Abstract
This study examined the chemical composition of A. blasiliensis and the chemical structural properties of an immuno-stimulating polysaccharide. The amino acids, free sugars, and organic acids by HPLC and fatty acids by GC were analyzed. The immuno-stimulating substance from A. blasiliensis was extracted with hot water and purified by ethanol precipitation. It underwent ion exchange chromatography on DEAE-cellulose and gel filtration on Toyopearl HW 65F. Through GP-HPLC, the substance was found to be homogeneous. Its chemical structure was determined by 13C-NMR. Fatty acids, organic acids, and sugar alcohol composition consisted exclusively of linoleic acid, fumaric acid and mannitol, respectively. The amino acids were mainly glutamic acid, glycine, and arginine. By 13C-NMR analysis, the immuno-stimulating substance was identified as β-(1→3) (1→6)-glucan, composed of a backbone with (1→3)-linked D-glucopyranosyl residues branching a (1→6)-linked D-glucopyranosyl residue. The β-glucan from A. blasiliensis showed pronounced immuno-stimulating activity on the antibody-production ability of B-lymphocytes by the hemolytic suspension assay. In these results, A. blasiliensis was estimated to have potent pharmacological properties and potential nutritional values.
Keywords: Agaricus blasiliensis, Chemical composition, Immuno-stimulating substance
Mushrooms have received much interest with the realization that they are a good source of delicious food with high nutritional attributes and that some have medicinal values as well (Mizuno, 1993; Kawagishi, 1994).
Chemical evaluation of some edible mushrooms has been the object of many investigations in subjects as varied as metabolism, nutrition, and medicine (Diez and Alvarez, 2001; Sanmee et al., 2003). The fatty acids, organic acids, and amino acids of mushrooms were significantly evaluated and analyzed for their nutritive and medicinal effects (Longvah and Deosthale 1998; Stephen et al., 2004). Recently, the carbohydrates in mushrooms have been evaluated to be dietary fibers with medicinal effects (Mizuno and Kwai, 1992; Mizuno 1993). The carbohydrates in mushroom are chiefly composed of low molecular saccharides such as trehalose and mannitol.
Polysaccharides derived from mushrooms have emerged as an important class of bioactive substances. Their antitumor, immunological, anticomplementary, anticoagulant, hypoglycemic, and antiviral activities have been investigated (Furukawa, 1995; Mizuno, 1993). Mushroom polysaccharides have been investigated with regard to their chemical structures and biological activities. Among the various polysaccharides, b-glucans has been shown to express the most interesting biological effects (Franz, 1989).
One edible mushroom with this kind of polysaccharide is Agaricus blasiliensis (A. blazei; see Wasser et al., 2002 for taxonomic discussion), distributed originally in Brazil and presently cultivated in other countries such as Korea, Japan, and China. Its fruiting body is used as a health food and home remedy in these areas. Its fresh basidiocarp consists of 85~87% water. When dehydrated, it is rich in protein (40~45%) and carbohydrates (3~4%). It also contains dietary fibers (6~8%), lipids (3~4%), and vitamins, especially B1, B2, and niacin (Mizuno, 1995, 2002).
From soluble residues in water from the fruiting body of A. blasiliensis, glucan-protein complexes were isolated (Gonzaga et al., 2004, Kawagishi et al., 1989, 1990). This protein-polysaccharide complex was characterized by growth inhibition of sarcoma-180 implanted in mice, developing immuno-modulatory properties (Itoh et al., 1994), possibly due to immunological mechanisms involving the action of various immunocompetent cells (Kaneno et al., 2004; Nakajima et al., 2002; Fujimiya et al., 1999).
This study examined composition related to the nutritional and pharmacological properties of A. blasiliensis cultivated to the Korean conventional method using fermented straws (Park et al., 1996). It aimed to analyze the carbohydrates, amino acids, and organic acids present in A. blasiliensis. It also investigated the chemical properties and immuno-stimulating activity of its polysaccharide.
Materials and Methods
Materials
The fruiting body of Agaricus blasiliensis ASI 1174 was cultivated by the Korean conventional method (Park et al., 1996), dried, and milled. The strains were conserved at the National Institute of Agricultural Science and Technology (NIAST) of the Rural Development Administration (RDA) of Korea.
Free sugar analysis
The free sugars were extracted with 10 ml of 85% ethanol in 1.0-g samples on the basis of dry weight for 24 h. The free sugars were analyzed by HPLC at the following conditions: column, high-performance carbohydrate column (4.6 × 250 mm, Waters Co.); column temperature, 35℃; detection, refractive index (Waters Model 410); mobile phase, 75% acetonitrile; flow rate, 1.2 ml/min. The free sugars were measured by the comparison of standards using the Millennium Program (Waters Co.).
Organic acid analysis
To obtain free organic acids, the study extracted samples (1.0 g) with 80% EtOH (10 ml) and purified by Amberlite IRC-50 column. The obtained free organic acids were analyzed by HPLC at the following conditions: column, Supelcogel C-610H (7.8 × 300 mm, Supelco Co.); detection, UV 210 nm (Waters Model 486); mobile phase, 0.1% phosphoric acid; flow rate, 0.5 ml/min. Each free organic acid was quantified by the calibration curve of authentic organic acids (Supelco Co.).
Amino acid analysis
The amino acid composition of the samples was determined by hydrolyzing them with 6 N HCl for 24 h at 105℃ and then deriving the amino acids in a Waters Pico-Tag work station (Pico-Tag System, Waters Co.). The derivative amino acids were analyzed by liquid chromatograph composed of Waters 515 pumps, Waters 486 UV detector, and Reodyne injector (Waters Co.), equipped with Waters Pico-Tag column (3.9 × 150 mm, Waters Co.). Amino acids were identified by comparing retention times and areas with those of an authentic standard mixture.
Fatty acid analysis
The fatty acid composition of the total lipids, extracted from dried samples according to Hamilton et al. (1992), was determined as fatty acid methyl esters (FAMEs) by gas chromatography using Hewlett-Packard, Model 5890 Series II gas chromatograph (Agilent Co.) equipped with a fused silica capillary column (SP-2560, with a 0.25 mm diameter, 100 m length, and 0.20 µm film thickness; Supelco Ltd.). The sample was injected into the GC using a Hewlett-Packard 7673 autoinjector (Agilent Co.). Temperature of the oven was programmed at 140℃ for 5 min, followed by ramping to 240℃ at 4℃/min and kept there for 15 min. Helium at a flow rate of 20 cm/s was used as the carrier gas. The injection port and the flame ionization detector oven temperatures were set at 260℃. FAMEs were identified by comparing retention times with those of an authentic standard mixture (Supelco 37 Component FAME Mix, Supelco Co.).
Isolation and identification of the polysaccharide
The polysaccharide from A. blasiliensis was extracted with hot water, purified by ethanol precipitation, and subjected to ion exchange chromatography on DEAE-cellulose and gel filtration on Toyopearl HW 65F. The purity of substance was identified to be homogeneous by analysis of GP-HPLC (Cho et al., 1999). The chemical structure was determined by 13C-NMR. Homogeneity was investigated by gel permeation (GP)-HPLC. The HPLC conditions were as follows: column, Tosoh GMPW column (7.8 × 300 mm); column temperature, 25℃; detection, refractive index (RI-8010, Tosoh, Japan) and UV detector (280 nm); mobile phase, 0.1M NaCl; and flow rate, 1 ml/min. NMR spectra were recorded on Varian UNITY 300 (400MHz, USA). Fifty milligrams of polysaccharides were dissolved in 1 ml of D2O and lyophilized. This process was repeated twice for further deuterium exchange. Finally, the polysaccharide was dissolved in 0.7 ml of D2O (Aldrich, USA). All spectra were recorded at 25℃. 13C-NMR spectra of the polysaccharides were recorded using sodium 3-(trimethylsily)-propane-1-sulphonate-d4 (TSP, 0.0 ppm) as the internal standard.
Immuno-stimulating activity
To determine the antibody production of B-lymphocytes, the study obtained spleen cells by gently disrupting the mice spleen and repeatedly pipetting (Kim et al., 1987). The number of cells was adjusted to 5.0 × 106 cells/ml for B cell activation; 0.5 ml of the adjusted cell suspension was distributed on a 48 well microplate. LPS was used as a positive control at final concentrations of 10 and 100 µg/ml and polysaccharides were added into the cell suspension quadruplicates at final concentrations of 10 and 100 µg/ml. All cultures were incubated with rocking (7~10 complete cycles) in an atmosphere of 10% CO2, 7% O2, and 83% N2 at 4~5 psi for 3 days (Mishell and Dutton, 1967). The antibody production of B cells was determined by hemolytic suspension assay as described previously (Han et al., 1996).
Results and Discussion
This study detected the sugars glycerol, mannitol, inositol, and trehalose in the fruiting bodies of A. blasiliensis (Table 1). Mannitol (21.8 g/100 g) had the highest concentration on a dry weight basis, thus concurring with previous studies that showed mushrooms had high levels of mannitol (Sanmee et al., 2003). Mannitol is the most abundant polyol in the sorocarps of Basidiomycetes and Ascomycetes (Lewis and Smith, 1967). Because of their mannitol contents, mushrooms are useful as diabetic foods.
Table 1.
Carbohydrate composition of the fruiting body of Agaricus blasiliensis

Values are means±S.D. of three samples each in triplicate; n=3.
The study found high amounts of fumaric and lactic acids in the A. blasiliensis fruiting body (Table 2). The other organic acids, in descending order by quantity, were acetic, malic, pyroglutamic, formic, citric, and oxalic acids. Most mushrooms whether wild or cultivated are especially rich in malic acid (Mizuno and Kawai, 1992). Organic acids contribute to the taste and flavor of mushrooms (Mizuno and Kawai, 1992).
Table 2.
Organic acid composition of the fruiting body of Agaricus blasiliensis
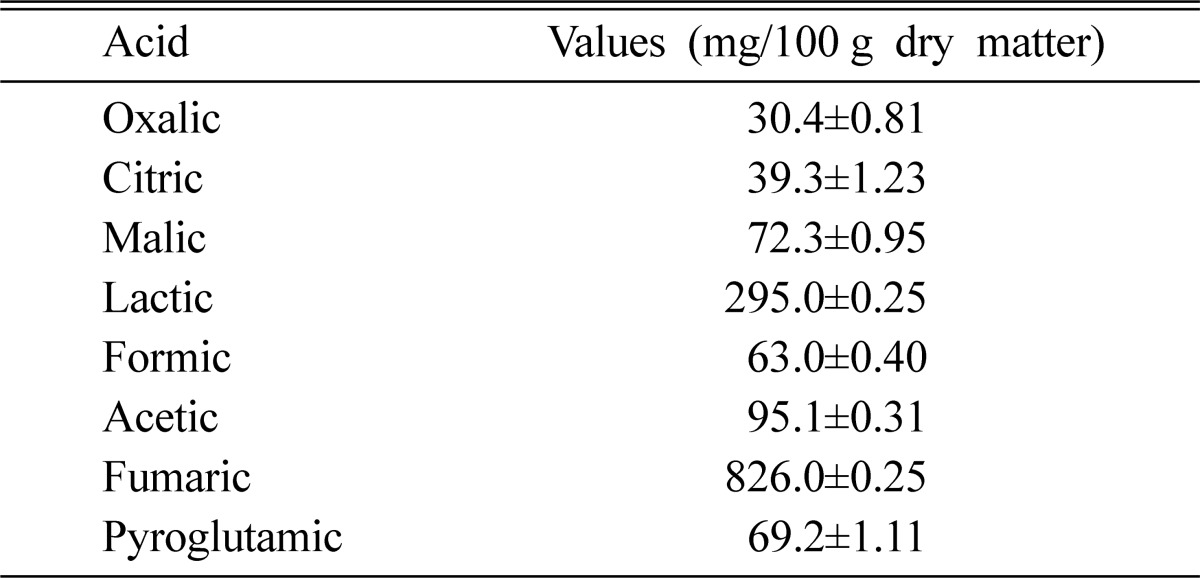
Values are means±S.D. of three samples each in triplicate; n=3.
The A. blasiliensis fruiting bodies were well balanced in their essential amino acid compositions (Table 3). The values were similar to those reported by Longvah and Deosthale (1988) for edible wild mushrooms, Schizophyllum commune and Lentinus edodes. The most abundant amino acid in A. blasiliensis fruiting body was arginine (9.3 µmole/g); followed by glycine, glutamic acid, proline, and alanine, in decreasing order. This study concurred with previous observations that mushrooms are deficient in sulphur-containing amino acids (Senatore et al., 1988; Senatore, 1990).
Table 3.
Amino acid composition of the fruiting body of Agaricus blasiliensis
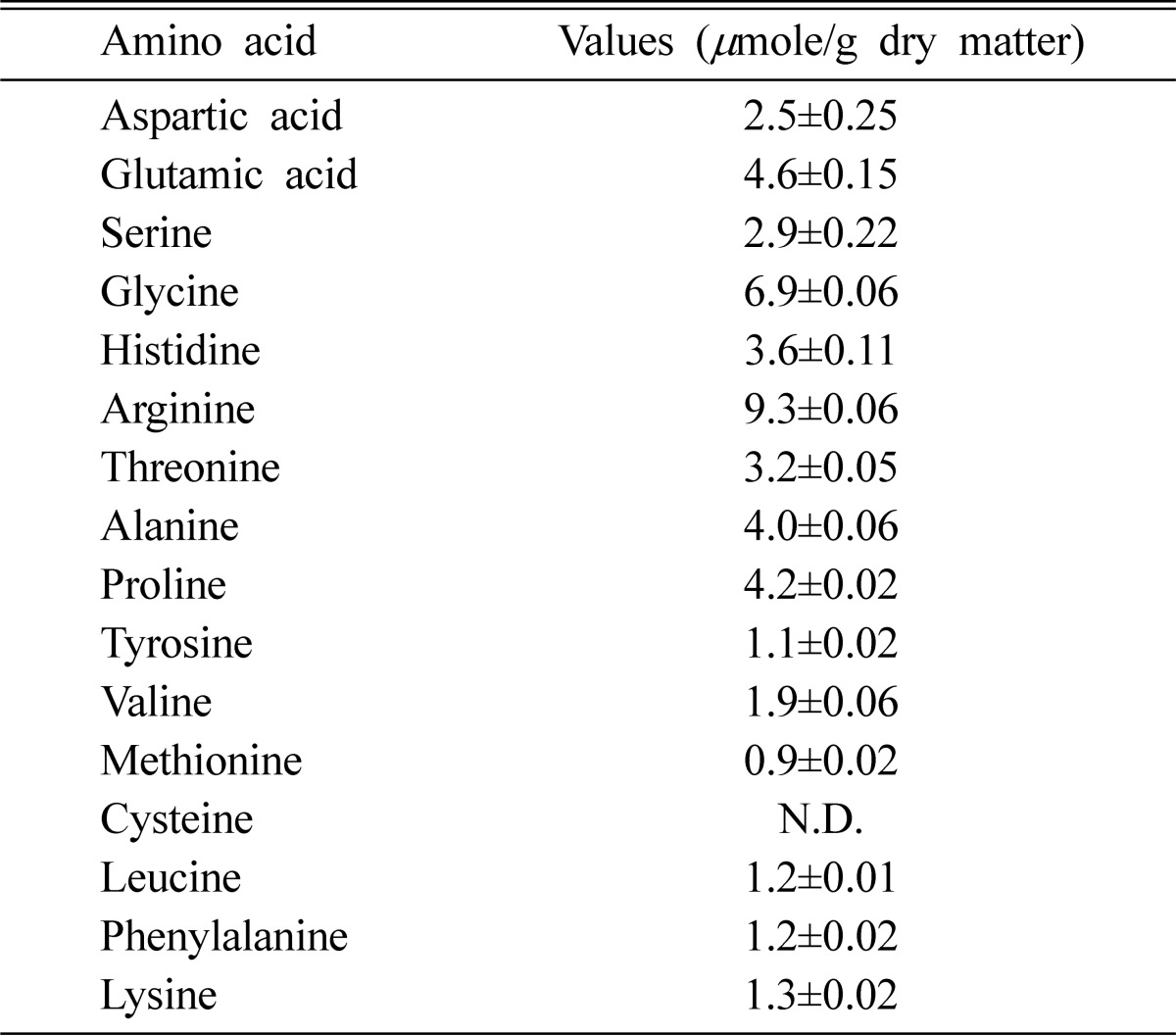
Values are means±S.D. of three samples each in triplicate; n=3.
The fruiting body of A. blasiliensis is rich in unsaturated fatty acids. In the oil extracted from the fruiting bodies, palmitic and linoleic acids accounted for more than 85% of the total fatty acids on a dry weight basis (Table 4). Linoleic acid accounted for 76% of the total fatty acids. The results concurred with previous studies that showed oils of some Basidiomycetes containing high levels of palmitic and linoleic acids (Sematore et al., 1988; Dembitsky et al., 1992; Diez and Alvarez, 2001). In this study, saturated acids accounted for 21.3% of the total fatty acids. The main saturated acids were pentadecanoic, palmitic, behenic, and stearic acids, with minute amounts of myristic and heptadecanoic acids.
Table 4.
Fatty acid composition of the fruiting body of Agaricus blasiliensisa
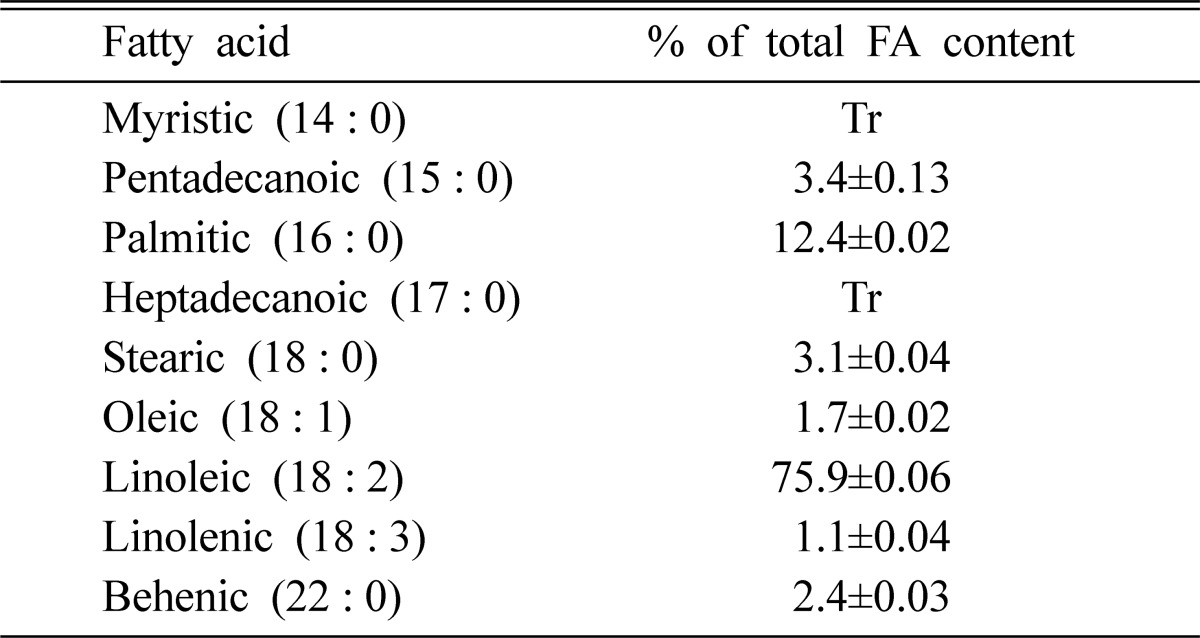
Tr, trace (≤ 0.05%).
aMeans of three determination±SD.
Therefore, the results showed that the A. blasiliensis fruiting body is a source of essential fatty acids such as linoleic acid (C18:2), with a good ratio of unsaturated/saturated fatty acids and n-6/n-3 fatty acids. It can be inferred that the A. blasiliensis fruiting body could be used as health food.
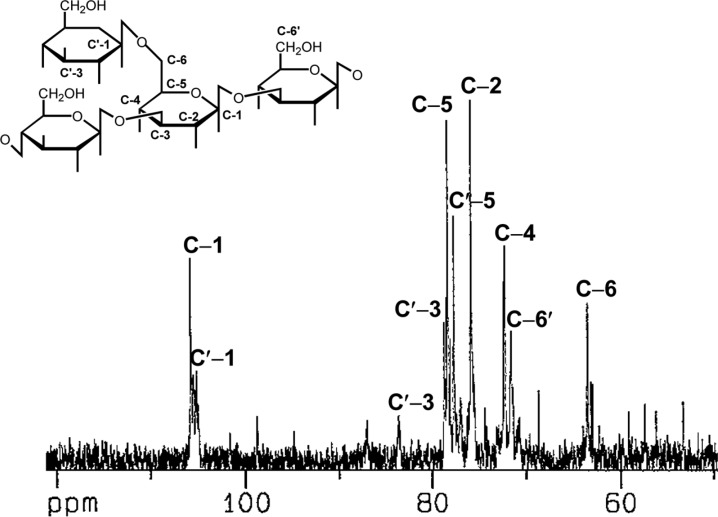
The 13C NMR spectrum of the polysaccharide (AG-6, Cho et al., 1999) isolated from the hot water extract of A. blasiliensis fruiting body (Fig. 1) provides useful information on its composition and sequence. The structure was in accordance with the values reported in previous literature (Schulz and Rapp, 1991; Gutierrez et al., 1996; Dong et al., 2002). Two closely located signals at 105.8 ppm (parts per million) and 105.2 ppm in the anomeric region indicated that all the anomeric carbons adopted the β configuration. The substituted C-6 signal could be identified at 71.7 ppm and the non-substituted C-6 signal at 63.5 ppm. The signals at 83.6 ppm and 78.7 ppm were assigned to the substituted C-3 and the non-substituted C-3, respectively. The other signals at 78.4 ppm, 77.7 ppm, 75.9 ppm, and 72.3 ppm suggested substituted adjacent glucose residues, non-substituted adjacent glucose residues with a branched β-glucopyranosyl, and two other non-branched β-glucopyranosyl residues, respectively. These results indicated β-(1→3)-(1→6)-glucan composed of a backbone with (1→3)-linked D-glucopyranosyl residues branching to a (1→6)-linked D-glucopyranosyl residue.
Fig. 1.
13C-NMR spectrum at 125MHz and a supposed structure of the polysaccharide isolated from the hot water extract of A. blasiliensis fruiting body at 25℃ in D2O.
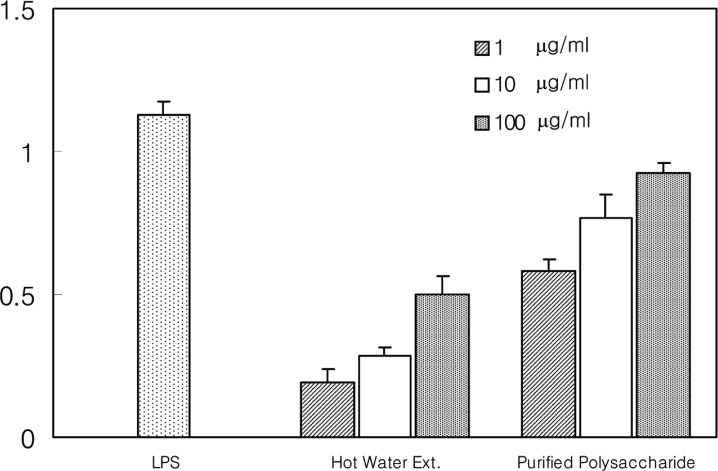
To find out its effect on the antibody production of B-lymphocytes, this study directly added the polysaccharide to the culture medium at concentrations of 1, 10, and 100 µg/ml and LPS (6.25 µg/ml) was used as the positive control. The polysaccharide increased the antibody production of B-lymphocytes (Fig. 2).
Fig. 2.
Immuno-stimulating activity on antibody forming ability of B-lymphocytes of the polysaccharide purified from hot-water extract of A. blasiliensis fruiting body by the hemolytic suspension assay.
The results of this study confirmed that the fruiting body of A. blasiliensis is a rich source of many important nutrients that appear to have positive effects on human health.
References
- 1.Cho SM, Park JS, Kim KP, Cha DY, Kim HM, Yoo ID. Chemical features and purification of immunostimulating polysaccharide from the fruit bodies of Agaricus blazei. Korean J Mycol. 1999;27:170–174. [Google Scholar]
- 2.Dembistsky VM, Shubina EE, Kashin AG. Phospholipid and fatty acid composition of some basidiomycetes. Phytochemistry. 1992;31:845–849. [Google Scholar]
- 3.Diez VA, Alvarez A. Compositional and nutritional studies on two wild edible mushrooms from northwest spain. Food Chem. 2001;75:417–422. [Google Scholar]
- 4.Dong Q, Yao J, Yang X, Fang J. Structural chariacterization of a water-soluble β-D-glucan from fruiting bodies of Agaricus blazei Murr. Carbohydr Res. 2002;337:1417–1421. doi: 10.1016/s0008-6215(02)00166-0. [DOI] [PubMed] [Google Scholar]
- 5.Franz G. Polysaccharides in pharmacy: current applications and future concepts. Planta Med. 1989;55:493–497. doi: 10.1055/s-2006-962078. [DOI] [PubMed] [Google Scholar]
- 6.Fujimiya Y, Susuki H, Katakura R, Ebina T. Tumor-specific cytotoxicity and immunopotentiating effects of relatively low molecular weight products derived from the basidiomycetes, Agaricus blazei Murrill. Anticancer Res. 1999;19:113–118. [PubMed] [Google Scholar]
- 7.Furukawa H. Mushroom science. Chem Biol. 1995;26:631–639. [Google Scholar]
- 8.Gonzaga MLC, Ricardo NMPS, Heatley F, Soares SA. Isolation and characterization of polysaccharides from Agaricus blazei Murill. Carbohydrate Polymers. 2005;60:43–49. [Google Scholar]
- 9.Gutiérrez A, Prieto A, Martinez A. Structural characterization of extracellular polysaccharides produced by fungi from the genus Pleurotus. Carbohydr Res. 1996;281:143–154. doi: 10.1016/0008-6215(95)00342-8. [DOI] [PubMed] [Google Scholar]
- 10.Han SB, Oh T, Yun YP, Min BK, Hyun BH, Kim HM. Rapid determination of in vivo and in vitro antibody responses by suspension hemolytic assay. J Pharmacol Toxicol Methods. 1996;36:33–40. doi: 10.1016/1056-8719(96)00070-6. [DOI] [PubMed] [Google Scholar]
- 11.Hamilton S, Hamilton RJ, Sewell P. Extraction of lipids and derivative formation. In: Hamilton S, Hamilton RJ, editors. Lipid Analysis. NY: Oxford University Press; 1992. pp. 47–48. [Google Scholar]
- 12.Itoh H, Amano H, Noda H. Inhibitory action of a (1-6)β-D-glucan-protein complex (FIII-2b) isolated from Agaricus blazei Murrill ("Himematsutake") on meth a fibrosarcoma bearing mice and its antitumor mechanism. Jpn J Pharmacol. 1994;66:265–271. doi: 10.1254/jjp.66.265. [DOI] [PubMed] [Google Scholar]
- 13.Kaneno R, Fontanari LM, Santos SA, Stasi LC, Filho ER, Eira AF. Effects of extracts from brazilian sun-mushroom (Agaricus blazei) on the NK activity and lymphoproliferative responsiveness of Ehrlich tumor-bearing mice. Food Chem Toxicol. 2004;42:909–916. doi: 10.1016/j.fct.2004.01.014. [DOI] [PubMed] [Google Scholar]
- 14.Kawagishi H. Cell-function regulating substance from mushrooms. Nippon Nogeikagaku Kaishi. 1994;68:1671–1677. [Google Scholar]
- 15.Kawagishi H, Inagaki R, Kano T, Shimura K, Ito H, Hagiwara T, Nakamura T. Fraction and antitumor activity of the water-insoluble residue of Agaricus blazei fruiting bodies. Carbohydrate Res. 1989;186:267–273. doi: 10.1016/0008-6215(89)84040-6. [DOI] [PubMed] [Google Scholar]
- 16.Kawagishi H, Inagaki R, Kano T, Shimura K, Ito H, Hagiwara T, Nakamura T. Formolysis of a potent antitumor (1-6)β-D-glucan-protein complex from Agaricus blazei fruiting bodies and antitumor activity of the resulting products. Carbohydrate Polymer. 1990;12:393–403. [Google Scholar]
- 17.Kim DH, Yang KH, Johnson KW, Holsapple MP. Suppression of in vitro antibody production by dimethylnitrosamine in mixed cultures of mouse primary hepatocytes and mouse splenocytes. Toxicol Appl Pharmacol. 1987;87:32–42. doi: 10.1016/0041-008x(87)90081-0. [DOI] [PubMed] [Google Scholar]
- 18.Lewis DH, Smith DC. Sugar alcohols (polyols) in fungi and green plants. New Phytologist. 1967;66:143–184. [Google Scholar]
- 19.Longvah T, Deosthale YG. Compositional and nutritional studies on edible wild mushroom from northeast India. Food Chem. 1998;63:331–334. [Google Scholar]
- 20.Mishell RI, Dutton RW. Immunization of dissociated spleen cell cultures from normal mice. J Exp Med. 1967;126:423–441. doi: 10.1084/jem.126.3.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mizuno T, Kwai M. Chemistry and Biochemistry of Mushroom Fungi. Tokyo: Gakai-shupan Center; 1992. [Google Scholar]
- 22.Mizuno T. Agaricus blazei Murill: Medicinal and dietary effects. Food Rev Int. 1995;11:167–172. [Google Scholar]
- 23.Mizuno T. Medicinal properties and clinical effects of culinary mushroom Agaricus blazei Murrill (Agaricomycetideae) Int J Med Mushrooms. 2002;4:299–312. [Google Scholar]
- 24.Mizuno T. Food function and medicinal effect of mushroom fungi. Food & Food Ingredients J. 1993;158:8–23. [Google Scholar]
- 25.Nakajima A, Ishita T, Koga M, Takeuchi T, Mazda O, Takeuchi M. Effect of hot water extract Agraricus blazei Murill on antibody-producing cells in mice. Int Immunopharmacol. 2002;2:1205–1211. doi: 10.1016/s1567-5769(02)00056-5. [DOI] [PubMed] [Google Scholar]
- 26.Park JS, Jang KY, Cha DY, Chun CS. Agricultural Biology Research, editors. National Institute of Agricultural Science & Technology. 1996. The study of cultivated physiology of Agaricus blazei; pp. 645–650. [Google Scholar]
- 27.Sanmee R, Dell B, Lumyong P, Izumori K, Lumyong S. Nutritive value of popular wild edible mushrooms from northern Thailand. Food Chem. 2003;82:527–532. [Google Scholar]
- 28.Schulz D, Rapp P. Properties of the polyalcohol prepared from the b-D-glucan schizophyllan by periodate oxidation and borohydride reduction. Carbohydr Res. 1991;222:223–231. doi: 10.1016/0008-6215(91)89020-g. [DOI] [PubMed] [Google Scholar]
- 29.Senatore F. Fatty acid and free amino acid content of some mushrooms. J Science Food Agriculture. 1990;51:91–96. [Google Scholar]
- 30.Senatore F, Dini A, Marino A, Schettino O. Chemical constituents of some basidiomycetes. J Sci Agric. 1988;45:335–345. [Google Scholar]
- 31.Stephen JMM, Mayunga HHN, Vitus AN, Isai TU. Amino acids composition of some Tanzanian mushrooms. Food Chem. 2004;86:179–182. [Google Scholar]
- 32.Wasser SP, Didukh MY, Amazonas MA, Nevo E, Stamets P, Eria AF. Is a widely cultivated culinary-medicinal royal sun Agaricus (the Himematsutake mushroom) indeed Agaricus blazei Murrill? Int J Med Mushrooms. 2002;4:267–290. [Google Scholar]