibitory agent is a heat-stable compound. In the case of LBS5, only the bacterial cell-containing culture broth was capable of inhibiting the formation of the fruiting body, whereas the cell-free supernatant did not, which suggests that an inhibitory agent generated by LBS5 is a protein or a heat-labile chemical compound, potentially a fungal cell wall-degrading enzyme. The culture broth of LBS1 was not inhibitory. However, its cell-free supernatant was capable of inhibiting the formation of fruiting bodies. This indicates that LBS1 may produce an inhibitory heat-stable chemical compound which is readily degraded by its own secreted enzyme.
Keywords: Commercial production, Mushroom, Pathogen, Pleurotus
Pleurotus eryngii, the king oyster mushroom, is one of the most commercially important edible mushrooms. It is responsible for more than 30% of the edible mushroom market in Korea. The commercial cultivation of the king oyster mushroom involves multi-step processes in accordance with growth stage. In general, the cultivation step can be categorized into the following 6 steps: 1) preparation of liquid spawn; 2) inoculation of the spawn into a bottled solid medium; 3) propagation of mycelia; 4) formation of primordia (pinning) and removal of excess pins; 5) growth of fruiting body; 6) harvest. The entire process requires 3 months and a great deal of human effort as well as massive input of resources, including substrates, facilities, and electricity.
Because the cultivation of edible mushrooms, including P. eryngii, is conducted under well-controlled environmental conditions, the yield is expected to be highly reliable. However, mushroom growers are frequently challenged by mushroom diseases of both bacterial and viral origin. Mycoviruses including La France Isometric Virus (LIV) and Mushroom Virus X (MVX) have been shown to constitute severe threats to the cultivation of the button mushroom, Agaricus bisporus (Goodin et al., 1992; Hollings, 1962; Grogan et al., 2003; Rao et al., 2007). Two popular edible mushrooms of the Pleurotus species, P. ostreatus and P. eryngii, are infected by a variety of mycoviruses such as Oyster Mushroom Spherical Virus (OMSV), Oyster Mushroom Isometric Virus OMIV, and Pleurotus eryngii Spherical Virus (PeSV) (Yu et al., 2003; Ro et al., 2006, 2007). The containment of these viruses is accomplished principally by the detection and removal of virally-infected spawns (Romaine and Schlagnhaufer, 1995). Bacteria have been reported to associate with a variety of fungi including mushrooms. Antifungal agents produced by some bacteria have shown to be beneficial to control pathogenic fungi (Kim, 2006; Chang and Kim, 2007). However, they are harmful for the mushroom industry in general. Pseudomonas tolaasii is particularly notorious for causing brown blotch disease in the cultivation of edible mushrooms, including Agaricus bisporus, P. ostreatus, P. eryngii, and Flammulina velutipes (Rainey et al., 1991). The causative agent of the disease was identified as a lipopeptide toxin, tolaasin, which has been shown to disrupt the cell membrane via the formation of membrane pores (Rainey et al., 1991; Nutkins et al., 1991). Unlike P. tolaasii, some bacteria belonging to the species Pseudomonas and Bacillus have been reported to exert promoting effects on the growth of mushrooms, including P. eryngii (Kim et al., in press), P. ostreatus (Cho et al, 2003), and A. biporus (Eger, 1972; Rainey et al., 1990). Therefore, the correct analysis of the bacterial community in association with the cultivated mushrooms becomes crucial for the mushroom cultivation industry.
In this study, we have attempted to isolate bacterial components from the diseased mycelia of the king oyster mushroom. The partial identities of these isolates were assessed via 16S rDNA sequence determination. We also assessed the inhibitory effects of the bacterial isolates on the growth of the mushroom mycelia and fruiting bodies.
Materials and Methods
Mushroom strain and culture conditions
P. eryngii KNR2501 was provided by the Gyeongnam Provincial Agricultural Research and Extension Services (GNARES). The mushroom mycelia were cultivated in a potato dextrose broth (Ventech Bio Co., Korea) for 2 weeks at 25℃ in order to prepare the liquid spawn and to assess the effects of bacterial culture broth on the mycelial growth. For the production of fruiting bodies, a substrate harboring poplar sawdust, 15% wheat bran, and rice bran was mixed, and the moisture of the mixture was adjusted to 65%. The formulated substrate was put into a polypropylene (PP) bottle (850 ml) and the bottle was sterilized for 1.5 h at 121℃. 15 ml of liquid spawn was then inoculated into the substrate bottle and the bottle was transferred to an incubation room conditioned at 22 ± 1℃, with a relative humidity of 60%. When the mycelia were fully propagated in the substrate bottle, the bottles were moved into an incubation room conditioned at 15 ± 1℃, with a relative humidity of 90% for the production of fruiting bodies.
Isolation of bacteria, 16S rDNA sequence determination and its analysis
The diseased mycelia in the substrate bottle were collected and the bacterial cells, which were associated with the mycelia, were released via the suspending the mycelia into the R2A broth media (Difco) containing yeast extract (0.5 g/l), proteose peptone (0.5 g/l), casamino acid (0.5 g/l), dextrose (0.5 g/l), soluble starch (0.5 g/l), sodium pyruvate (0.3 g/l), dipotassium phosphate (0.3 g/l), magnesium sulfate (0.05 g/l). 300 ml of the serially diluted broths were plated onto R2A agar plates and the plates were incubated for 3 days at 30℃. The bacterial colonies appearing on the plate were picked and inoculated into the R2A broth. In order to determine the 16S rDNA sequence of the isolated bacteria, the bacterial total cellular DNA was isolated with a genomic DNA extraction kit (Intron Biotech. Co., Korea). The extracted DNA was subjected to the polymerase chain reaction (PCR) using a forward primer (27F, 5'-AGAGTTTGATCMTGGCTCAG-3') and a reverse primer (1492R, 5'-TACGGYTACCTTGTTACGACTT-3') to amplify the 16S rDNA sequence (Lane, 1991). PCR was conducted with a Taq DNA polymerase (Solgent, Korea) with a thermal cycler (Px2 thermal cycler, Thermo Electron Co., USA): 94℃ for 5 min; 30 cycles at 94℃ for 30 sec, 60℃ for 30 sec, and 72℃ for 1.5 min; 72℃ for 10 min. The resultant PCR product was purified using a PCR prep kit (Solgent Co., Korea) and sent out to a commercial sequencing service company (Bioneer, Korea) for DNA sequence determination. The resulting 16s RDNA sequences were analyzed using ARB program (http://www.arb-home.de/).
Preparation of cell-free supernatant of bacterial culture broth
Bacteria were cultured for 2 days in the R2A broth media at 30℃. The optical density at 600 nm of the broths reached approximately 3. In order to prepare the cell-free supernatant of the broth, the bacterial cells in the broth were removed via 30 minutes of high-speed centrifugation at 13,000 rpm. The supernatant was collected and incubated for 30 min at 95℃. The heat-treated solution was then further clarified via 30 minutes of high-speed centrifugation at 13,000 rpm.
Inhibitory activity of the bacteria on the mushroom growth
In order to investigate the effect of the bacteria on the mushroom mycelia growth, a mycelia disc was positioned at the center of the PDA plate and incubated until the mycelia propagated 1 cm in length from the center of the disc. Bacterial isolate was then streaked in single line and the plate was incubated for 3 days at 25℃. The effect of the bacterial broth on the fruiting body development and growth was assessed by the application of 5 ml of the bacterial culture broth or the cell-free supernatant to the substrate bottle, in which the mushroom mycelia propagated thoroughly. The broth or the cell-free supernatant was applied after the removal of the top layer of the substrate approximately 1 cm in depth. The bottle was incubated for 5 days at 15℃, with a relative humidity of 90%. Each treatment was triplicated.
Results
Full mycelial propagation within the substrate bottle requires at least 30 days under controlled environmental conditions, including humidity and temperature. During incubation, it has been frequently observed that parts of the mycelia assume a brownish color as the result of bacterial contamination (Fig. 1A and B). Once this occurs, the contaminated mycelia are not able to generate fruiting bodies, or they generate fruiting bodies with a significantly reduced half-life. In some farmland areas, more than 30% of the inoculated bottles are reportedly contaminated, and this results in a significant loss of mushroom productivity. In order to investigate the nature of the contamination, we attempted to isolate the contaminated bacteria from the diseased mycelia.
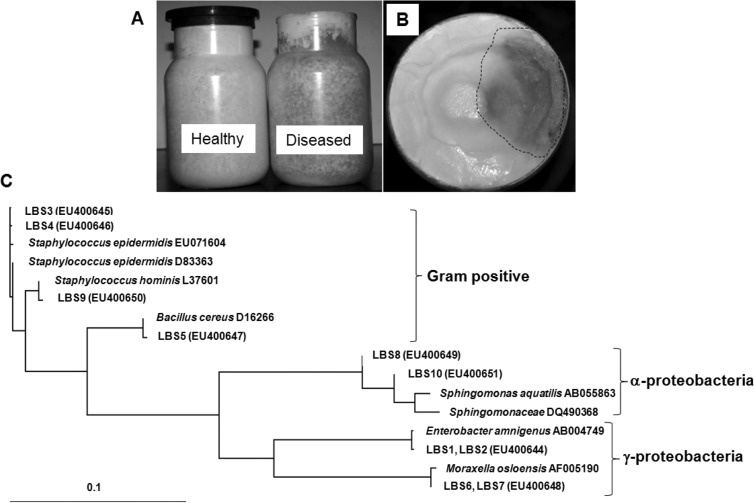
Fig. 1.
Isolation of bacteria from the diseased mycelia of P. eryngii. A) The mycelia propagated in the substrate bottle. B) Top view of the substrate bottle. The area included within the dotted line is the contaminated mycelia. C) Phylogenetic analysis on the basis of 16S rDNA sequence of the bacterial isolates. Gene bank accession numbers of the 16S rDNA sequences of the isolates are written in the parenthesis after the code name.
By spreading a serially diluted solution of the mycelial suspension onto the R2A agar, we were able to isolate 10 morphologically distinct bacteria. The 16S rDNA sequences of the isolates were then determined. Phylogenetic analysis of the 16S rDNA sequences indicated that the bacteria belonged to a variety of bacterial genera, including Bacillus (LBS5), Enterobacter (LBS1 and LBS2), Sphingomonas (LBS8 and LBS10), Staphylococcus (LBS3, LBS4 and LBS9) and Moraxella (LBS6 and LBS7) (Fig. 1C). Our analysis also demonstrated that LBS1 and LBS2 were identical and could be identified as a strain of E. amnigenus. The 16S rDNA sequences of LBS3 and LBS4 were identical and both belonged to the species S. epidermidis. However, these two were distinguished with regard to the color of colonies and the inhibitory effect on mushroom mycelial growth. LBS3 formed yellowish colonies and did not inhibit the growth of mushroom mycelia, whereas LBS4 formed white colonies and inhibited mycelia growth (Fig. 1C). LBS5 was determined to be a species of B. cereus, evidencing 99% identity in terms of its 16S rDNA sequence. LBS6 and LBS7 were identical, and were belonged to Moraxella osloensis. Both LBS8 and LBS10 were species of Sphingomonas with slight differences in the colors of their colonies. LBS8 formed red colonies, while the latter formed yellow colonies.
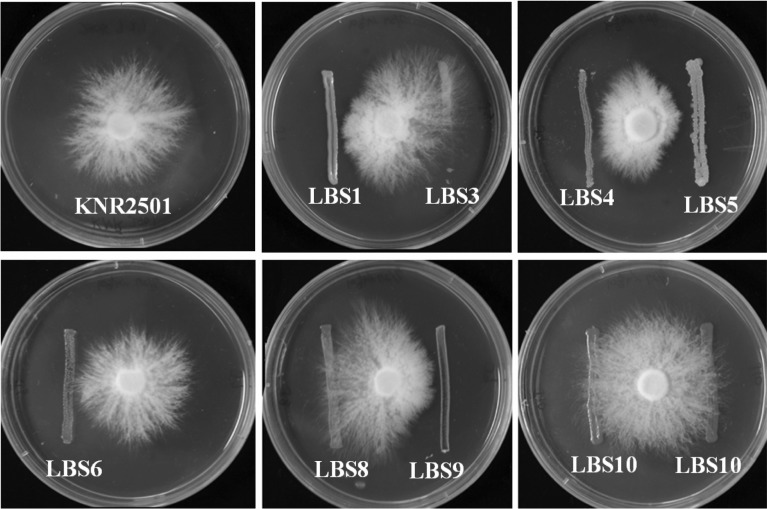
In order to confirm which of the isolates are responsible for the mycelia disease, we assessed the effects of the bacteria on the growth of mushroom mycelia on the solid media. As is shown in Fig. 2, the control mushroom mycelia grew well in all directions. However, when the mycelia met the lines of bacteria including LBS1, LBS4, LBS5, and LBS9, mycelial growth was significantly retarded, whereas LBS3, LBS6, LBS8, and LBS10 evidenced no inhibitory effects (Fig. 2).
Fig. 2.
Inhibitory activity of the bacteria on the mushroom mycelial growth. A mycelia disc was positioned at the center of the PDA plate and incubated until the mycelia propagated 1 cm in length from the center of the disc. Bacterial isolate was then streaked in single line. The closest distance of the bacterial line from the center was 2 cm. The plate was incubated for 3 days at 25℃.
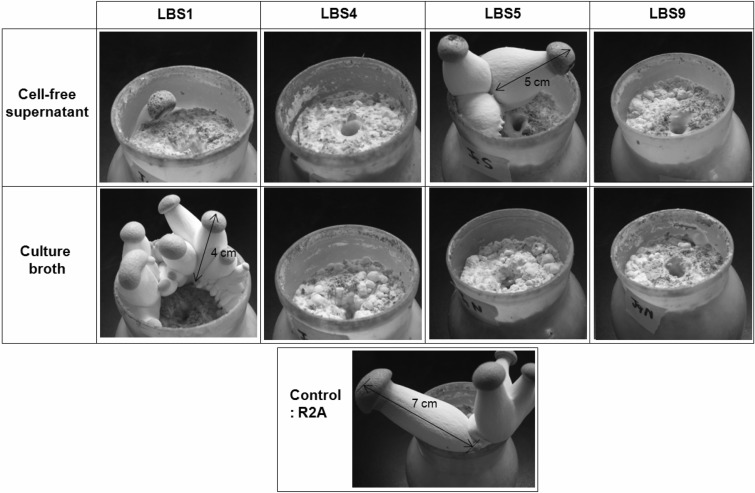
We then challenged the bacteria to the development of mushroom fruiting bodies. 5 ml of the bacterial culture broths or the cell-free supernatants of LBS1, LBS4, LBS5, and LBS9 were applied to the substrate bottle, in which the mushroom mycelia propagated thoroughly. In the control experiment, in which only fresh R2A media was applied, primordia were observed within 3 days of incubation. They grew into young fruiting bodies with an approximate height of 7 cm in 5 days (See Fig. 3, Control). On the other hand, treatment of the culture broths or the cell-free supernatants of LBS4 or LBS9 significantly retarded the formation of primordia and completely inhibited the formation of fruiting bodies (See Fig. 3, LBS4 and LBS9). In the case of LBS5, only the bacterial cell-containing culture broth was capable of inhibiting the formation of fruiting bodies, whereas the cell-free supernatant did not. Therefore, it appears that the inhibitory agent generated by LBS5 is a protein or a heat-labile chemical compound. The most peculiar case was LBS1. The culture broth itself was not inhibitory. However, the cell-free supernatant of LBS1 was capable of inhibiting the formation of fruiting bodies slightly less efficiently than was the case with LBS4 or LBS9.
Fig. 3.
Inhibitory activity of the bacteria on the fruiting body formation. 5 ml of the bacterial culture broths or the cell-free supernatants were applied to the substrate bottle, in which the mushroom mycelia propagated thoroughly. The fresh R2A media was applied for the control experiment. The bottles were incubated for 5 days at 15℃, with a relative humidity of 90%.
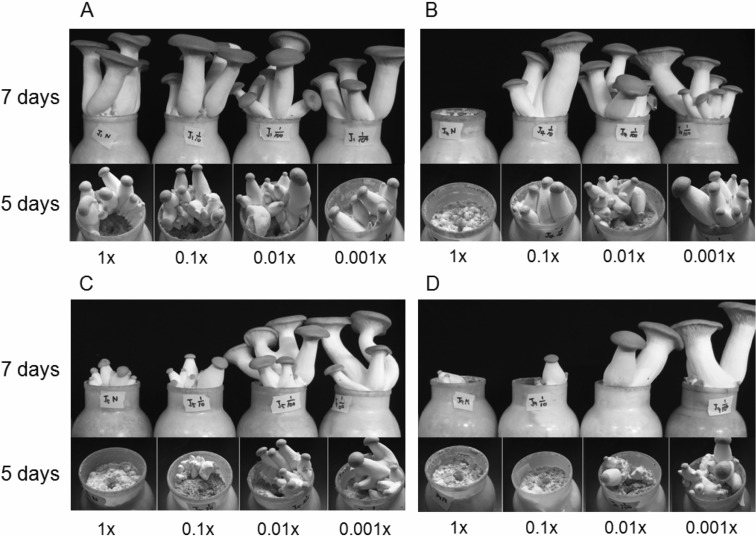
We subsequently investigated the effects of the serially diluted bacterial culture broths (1×, 0.1×, 0.01× and 0.001×) on the growth of the fruiting bodies. The culture broth of LBS1 did not influence the growth, as mentioned above (Fig. 4A). 1× broth of LBS4 was capable of inhibiting the growth, but the diluted broth was not (Fig. 4B). LBS5 evidenced inhibition ability with 0.1× diluted broth (Fig. 4C). The LBS9 culture broth evidenced the most favorable results in the inhibition of fruiting body growth. 0.01× of the broth still manifested some inhibitory activity (Fig. 4D).
Fig. 4.
Effects of the serially diluted bacterial broths (1×, 0.1×, 0.01× and 0.001×) on the growth of mushroom fruiting bodies. 5 ml of the serially diluted bacterial culture broths of LBS1 (A), LBS4 (B), LBS5 (C), and LBS9 (D) were applied. The pictures were taken 5 days and 7 days after the inoculation of broths. The dilution rates are written under the pictures.
Discussion
Although the bottled culture of P. eryngii mycelia is conducted under aseptic conditions, the deterioration of the mycelia by bacterial contamination occurs frequently. The source of contamination remains unclear, but the infiltration of contaminated air into the substrate bottle during the chilling process immediately after the sterilization process is one of the suspected sources of contamination.
In order to confirm the nature of the contamination, we have isolated 8 distinct bacteria, all of which are associated with the diseased mushroom mycelia. Among them, 4 bacteria, including strains of E. amnigenus (LBS1), S. epidermidis (LBS4), Staphylococcus sp. (LBS9), and B. cereus (LBS5) evidenced inhibitory effects on the growth of mycelia and fruiting bodies. None of these have thus far been implicated in the mushroom disease. However, in the case of B. cereus, antifungal activities against plant pathogenic fungi have been reported via the secretion of fungal cell wall-degrading enzymes including chitinase and glucan β-glucosidase (Huang et al., 2005; Kishore et al., 2007; Chang et al., 2007). Therefore, the inhibition of growth by B. cereus (LBS5) conceivably originates from the cell wall-degrading enzymes generated by the bacteria. Loss of the inhibitory activity by heat treatment, which deactivates the enzyme, further supports this hypothesis (Fig. 3, LBS5). Unlike B. cereus (LBS5), two staphylococcal culture broths (LBS4 and LBS9) retained their inhibitory activity even after heat treatment, thereby indicating that a heat-stable, potentially non-proteinaceous, compound is responsible for this inhibition. The culture broth of Enterobacter amnigenus (LBS1) was not inhibitory, but the heat-treated cell-free supernatant was inhibitory. This suggests that LBS1 may generate an inhibitory heat-stable chemical compound which can be readily degraded by its own secreted enzyme.
Acknowledgements
This study was carried out with the support of "On-Site Cooperative Agriculture Research Project (Project No. 20070201080016)", RDA and Technology Development Program for Agriculture and Forestry, Ministry of Agriculture and Forestry, Republic of Korea.
References
- 1.Chang I, Kim JD. Inhibition of aflatoxin production of Aspergillus flavus by Lactobacillus casei. Mycobiology. 2007;35:76–81. doi: 10.4489/MYCO.2007.35.2.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chang WT, Chen YC, Jao CL. Antifungal activity and enhancement of plant growth by Bacillus cereus grown on shellfish chitin wastes. Bioresour Technol. 2007;98:1224–1230. doi: 10.1016/j.biortech.2006.05.005. [DOI] [PubMed] [Google Scholar]
- 3.Cho YS, Kim JS, Crowley DE, Cho BG. Growth promotion of the edible fungus Pleurotus ostreatus by fluorescent pseudomonads. FEMS Microbiol Lett. 2003;218:271–276. doi: 10.1016/S0378-1097(02)01144-8. [DOI] [PubMed] [Google Scholar]
- 4.Eger G. Experiments and comments on the action of bacteria on sporophore initiation in in A. bisporus. Mushroom Science. 1972;8:719–725. [Google Scholar]
- 5.Goodin MM, Schlagnhaufer B, Romaine CP. Encapsidation of the La France disease-specific double-stranded RNAs in 36 nm isometric virus-like particles. Phytopathol. 1992;82:285–290. [Google Scholar]
- 6.Grogan HM, Adie BA, Gaze RH, Challen MP, Mills PR. Double-stranded RNA elements associated with the MVX disease of Agaricus bisporus. Mycol Res. 2003;107:147–154. doi: 10.1017/s0953756203007202. [DOI] [PubMed] [Google Scholar]
- 7.Hollings M. Viruses associated with dieback disease of cultivated mushrooms. Nature. 1962;196:962–965. [Google Scholar]
- 8.Huang CJ, Wang TK, Chung SC, Chen CY. Identification of an antifungal chitinase from a potential biocontrol agent, Bacillus cereus 28-9. J Biochem Mol Biol. 2005;38:82–88. doi: 10.5483/bmbrep.2005.38.1.082. [DOI] [PubMed] [Google Scholar]
- 9.Kim JD. Screening of cyanobacteria from rice paddy soil for antifungal activity against plant pathogenic fungi. Mycobiology. 2006;34:138–142. doi: 10.4489/MYCO.2006.34.3.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kim MK, Math RK, Cho KM, Shin KJ, Kim JO, Ryu JS, Lee YH, Yun HD. Effect of Pseudomonas sp. P7014 on the growth of edible mushroom Pleurotus eryngii in bottle culture for commercial production. Bioresource Technol. 2007 doi: 10.1016/j.biortech.2007.06.039. doi: 10.1016/j.biortech.2007.06.039. [DOI] [PubMed] [Google Scholar]
- 11.Kishore GK, Pande S. Chitin-supplemented foliar application of chitinolytic Bacillus cereus reduces severity of Botrytis gray mold disease in chickpea under controlled conditions. Lett Appl Microbiol. 2007;44:98–105. doi: 10.1111/j.1472-765X.2006.02022.x. [DOI] [PubMed] [Google Scholar]
- 12.Lane DJ. 16S/23S rRNA sequencing. In: Stackebrandt E, Goodfellow M, editors. Nucleic Acid Techniques in Bacterial Systematic. New York, NY: John Wiley and Sons; 1991. pp. 115–175. [Google Scholar]
- 13.Nutkins JC, Mortishire RJ, Packman LC, Brodey CL, Rainey RB, Johnstone K, Williams DH. Structure determination of tolaasin, an extracellular lipopeptide produced by the mushroom pathogen Pseudomonas tolaasii paine. J Amer Chem Soc. 1991;113:2621–2627. [Google Scholar]
- 14.Rainey PB, Cole AL, Fermor TR, Wood DA. A model system for examining involvement of bacteria in basidiome initiation of Agaricus bisporus. Mycol Res. 1990;94:191–195. [Google Scholar]
- 15.Rainey RB, Brodey CL, Johnstone K. Biological properties and spectrum of activity of tolaasin, a lipopeptide toxin produced by the mushroom pathogen Pseudomonas tolaasii. Physiol Mol Plant Pathol. 1991;39:57–70. [Google Scholar]
- 16.Rao JR, Nelson DW, McClean S. The enigma of double-stranded RNA (dsRNA) associated with mushroom virus X (MVX) Curr Issues Mol Biol. 2007;9:103–121. [PubMed] [Google Scholar]
- 17.Ro HS, Lee NJ, Lee CW, Lee HS. Isolation of a novel mycovirus OMIV in Pleurotus ostreatus and its detection using a triple antibody sandwich-ELISA. J Virol Methods. 2006;138:24–29. doi: 10.1016/j.jviromet.2006.07.016. [DOI] [PubMed] [Google Scholar]
- 18.Ro HS, Kang EJ, Yu JS, Lee TS, Lee CW, Lee HS. Isolation and characterization of a novel mycovirus, PeSV, in Pleurotus eryngii and the development of a diagnostic system for it. Biotechnol Lett. 2007;29:129–135. doi: 10.1007/s10529-006-9206-4. [DOI] [PubMed] [Google Scholar]
- 19.Romaine CP, Schlagnhaufer B. PCR analysis of the viral complex associated with La France disease of Agaricus bisporus. Appl Environ Microbiol. 1995;61:2322–2325. doi: 10.1128/aem.61.6.2322-2325.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yu HJ, Lim D, Lee HS. Characterization of a novel single stranded RNA mycovirus in Pleurotus ostreatus. Virology. 2003;314:9–15. doi: 10.1016/s0042-6822(03)00382-9. [DOI] [PubMed] [Google Scholar]