Abstract
Ganoderma lucidum (Fr.) Karst (Polyporaceae), belonging to basidiomycota, is one of the most famous medicinal mushrooms. This study was carried out to investigate favorable mycelial growth conditions, such as pH, temperature, growth media, carbon sources and nitrogen sources of Korean strains in G. lucidum. The most suitable temperature for the mycelial growth was obtained at 30℃. In general, optimal temperature range for the mycelial growth was found at 25~30℃. This Mushroom has a broad pH range (5~9) for its mycelial growth and mostly favorable growth was found at pH 5. Generally, Hamada, Glucose peptone, YM, Mushroom complete and Lilly media were the most suitable for the mycelial growth of G. lucidum. Among 10 different carbon sources, dextrin, galactose and fructose were best but the rest of other carbon sources also facilitated the growth of mycelia. The most suitable nitrogen sources were ammonium acetate, glycine, arginine and calcium nitrate, but to a certain extent, all of the supplemented nitrogen sources also stimulated the mycelial growth.
Keywords: Culture condition, Ganoderma lucidum, Media, Mycelial growth, Nutrition
Ganoderma lucidum (Fr.) Karst (Polyporaceae), a basidiomycota, is one of the most famous medicinal mushrooms in Asian countries. Its fruiting body is called 'Bulnocho' in Korea, 'Lingzhi' in China, and 'Reishi' in Japan. In Korea, China and Japan, G. lucidum has been a popular medicinal mushroom used for treating many diseases, such as hepatitis, hypertension, hypercholesterolemia and gastric cancer (Mizuno et al., 1995). Several biological active triterpenes and sterols have been isolated from this mushroom and showed cytotoxic, (Toth et al., 1983; Kohda et al., 1985) antiviral (Lindequist et al., 1989) and anti inflammatory activities (Tasaka et al., 1988). Polysaccharides and glycoproteins possessing hypoglycemic (Hikino and Mizuno 1989) and immunostimulant (Kino et al., 1989) activities have also been found from the water extract of the fruiting body in Taiwan. In recent years, submerged cultivation of G. lucidum has been developed to obtain mycelial biomass, ganoderic acid and polysaccharides which can be used to produce medicinal products (Yang and Liau, 1998; Yang et al., 2000). To accelerate mycelial growth and metabolite production, the major concerns were to find environmentally good and economically feasible compounds that stimulate mycelial growth and metabolite production of G. lucidum (Yang et al., 2000). Therefore, the submerged culture of G. lucidum has received great attention as a promising alternative for efficient production of its valuable metabolites, especially polysaccharide (Lee et al., 1999a; Yang and Liau, 1998) and ganoderic acid (Tsujikura et al., 1992).
Thus, it is necessary to find optimal nutritional and environmental conditions for culturing mycelia in the liquid and solid media. Because of that, this study was conducted to find out important parameters that affect mycelia growth of Korean strains in G. lucidum.
Materials and Methods
Collection, identification and isolation
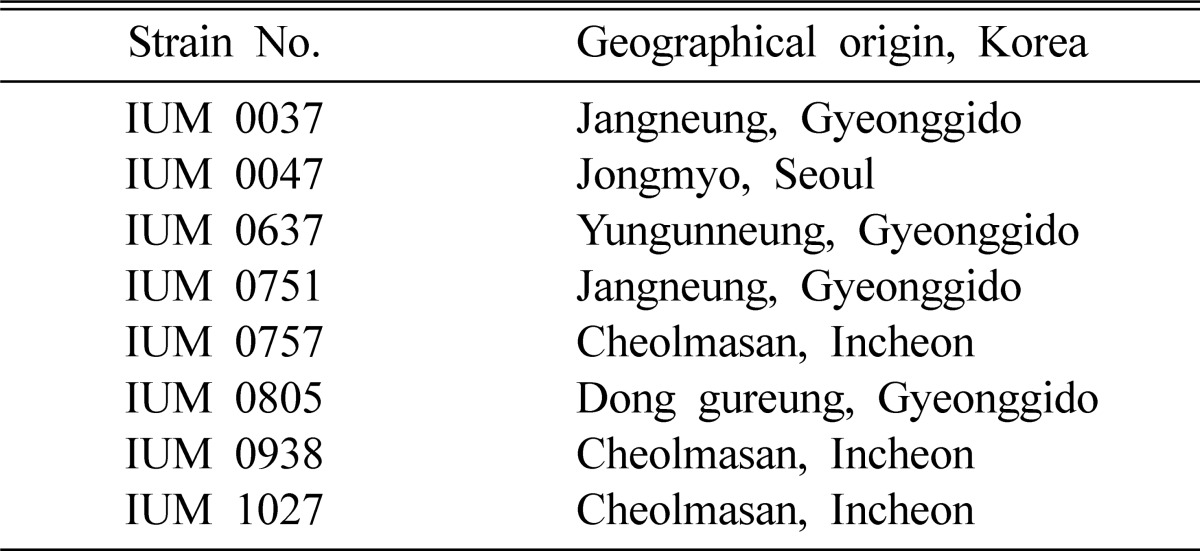
The fruiting bodies of 10 strains of Ganoderma lucidum were collected from different parts of Korea (Table 1). After identification, mycelia were isolated using standard potato dextrose agar (PDA) method and incubated for 10 days at 25℃. After isolation, pure culture of the mycelia was stored at 4℃ for further study.
Table 1.
List of G. lucidum strains used in this study
Effect of temperature
To find out the optimum temperature for the favorable mycelial growth of G. lucidum, 15 ml of molten PDA was dispensed into each of 9 cm sterile Petri dish. Disc (5 mm in diameter) taken from advancing margin of 10 day old cultures of the isolates of each strain by the aid of a cork borer were separately placed each at the centre of the dish. The inoculated dishes were incubated at 15~35℃ (5℃ intervals) for 10 days under dark condition. Mycelial growths on agar plates were measured on the basis of mean colony diameter and density. Each test was replicated 4 times.
Effect of pH
To find out optimum pH for the favorable mycelial growth of G. lucidum, 15ml of molten PDA was dispensed into each of 9 cm sterile Petri dish. Dishes (5 mm in diameter) was taken from 10 day old PDA culture and placed on the center of the plate similarly. The medium was adjusted to pH of 5, 6, 7, 8 and 9 with the addition of 1 N NaOH or HCl and incubated for 10 days at 25℃ under dark condition. The measurement of mycelial growth and density was performed as described earlier.
Favorable culture media
Disc (5 mm in diameter) was taken and placed on the center of each plate separately filled with 10 different solid culture media namely Czapek dox, Hamada, Hennerberg, Hoppkins, Glucose peptone, Glucose tryptone, Lilly, Mushroom complete, PDA and YM (Yeast-malt extract) (Table 2). The inoculated dishes were replicated four times and incubated at 25℃ for 10 days under dark condition. Mycelial growth on agar plate was measured following same manner described previously.
Table 2.
Media and their compositions used in this study
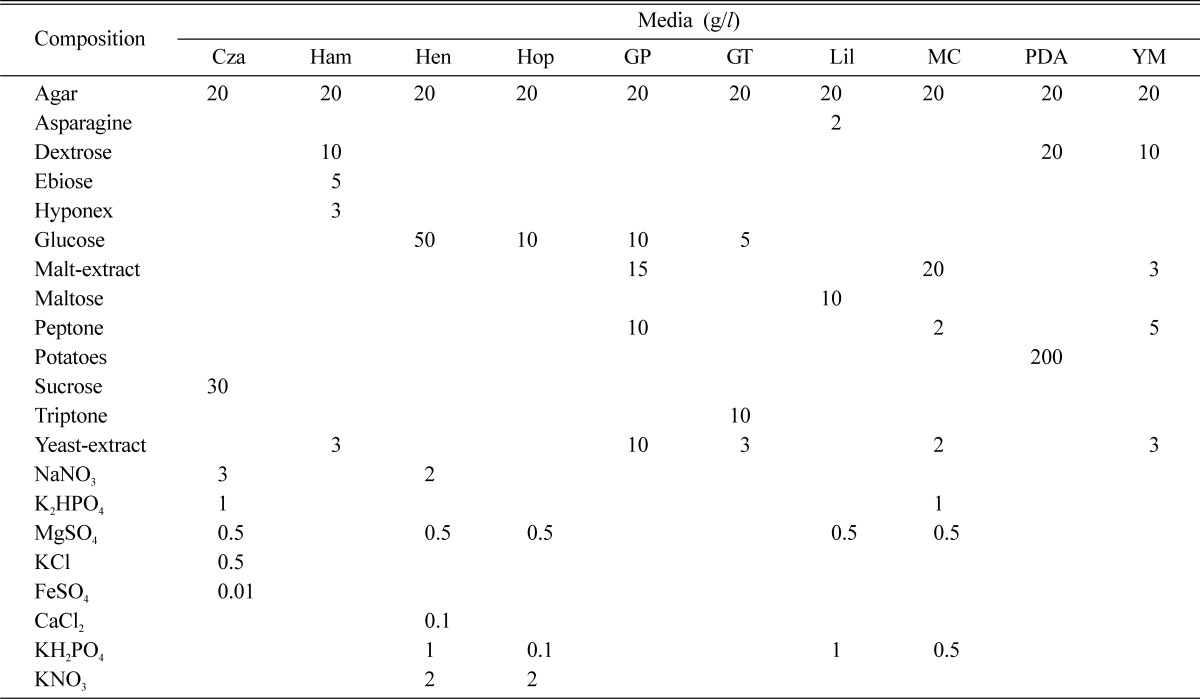
Cza: Czapek dox, Ham: Hamada, Hen: Hennerberg, Hop: Hoppkins, GP: glucose peptone, GT: glucose tryptone, Lil: Lilly, MC: mushroom complete, PDA: potato dextrose agar and YM: yeast-malt extract
Effect of carbon and nitrogen sources
Tests were performed in the basal medium (Sung et al., 1993) to screen carbon and nitrogen sources favorable for the mycelial growth of selected G. lucidum strains, supplemented with each of 10 carbon and 10 nitrogen sources. The basal medium was composed of MgSO4 (0.05 g), KH2PO4 (0.46 g), K2HPO4 (1 g), Thiamine-HCl (120 µg), Agar (20 g) and distilled water (1000 ml). To screen for the best carbon source favorable for the mycelial growth, each carbon source supplemented with 5 g of peptone to the basal medium at the concentration of 0.1M per 1000 ml and mixed thoroughly (Shim et al., 1997). The basal medium which was used for screening the favorable nitrogen source was made of same additives as those described by Sung et al. (1993). Each nitrogen source with 20 g of glucose was added to the basal medium at the concentration of 0.02M (Shim et al., 1997). In both cases, the basal medium was adjusted to pH 6 and autoclaved for 15 minutes at 121℃, poured into plates. The inoculated dishes were replicated four times and incubated at 25℃ for 10 days under dark condition. Mycelial growth was measured as describe before.
Results and Discussion
Effect of temperature
In general, the minimum and maximum cardinal temperatures for the mycelial growth and density of G. lucidum were 15 and 35℃, respectively. The best mycelial growth was (87 mm) obtained at 30℃ followed by 35℃ (Table 3). Nevertheless, some of strains (IUM 637, 751, 757 and 1027) showed optimum growth at 25℃. However, IUM 938 always showed slow growth at all temperatures. No mycelial growth was found at 15℃ (IUM805 and 938) and 35℃ (IUM721, 757 and 1027). But, incubation at 20℃ showed considerable mycelial growth for all strains of G. lucidum. Therefore, experimental results indicated that, optimum temperature range was 25~30℃ for the mycelial growth of 8 strains of G. lucidum. Lee et al. (1999b) and Shim et al. (2003) reported that the mycelial growth of Paecilomyces fumosoroseus had been expedited gradually in proportion to the rise of temperature and was the most suitable at 25℃. Even though the mycelial growth of P. fumosoroseus was favorable at the range of 20~25℃ and had been expedited in proportion to the rise of temperature, the mycelial growth appeared to be suppressed at the temperature higher than 30℃. Similarly, there were slow growth at 15 and 35℃. This could be due to the denaturation and inactivation of important enzymes which catalyze metabolic processes of tested mushroom strains. Jonathan (2002) also reported that the growth of S. commune was inhibited at 45 and 50℃. Jonathan and Fasidi (2003) found that Psathyrella atroumbonatai grew fairly well within the temperature range of 25~35℃.
Table 3.
Effect of temperatures on the mycelial growth and density of different strains of G. lucidum
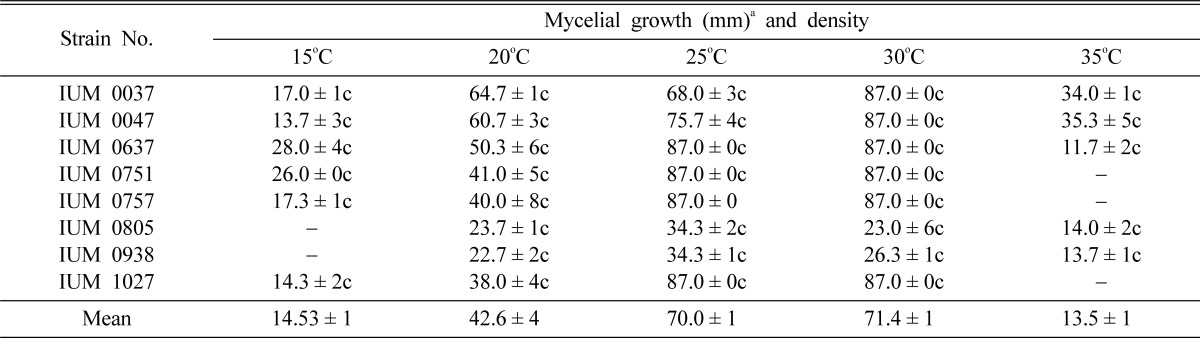
aMean of four replications. Temperature and pH effects were conducted in potato dextrose agar medium (PDA). c: Compact, sc: Somewhat compact, t: Thin and st: Somewhat thin.
Effect of pH
Favorable mycelial growth of G. lucidum was obtained at the pH range of 5~9 (Table 4). Among the eight strains, optimal mycelial growth was found at pH 5 (IUM 37, 47, 805 and 938), 8 (IUM637) and 9 (IUM751, 757 and 1027). But, 8 strains of G. lucidum also showed good mycelial growth and density in the rest of pH. Therefore, the results indicated that G. lucidum strains can grow at broad pH range, such as pH 5~9. This result also implies that different G. lucidum strains prefer different pH values tending toward neutrality. Likewise, Chandra and Purkayastha (1977) and Jonathan et al. (2004) obtained very good mycelial growth of Agaricus campestris and Volvariella esculenta at pH 6. Fasola et al. (2007) also reported that V. speciosa grew over a wide range of pH but optimum growth was obtained at pH 6. Adejoye et al. (2007) also reported S. commune showed favorable growth at pH 5.5. Akinyele and Adetuyi (2005) reported that pH range for the mycelial growth of V. volvacea was 5.5~8.5, while Kuforiji and Fasidi (1998) obtained an optimal pH for mycelial growth of Pleurotus tuberregium, Phellinus japonica and P. linteus at 5~7, 6~7 and 7, respectively. They suggested that mushrooms may have a broad pH range for their favorable mycelial growth.
Table 4.
Effect of pH on the mycelial growth and density of different strains of G. lucidum
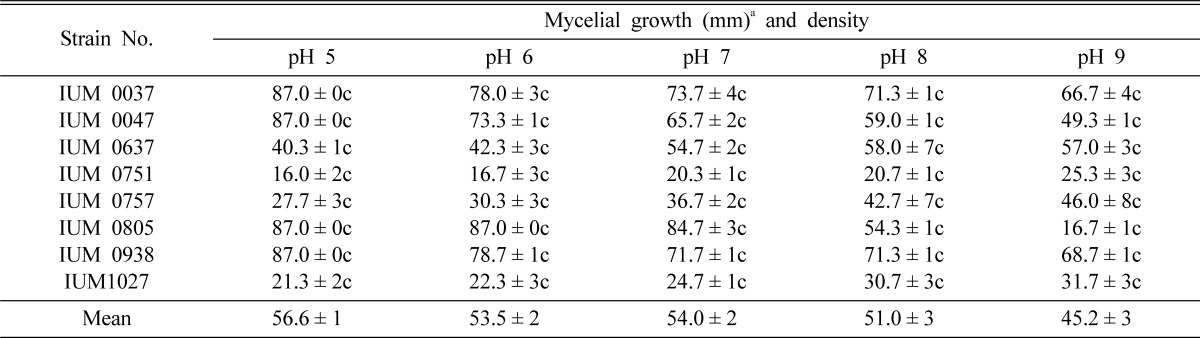
aMean of four replications. Temperature and pH effects were conducted in potato dextrose agar medium (PDA). c: Compact, sc: Somewhat compact, t: Thin and st: Somewhat thin.
Screening for favorable culture media
Ten different culture media were used to screen the optimal mycelial growth of 8 different strains of G. lucidum. The result showed that Hamada, Glucose peptone, YM, Mushroom complete and Lilly media were the most suitable and Hoppkins was the most unfavorable for mycelial growth of G. lucidum. But remaining media were also showed comparatively good mycelial growth for G. lucidum (Table 5). This result is corresponded with that of P. sinclairii and P. fumosoroseus which had been studied by Shim et al. (2003) where mycelial growth was optimal on Hamada medium. Shim et al. (2005) also reported that PDA, YM, Mushroom complete and Hamada were the most suitable, where Czapex dox and Glucose peptone were unfavorable to mycelial growth of Macrolepiota procera.
Table 5.
Effect of media on the mycelial growth and density of different strains of G. lucidum
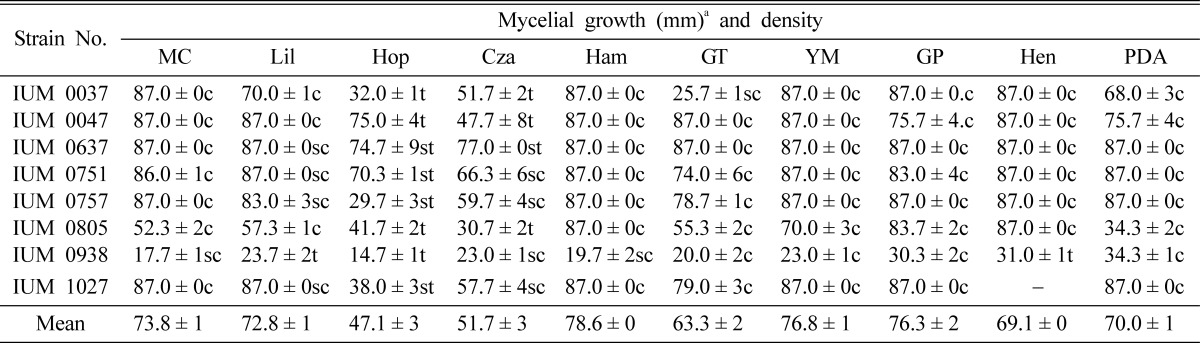
aMean of four replications. Cza: Czapek dox, Ham: Hamada, Hen: Hennerberg, Hop: Hoppkins, GP: glucose peptone, GT: glucose tryptone, Lil: Lilly, MC: mushroom complete, PDA: potato dextrose agar and YM: yeast-malt extract. c: Compact, sc: Somewhat compact, t: Thin and st: Somewhat thin.
Effect of carbon sources
It was found that dextrin was the best carbon source for mycelial growth of G. lucidum (Table 6). This was closely followed by galactose and fructose which were not considerably different each other. On the other hand, mannose, maltose, sucrose and xylose showed moderate mycelial growth, while sorbitol lactose and glucose showed slow level of mycelial growth in G. lucidum. But in case of IUM 751, 757 and 1027, optimum mycelial growth was obtained at galactose, while IUM 938, optimum mycelial growth was found on fructose. However, Chandra and Purkayastha (1977) reported that most of the tropical edible macrofungi were in favor of utilizing glucose than other carbon sources. The preference of glucose over other carbon compounds may be due to the fast metabolization of glucose by the fungi to produce cellular energy easily (Garraway and Evans, 1984). dum strains, is an isomer of glucose which can be easily transformed to glucose during metabolic pathway (Morrison and Boyd, 1992). Griffin (1994) suggested that mannose and fructose are the most commonly utilized sugars after glucose. Shim et al. (2005) proved that maltose, dextrin, sucrose and mannose were effective where lactose was highly negative for mycelial growth of M. procera. Shim et al. (1997) reported that mycelial growth of Grifola umbellata was favorable to all tested carbon sources except salicin, cellobiose and lactose. Shim et al. (2003) revealed that dextrin was suitable for mycelial growth of P. fumosoroseus. Those results are partially similar to our findings, but they showed that mycelial density in all carbon sources is thin where our result is opposite.
Table 6.
Effect of carbon sources on the mycelial growth and density of different strains of G. lucidum
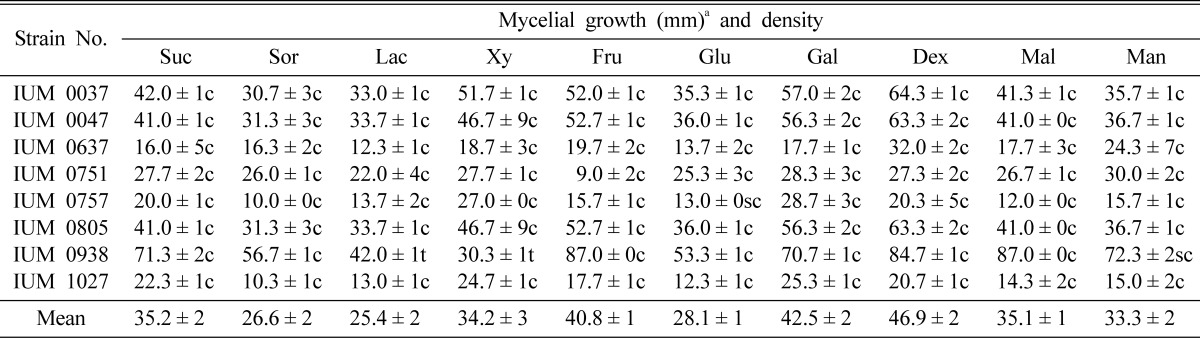
aMean of four replications. Dex: dextrin, Fr: fructose, Ga: galactose, Gl: glucose, Lac: lactose, Mal: maltose, Man: mannose, Sor: sorbitol, Suc: sucrose and Xy: xylose. Each carbon source was added to the basal medium at the concentration of 0.1M. c: Compact, sc: Somewhat compact, t: Thin and st: Somewhat thin
Effect of nitrogen sources
According to the experimental results, eight strains of G. lucidum showed optimum mycelial growth on ammonium acetate, glycine, arginine and calcium nitrate. However, remaining other nitrogen sources also facilitated considerable mycelial growth of G. lucidum (Table 7). In case of IUM 757 and 1027, these strains showed no mycelial growth on histidine, urea and ammonium phosphate. The strain of IUM 751 also showed no mycelial growth on histidine. Shim et al. (2005) clarified that glycine was the most favorable and histidine, arginine and ammonium oxalate were the most unfavorable nitrogen sources for the mycelial growth of M. procera. In general, organic nitrogen sources are more effective than inorganic nitrogen source for mycelial growth. But from this experiment, it was observed that inorganic nitrogen sources also enhanced the mycelial growth of G. lucidum. Therefore, the present findings are very similar to the observations for Lentinus lepideus (Kim et al., 1994) and V. esculenta (Jonathan et al., 2004). They reported that mycelial growth of these 2 species was more favorable on the culture media containing organic nitrogen sources than inorganic nitrogen sources.
Table 7.
Effect of nitrogen sources on the mycelial growth and density of different strains of G. lucidum
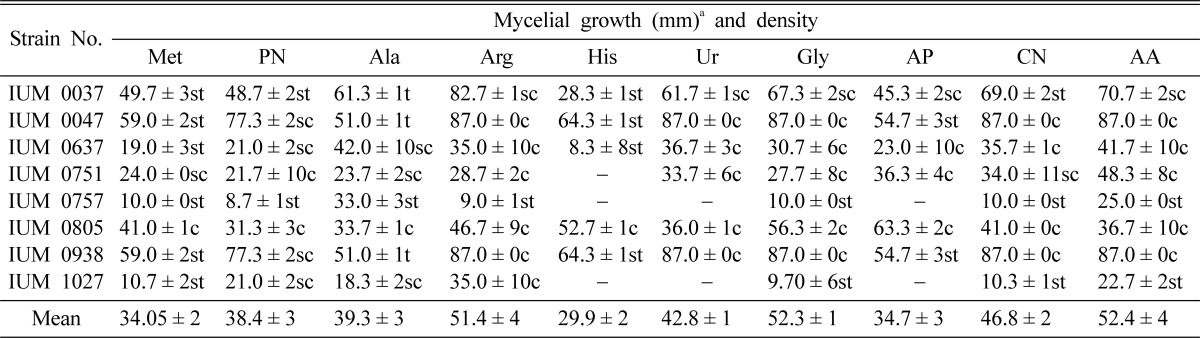
aMean of four replications. Ala: alanine, AA: ammonium acetate, AP: ammonium phosphate, Arg: arginine, CN: calcium nitrate, Gly: glycine, His: histidine, Met: methionine, PN: potassium nitrate and Ur: urea. Each nitrogen source was added to the basal medium at the concentration of 0.02M. c: Compact, sc: Somewhat compact, t: Thin and st: Somewhat thin.
Acknowledgements
This research was supported by research grant from University of Incheon, Korea (2006).
References
- 1.Adejoye OD, Adebayo-Tayo BC, Ogunjobi AA, Afolabi OO. Physicochemical studies on Schizophyllum commune (Fries), a Nigerian edible fungus. World Appl Sci J. 2007;2:73–76. [Google Scholar]
- 2.Akinyele BJ, Adetuyi FC. Effect of agrowastes, pH and temperature variation on the growth of Volvariella volvacea. Afr J Biotech. 2005;4:1390–1395. [Google Scholar]
- 3.Chandra A, Purkayastha RP. Physiological studies on Indian mushrooms. Trans Br Mycol Soc. 1977;69:63–70. [Google Scholar]
- 4.Fasola TR, Gbolagade JS, Fasidi IO. Nutritional requirements of Volvariella speciosa (Fr. Ex. Fr.) Singer, a Nigerian edible mushroom. Food Chem. 2007;100:904–908. [Google Scholar]
- 5.Griffin DH. Fungal Physiology. 2nd edition. New York: Wiley Liss; 1994. Chemical requirement for growth; pp. 130–157. [Google Scholar]
- 6.Garraway OM, Evans CR. Fungal Nutrition and Physiology. New York: John Wiley and Sons; 1984. [Google Scholar]
- 7.Hikino H, Mizuno T. Hypoglycemic actions of some heteroglycans of Ganoderma lucidum fruit bodies. Planta Medica. 1989;55:385–389. doi: 10.1055/s-2006-962033. [DOI] [PubMed] [Google Scholar]
- 8.Jonathan SG, Fasidi IO, Ajayi EJ. Physico-chemical studies on Volvariella esculent Mass (Singer), a Nigerian edible fungus. Food Chem. 2004;85:339–342. [Google Scholar]
- 9.Jonathan SG, Fasidi IO. Studies on Psathyrella atroumbonatai (Pegler), a Nigerian edible fungus. Food Chem. 2003;81:481–484. [Google Scholar]
- 10.Jonathan SG. Vegetative growth requirements and antimicrobial activities of some higher fungi in Nigeria. Ibadan, Nigeria: University of Ibadan; 2002. Ph. D. Thesis. [Google Scholar]
- 11.Kim HK, Park JS, Cha DY, Kim YS, Moon BJ. Study on the artificial cultivation of Lentinus lepideus (Fr. ex. Fr.) Korean J Mycol. 1994;22:145–149. [Google Scholar]
- 12.Kino K, Yamashita A, Yamaoka K, Watanabe J, Tanaka S, Ko K, Shimizu K, Tsuno H. Isolation and characterization of a new immunomodulatory protein, Ling Zhi-8 (LZ-8), from Ganoderma lucidium. J Biol Chem. 1989;264:472–478. [PubMed] [Google Scholar]
- 13.Kohda H, Tokumoto W, Sakamoto K, Fujii M, Hirai Y, Yamasaki K, Komoda Y, Nakamura H, Ishihara S, Uchida M. The biologically active constituents of Ganoderma lucidum (Fr.) Karst. histamine release-inhibitory triterpenes. Chem Pharm Bull. 1985;33:1367–1374. doi: 10.1248/cpb.33.1367. [DOI] [PubMed] [Google Scholar]
- 14.Kuforiji OO, Fasidi IO. Growth requirement of Pleuroteus tuberregium, a Nigerian mushroom. Proc Nigerian Soci Micro. 1998;1:73–78. [Google Scholar]
- 15.Lee KM, Lee SY, Lee HY. Bistage control of pH for improving exopolysaccharide production from mycelia of Ganoderma lucidum in an air-lift fermentor. J Biosci Bioeng. 1999a;88:646–650. doi: 10.1016/s1389-1723(00)87094-2. [DOI] [PubMed] [Google Scholar]
- 16.Lee IK, Shim HJ, Woo SD, Je YH, Yang Z, Kang SK. Variation in growth and pathogenicity of Beauveria barssiana and Paecilomyces fumosoroseus pathogenic to the pine gall midge, Thecodiplosis japonensis. Korean J Appl Microbiol Bioeng. 1999b;27:415–418. [Google Scholar]
- 17.Lindequist U, Lesnau A, Teuscher E, Pilgrim H. Untersuchungen zur antiviralen Wirksamkeit von Ergosterolperoxid. Pharmazie. 1989;44:579–580. [PubMed] [Google Scholar]
- 18.Mizuno T, Wang G, Zhang J, Kawagishi H, Nishitoba T, Li J. Reishi, Ganoderma lucidum and Ganoderma tsugae: bioactive substances and medicinal effects. Food Rev Intl. 1995;11:151–166. [Google Scholar]
- 19.Morrison R, Boyd RN. Organic Chemistry. New Jersey: Englewood Cliffs, Prentice Hall; 1992. Carbohydrates I. Monosaccharides; pp. 1144–1146. [Google Scholar]
- 20.Shim JO, Son SG, Kim YH, Lee YS, Lee JY, Lee TS, Lee SS, Lee MW. The culture conditions affecting the mycelial growth of Grifola umbellata. Korean J Mycol. 1997;25:209–218. [Google Scholar]
- 21.Shim SM, Lee KR, Kim SH, Im KH, Kim JW, Lee UY, Shim JO, Lee MW, Lee TS. The optimal culture conditions affecting the mycelial growth and fruiting body formation of Paecilomyces fumosoroseus. Mycobiology. 2003;31:214–220. [Google Scholar]
- 22.Shim SM, Oh YH, Lee KR, Kim SH, Im KH, Kim JW, Lee UY, Shim JO, Shim MJ, Lee MW, Ro HS, Lee HS, Lee TS. The characteristics of culture conditions for the mycelial growth of Macrolepiota procera. Mycobiology. 2005;33:15–18. doi: 10.4489/MYCO.2005.33.1.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sung JM, Kim CH, Yang KJ, Lee HK, Kim YS. Studies on distribution and utilization of Cordyceps militaris and C. nutans. Korean J Mycol. 1993;21:94–105. [Google Scholar]
- 24.Tasaka K, Akagi M, Miyoshi K, Mio M, Makino T. Antiallergic constituents in the culture medium of Ganoderma lucidum. (I) Inhibitory effect of cyclooctasulfur on histamine release. Agents Actions. 1988;23:153–156. doi: 10.1007/BF02142526. [DOI] [PubMed] [Google Scholar]
- 25.Toth JO, Luu B, Ourisson G. Ganoderic acid T and Z: cytotoxic triterpenes from Ganoderma lucidum. Tetrahedron Lett. 1983;24:1081–1084. [Google Scholar]
- 26.Tsujikura Y, Higuchi T, Miyamoto Y, Sato S. Jpn Kokai Tokkyo Koho. 1992. Manufacture of ganoderic acid by fermentation of Ganoderma lucidum; p. JP04304890. [Google Scholar]
- 27.Yang FC, Liau CB. The influence of environmental conditions on polysaccharide formation by Ganoderma lucidum in submerged fermentations. Process Biochem. 1998;33:547–553. [Google Scholar]
- 28.Yang FC, Ke YF, Kuo SS. Effect of fatty acids on the mycelia growth and polysaccharide formation by Ganoderma lucidum in shake flask cultures. Enz Microb Technol. 2000;27:295–301. doi: 10.1016/s0141-0229(00)00213-1. [DOI] [PubMed] [Google Scholar]


