Abstract
Schizophyllum commune is an edible and medicinal mushroom widely distributed in the world. The optimal growth conditions for the mycelia of 10 strains of the fungus were investigated. The temperature suitable for the mycelial growth and density was obtained at 30~35℃. Among the tested conditions, the minimum mycelial growth was found at 15℃. In case of pH, the most favorable growth was found at pH 5. The results indicated that this mushroom well adapted to high temperature and low pH for its mycelial growth. Considering growth phenotype of mycelia, Hamada, Hennerberg, PDA and YM were the most suitable and Lilly, Glucose triptone, Glucose peptone and Hoppkins were the most unfavorable among tested media for the mycelial growth of S. commune. Out of tested carbon sources, dextrin and fructose were the most suitable and lactose, mannose and sorbitol were the unsuitable for the fungus. Compact mycelial density was obtained from most of the carbon sources. Among used nitrogen sources, calcium nitrate, potassium nitrate and alanine were the most appropriate and the most incompatible were ammonium phosphate, histidine, urea and arginine for mycelial growth of S. commune on the culture media. Calcium nitrate, histidine and potassium nitrate showed moderately thin or thin, and rest of nitrogen sources showed compact or moderately compact mycelial density.
Keywords: Culture conditions, Growth phenotype, Media, Mycelial density, Nutrition
INTRODUCTION
Schizophyllum commune is one of the most common and widely distributed mushrooms in the world. It is found in every continent except Antarctica, where there is no wood to be used as a substrate. The genus Schizophyllum means "split gill," and thus this is the split gill fungus. It does not appear to be very closely related to the other gilled mushrooms. It is a very drastic wood decay fungus that causes a white rot. Interestingly, this fungus is consumed for food in southern part of Asian countries such as Thailand, Taiwan, Malaysia, Vietnam and southern China. It is also known to cause a human mycosis in just a few cases involving immuno-incompetent people, especially children. The fungus had grown through the soft palate of a child's mouth and was actually forming fruiting bodies in sinuses (Kuo, 2003). Iizasa et al. (2001) studied pulmonary mucous consolidative lesion caused by colonization of S. commune, and recommended that this fungus is more readily considered as a potential pathogen in the lower respiratory tract. Different strains of S. commune showed different results not only for biomass, but also for biopolymer production. The data confirm the diversity of exopolysaccharide production among different strains in submerged culture (Maziero et al., 1999). Another strain of S. commune was studied with the same growing conditions (Cavazzoni and Adami, 1992). A practical aspect and characterization of exopolysaccharide is the availability of data for the investigation of its physiological and ecological importance. In addition, this biopolymer may have potential industrial applications. The exopolysaccharide polymer known as schizophyllan is soluble in water, which forms a viscous solution with high thermal stability. The possible application of this biopolymer is in human health. There is an intensive research on polysaccharides of this fungus as antitumor agents (Jong and Birgmingham, 1992; 1993).
Therefore, this study has been conducted to screen a suitable growth condition for the 10 strains of S. commune. The different physicochemical factors were used to assess the optimal culture conditions for the mycelial growth and density of this fungus.
Materials and Methods
Strains used
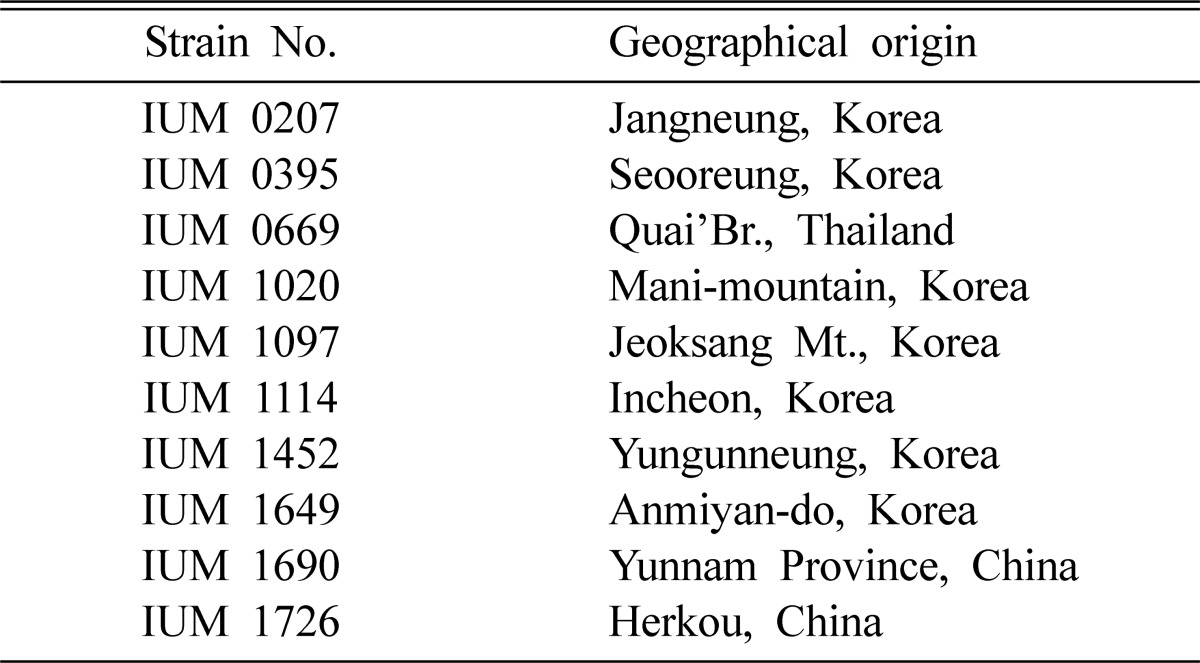
The fruiting bodies of 10 strains of Schizophyllum commune were collected from different geographical origins of Korea, China and Thailand (Table 1). After identification, the mycelia of the mushroom were cultured on potato dextrose agar (PDA) medium and incubated at 25℃ for further study. The pure cultures of the mushroom were deposited in 'Culture Collection of Wild Mushroom Species (CCWM)' and acquired accession number of IUM (Incheon University Mushroom) established in the Department of Biology, University of Incheon, Korea. The strains used in this experiment were performed with 4 replications.
Table 1.
List of Schizophyllum commune strains used in this study
Effect of temperature
To screen the optimum temperature for the mycelial growth of the mushrooms, 5 different temperatures (15, 20, 25, 30 and 35℃) were studied. A 5 mm diameter agar plug removed from 10 day old cultures grown on PDA and placed in the centre of each plate filled with 20 ml of PDA. The medium was adjusted to pH 6 and incubated for 10 days at 15, 20, 25, 30 and 35℃ separately. Radial growth of mycelia on each Petri dish was measured at 3 directions and average value was calculated out of those 3 measurements. To calculate final mean value of mycelial growth of each strain 4 replications were used.
Effect of pH
A 5 mm diameter agar plug of an inoculum was removed with cork borer from 10 day old cultures grown on PDA and placed in the centre of each agar plate. The medium was adjusted to pH 5, 6, 7, 8 and 9 with the addition of 1 N NaOH or HCl, and incubated for 10 days at 25℃. The measurement of mycelial growth was performed following same technique as optimum temperature tests.
Screening of favorable culture media
Ten different culture media (Czapek Dox, Hamada, Hennerberg, Hoppkins, Glucose peptone, Glucose tryptone, Lilly, Mushroom complete, PDA and YM) were prepared to investigate the mycelial growth of the strains (Table 2). The media were adjusted to pH 6 before autoclave. A 5 mm diameter plug of an inoculum was removed from 10 days old culture grown on PDA and placed in the centre of each plate of 10 different culture media. After 10 days of incubation at 25℃, measurement of mycelial growth and density was performed with same manner.
Table 2.
Culture media and their constituents used in this study
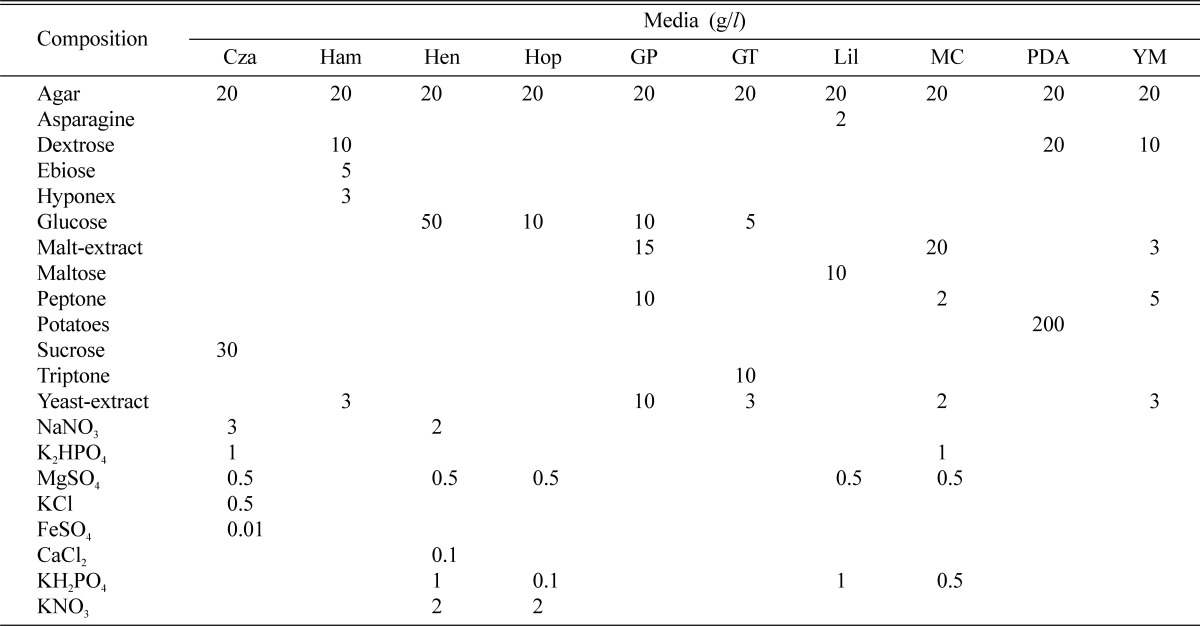
Cza: Czapek Dox, Ham: Hamada, Hen: Hennerberg, Hop: Hoppkins, GP: Glucose peptone, GT: Glucose tryptone, Lil: Lilly, MC: Mushroom complete, PDA: Potato dextrose agar and YM: Yeast-malt extract.
Effect of carbon and nitrogen sources
To screen carbon and nitrogen sources favorable for the mycelial growth of selected mushroom strains, the tests were performed on the basal medium (Sung et al., 1993) supplemented with each of 10 carbon (Dextrin, Fructose, Galactose, Glucose, Lactose, Maltose, Mannose, Sorbitol, Sucrose and Xylose) and 10 nitrogen (Alanine, Ammonium acetate, Ammonium phosphate, Arginine, Calcium nitrate, Glycine, Histidine, Methionine, Potassium nitrate and Urea) sources separately. The basal medium was composed of MgSO4 0.05 g, KH2PO4 0.46 g, K2HPO4 1.0 g, thiamine-HCl 120 µg, agar 20 g and 1000 ml of distilled water. To screen carbon source favorable to the mycelial growth, each carbon source with 5 g of peptone was added to the basal medium separately at the concentration of 0.1M per 1000 ml and mixed thoroughly (Shim et al., 1997). The basal medium which was used for screening a favorable nitrogen sources was made of same additive as those described by Sung et al. (1993). Each nitrogen source with 20 g of glucose was added to the basal medium at the concentration of 0.02M (Shim et al., 1997). In both cases, the basal medium was adjusted to pH 6 before autoclave. To measure the colony diameter on the media, all plates were incubated for 10 days at 25℃. Radial mycelial growth and density were measured following same method.
Results and Discussion
Effect of temperature
The optimum temperature for the mycelial growth and density of tested fungal strains was obtained at 30~35℃ and the lowest mycelial growth and density were recorded at 15℃. In case of IUM1452, no mycelial growth was found at 15℃. The different strains of S. commune have optimal mycelial growth and density at high temperature (Table 3). Shim et al. (2005) and Sung et al. (1999) stated that the favorable mycelial growth of Macrolepiota procera and Pleurotus ostreatus was at 30℃, respectively. Shim et al. (2003) reported that the mycelial growth of Paecilomyces fumosoroseus had been expedited gradually in proportion to the rise of temperature and was the most suitable at 25℃. Even though the mycelial growth of P. fumosoroseus was favorable at the range of 20~25℃ and had been expedited in proportion to the rise of temperature, the mycelial growth appeared to be suppressed at the temperature higher than 30℃. Therefore, this result is partially similar to Shim et al. (2005) and Sung et al. (1999) and incompatible to Shim et al. (2003).
Table 3.
Effect of temperature on the mycelial growth and density of different strains of Schizophyllum commune
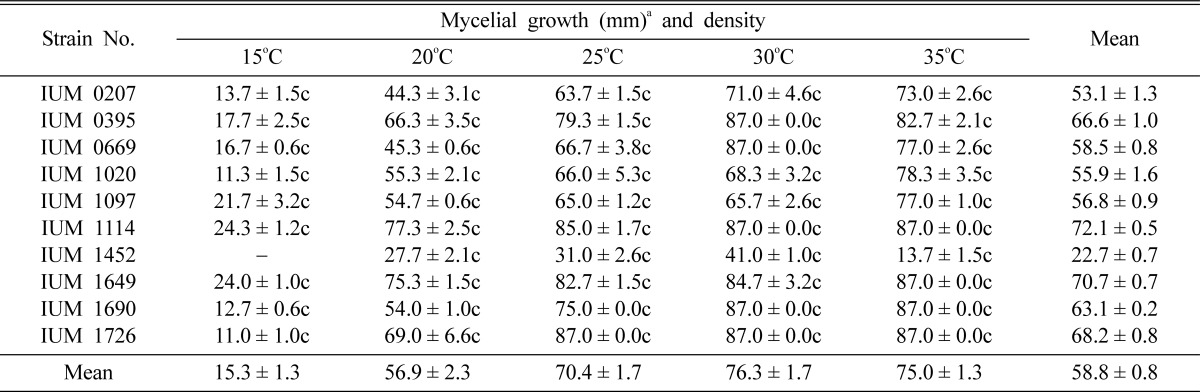
aMean of 4 replications. c: Compact, sc: Moderately compact, st: Moderately thin and t: Thin. Temperature and pH effects were conducted in potato dextrose agar medium (PDA).
Effect of pH
To screen suitable pH for mycelial growth and density of S. commune, the pH range of 5~9 was observed. The maximum and minimum growth was found at pH 5 and 9, respectively. In case of IUM1649, IUM1690 and IUM1726, the optimal growth was obtained at pH 5~6. Rest of the pH also showed good mycelial growth and density of different strains of S. commune (Table 4). Choi et al. (1999) reported that mycelial growth of Phellinus japonica and Phellinus linteus was optimal at pH 7 and 6~7, respectively. Shim et al. (2005) revealed that pH 7 is the most suitable for the mycelial growth of M. procera. Shim et al (2003) showed that optimal pH of Paecilomyces sinclairii was 8. Shim et al. (1997) also reported that the most favorable and most unfavorable pH of Grifola umbellata was 4 and 9, respectively. This result indicated that mushroom collected from different ecological origins may have different pH values for their optimal mycelial growth.
Table 4.
Effect of pH on the mycelial growth and density of different strains of Schizophyllum commune
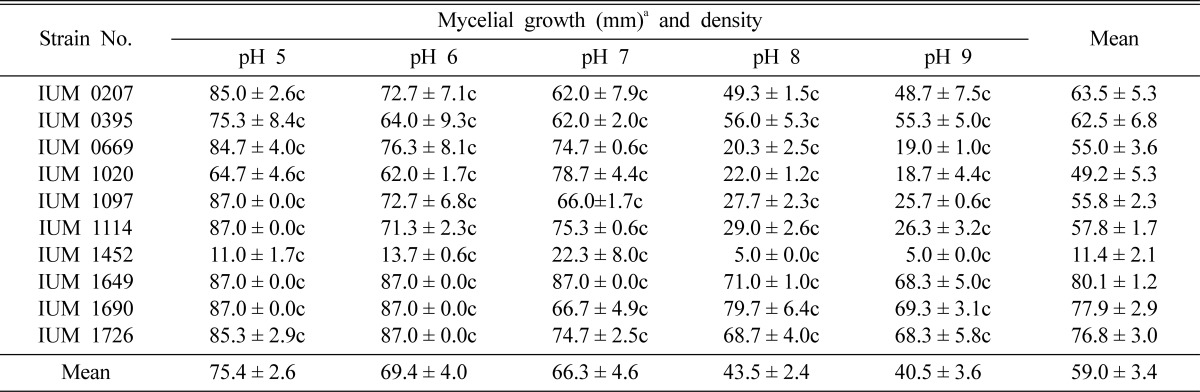
aMean of 4 replications. c: Compact, sc: Moderately compact, st: Moderately thin and t: Thin. Temperature and pH effects were conducted in potato dextrose agar medium (PDA).
Screening of favorable culture media
Ten diverse culture media were used to screen the optimal mycelial growth of 10 different strains of S. commune. According to mycelial growth, Hamada, Hennerberg, PDA and YM were the most suitable and Lily, Glucose triptone, Glucose peptone and Hoppkins were the most unfavorable for mycelial growth of S. commune. Mycelial density was found to be thin in Czapek Dox and Hoppkins, moderately compact in Glucose peptone, Glucose triptone, Lilly and YM as well as compact in PDA, Mushroom complete, Hamada and Hennerberg media (Table 5). This result is analogous to that of P. sinclairii and P. fumosoroseus which reported by Shim et al. (2003) where mycelial growth was optimal on Hamada medium. Shim et al. (2005) also reported that PDA, YM, Mushroom complete and Hamada were the most suitable, where Czapek dox and Glucose peptone were unfavorable to mycelial growth of M. procera.
Table 5.
Effect of media on the mycelial growth and density of different strains of Schizophyllum commune
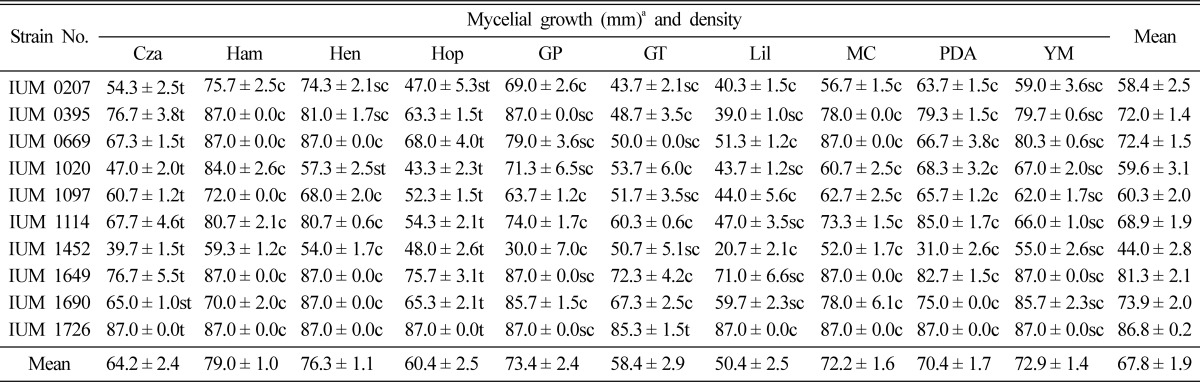
aMean of 4 replications. Cza: Czapek Dox, Ham: Hamada, Hen: Hennerberg, Hop: Hoppkins, GP: Glucose peptone, GT: Glucose tryptone, Lil: Lilly, MC: Mushroom complete, PDA: Potato dextrose agar and YM: Yeast-malt extract. c: Compact, sc: Moderately compact, st: Moderately thin and t: Thin.
Effect of carbon sources
Different carbon sources were used to monitor the most advantageous mycelial growth. The suitable mycelial growth was found in dextrin and fructose. Glucose, sucrose and xylose showed moderate mycelial growth of S. commune. The lowest growth of mycelium was obtained in lactose, mannose and sorbitol. Most of the carbon sources showed compact mycelial density. Considering mycelial phenotype, dextrin and fructose were the best among 10 carbon sources (Table 6). This result is partially similar to Shim et al. (2005) where they found that maltose, dextrin, sucrose and mannose were positive where lactose was highly negative. Shim et al. (1997) studied 19 carbon sources and reported that G. umbellata was favorable to used carbon sources except salicin, cellobiose and lactose. Shim et al. (2003) found that dextrin was suitable for mycelial growth of P. fumosoroseus which is parallel to our findings but they showed that in all carbon sources, mycelial density is thin where our result is contradictory.
Table 6.
Effect of carbon sources on the mycelial growth and density of different strains of Schizophyllum commune
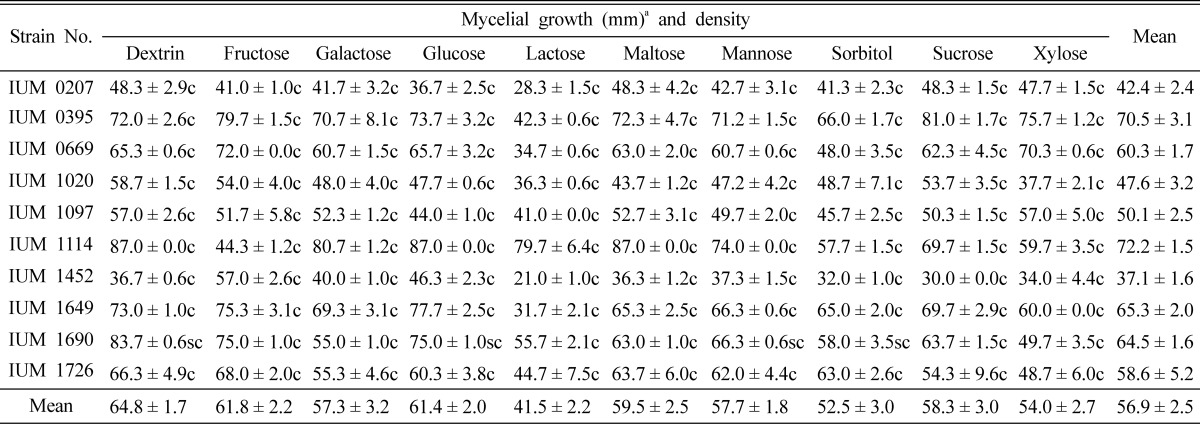
aMean of 4 replications. c: Compact, sc: Moderately compact, st: Moderately thin and t: Thin. Each carbon source was added to the basal medium at the concentration of 0.1M.
Effect of nitrogen sources
To find out the effects of nitrogen sources for mycelial growth, 10 nitrogen sources were used in this study. The most suitable nitrogen sources were calcium nitrate, potassium nitrate, and alanine, and the most unsuitable were ammonium phosphate, histidine, urea and arginine for mycelial growth of S. commune on the culture media. Calcium nitrate histidine and potassium nitrate showed moderately thin or thin mycelial density. Compact or moderately compact mycelial density was found in the rest of nitrogen sources (Table 7). Shim et al. (2005) reported that glycine was the most favorable nitrogen source which is reverse to our result. They also clarified that histidine, arginine and ammonium oxalate were the most unfavorable for the mycelial growth of M. procera on the culture media which is similar to our findings. Lee and Han (2005) showed that soytone, malt extract, yeast extract and bacto-peptone were the most favorable but NaNO3 and urea were the most unfavorable for the mycelial growth of Ramaria botrytis. In general, organic nitrogen sources are more effective than inorganic nitrogen sources.
Table 7.
Effect of nitrogen sources on the mycelial growth and density of different strains of Schizophyllum commune
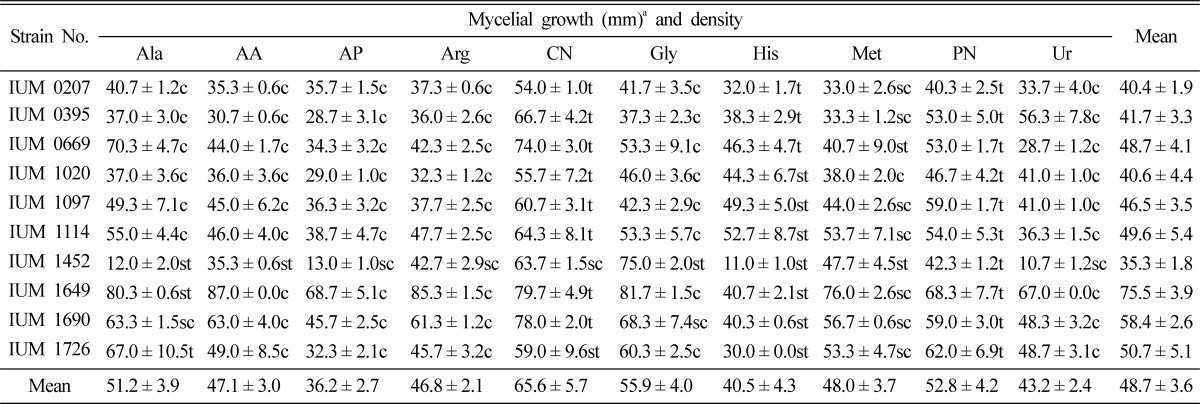
aMean of 4 replications. Ala: Alanine, AA: Ammonium acetate, AP: Ammonium phosphate, Arg: Arginine, CN: Calcium nitrate, Gly: Glycine, His: Histidine, Met: Methionine, PN: Potassium nitrate and Ur: Urea. Each nitrogen source was added to the basal medium at the concentration of 0.02M. c: Compact, sc: Moderately compact, st: Moderately thin and t: Thin.
To obtain factors affecting mycelial growth phenotype and density of 10 strains of S. commune, this research was conducted. In conclusion, it could be focused that the obtained results for vegetative growth and density of mycelia could be useful for promote extensive study of S. commune.
Acknowledgements
This work was supported by a research grant (no. 20070201080016) from Rural Development Administration in the Ministry of Agriculture and Forestry, Korea.
References
- 1.Cavazzoni V, Adami A. Exopolysaccharides produced by mycelial edible mushrooms. Ital J Food Sci. 1992;1:9–15. [Google Scholar]
- 2.Choi YJ, Hwang HK, Lee WH. The production of artificial fruiting body of Paecilomyces japonica. Korean J Mycol. 1999;27:87–93. [Google Scholar]
- 3.Iizasa T, Kamei K, Chiyo M, Suzuki M, Baba M, Toyosaki T, Hiroshima K, Ohwada H, Kanno S, Nishimura K, Fujisawa T. Colonization with Schizophyllum commune of localized honeycomb lung with mucus. Respiration. 2001;68:201–203. doi: 10.1159/000050493. [DOI] [PubMed] [Google Scholar]
- 4.Jong SC, Birgmingham JM. Medicinal benefits of the mushroom Ganoderma. Adv Appl Microbiol. 1992;37:101–134. doi: 10.1016/s0065-2164(08)70253-3. [DOI] [PubMed] [Google Scholar]
- 5.Jong SC, Birgmingham JM. Medicinal and therapeutic value of the shiitake mushroom. Adv Appl Microbiol. 1993;39:153–184. doi: 10.1016/s0065-2164(08)70595-1. [DOI] [PubMed] [Google Scholar]
- 6.Kuo M. Schizophyllum commune. Retrieved from the MushroomExpert.Com. 2003. Web site: http://www.mushroomexpert.com/hericium_erinaceus.html.
- 7.Lee TH, Han HH. Cultural characteristics for the enhanced mycelial growth of Ramaria botrytis. Mycobiology. 2005;33:12–14. doi: 10.4489/MYCO.2005.33.1.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Maziero R, Cavazzoni V, Bononi VLR. Screening of basidiomycetes for the production of exopolysaccharide and biomass in submerged culture. Rev Microbiol. 1999;30:77–84. [Google Scholar]
- 9.Shim JO, Son SG, Kim YH, Lee YS, Lee JY, Lee TS, Lee SS, Lee MW. The culture conditions affecting the mycelial growth of Grifola umbellata. Korean J Mycol. 1997;25:209–218. [Google Scholar]
- 10.Shim SM, Lee KR, Kim SH, Im KH, Kim JW, Lee UY, Shim JO, Lee MW, Lee TS. The optimal culture conditions affecting the mycelial growth and fruiting body formation of Paecilomyces fumosoroseus. Mycobiology. 2003;31:214–220. [Google Scholar]
- 11.Shim SM, Oh YH, Lee KR, Kim SH, Im KH, Kim JW, Lee UY, Shim JO, Shim MJ, Lee MW, Ro HS, Lee HS, Lee TS. The characteristics of culture conditions for the mycelial growth of Macrolepiota procera. Mycobiology. 2005;33:15–18. doi: 10.4489/MYCO.2005.33.1.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sung JM, Kim CH, Yang KJ, Lee HK, Kim YS. Studies on distribution and utilization of Cordyceps militaris and C. nutans. Korean J Mycol. 1993;21:94–105. [Google Scholar]
- 13.Sung JM, Moon HW, Park DH. Growth condition of liquid culture by Pleurotus ostreatus. Korean J Mycol. 1999;27:1–9. [Google Scholar]


