Abstract
Interoceptive sensitivity is an essential component of recent models of ‘the self’. Increased focus on the self (e.g. self-observation in a mirror) can enhance aspects of self-processing. We examined whether self-observation also enhances interoceptive sensitivity. Participants performed a heartbeat detection task while looking at their own face in a mirror or at a black screen. There was significant improvement in interoceptive sensitivity in the mirror condition for those participants with lower interoceptive sensitivity at baseline. This effect was independent of the order of conditions, gender, age, body mass index, habitual exercise and changes in heart rate. Our results suggest that self-observation may represent a viable way of manipulating individuals’ interoceptive sensitivity, in order to directly test causal relations between interoceptive sensitivity and exteroceptive self-processing.
Introduction
Recent models of the self have emphasised the fundamental role of afferent interoceptive signals, which provide information about the physiological state of the body. Interoceptive body-mapping is thought to be the foundation of the elementary feelings that we exist (Damasio, 2010) and it is further proposed that the remapping of interoceptive signals in the cortex - underpins our sense of self (Craig, 2010). However, individuals differ in the extent to which they are consciously aware of internal body states. Individual ‘interoceptive sensitivity’ is usually assessed behaviorally with a heartbeat detection task (Schandry, 1981; Whitehead & Drescher, 1980). A substantial body of research has studied the behavioral correlates of differences in interoceptive sensitivity, particularly in relation to emotional experience. For example, individuals with high interoceptive sensitivity have been shown to report more subjective emotional arousal for the same level of objective bodily arousal, despite reporting similar valence for the emotion (Dunn et al., 2010; Wiens, Mezzacappa & Katkin, 2000). Interoceptive sensitivity has also been linked to several clinical conditions, including a positive association between high interoception, anxiety and panic disorder (see Domschke, Stevens, Pfleiderer & Gerlach, 2010, for a review). However, low interoception may be equally significant and has been recently related to anorexia nervosa (Pollatos et al., 2008), alexithymia (Herbert, Herbert & Pollatos, 2011) and moderate depression (Dunn, Dalgleish, Ogilvie & Lawrence, 2007). There is also evidence for important links with cognition, as shown by the way in which interoceptive sensitivity modulates intuitive decision-making (Dunn et al., 2010; Werner, Jung, Duschek & Schandry, 2009), probably because ‘gut feelings’ depend upon preconscious bodily signals. In a potentially similar manner, high interoceptive sensitivity is associated with both responsiveness to masked fear conditioning (Katkin, Wiens & Ohman, 2001) and implicit memory for emotionally laden words (Werner, Peres, Duschek & Schandry, 2010).
Unfortunately, research on interoceptive sensitivity has been unable to establish directions of causality (Ehlers & Breuer, 1992), for example, whether high interoceptive sensitivity is the cause or the result of anxiety, or whether the two co-occur without a causal relationship, because experimental attempts at manipulation have generally been ineffective. Similarly, experimental attempts to alter people’s interoceptive sensitivity have generally been ineffective. Fairclough and Goodwin (2007) found no improvement when participants engaged in a yogic breathing pattern, although interoceptive sensitivity (for women only) was reduced by a mental stressor (possibly due to fatigue or divided attention). Khalsa, et al. (2008) likewise, found neither evidence of heightened interoceptive sensitivity in highly experienced meditators, nor any improvement after Ujjai breathing. Similarly, Stevens, et al. (2011) found no effect of anticipated social anxiety. Interoceptive sensitivity has therefore been considered a robust trait variable with good test-retest reliability (Mussgay, Klinkenberg & Ruddel, 1999). The aforementioned studies, however, compared changes in mean interoceptive sensitivity for the whole group of participants, between conditions, but did not investigate whether baseline individual differences in sensitivity (e.g. high versus low accuracy) might have influenced the extent of change for individuals under the experimental conditions. Given the substantial and growing literature on interoception, and its link with clinical symptoms, the ability to manipulate interoceptive sensitivity experimentally and to record the resulting effects on other, supposedly linked, aspects of self-processing and self-experience would be highly desirable.
Our experimental attempt to alter interoceptive sensitivity was prompted by studies in social psychology which have long used mirror self-observation as an attempt to increase the so-called ‘self-focus’ of individuals (Fejfar & Hoyle, 2000). For example, self-reported arousal is less influenced by experimental instructions when participants are exposed to a mirror (Scheier, Carver & Gibbons, 1979). Similarly, when given mirror access, participants report fewer illusory symptoms in response to a placebo (Gibbons, Carver & Scheier, 1979). An early study (Weisz, Balazs & Adam, 1988) attempted to manipulate interoceptive sensitivity, using the (apparently accidental) presence of a mirror to increase self-focus during two different heartbeat detection tasks, but did not provide conclusive evidence. Participants had to tap with their index finger immediately after each beat (heartbeat tracking) or detect discrepancies between the rhythm of their heartbeat and the rhythm of presented tones (heartbeat discrimination). The mere presence of a mirror improved performance in the discrimination, but not in the tapping task. However, that study did not control for whether participants truly looked at themselves in the mirror, nor did it investigate the potentially differential effects on individuals with high or low interoceptive sensitivity.
Our study aimed to investigate interoceptive sensitivity from the perspective of ‘the self’ by studying the effect of self-observation as a means of heightening interoceptive accuracy. We used instructed and controlled self-observation and employed a well-validated heartbeat detection task (Schandry, 1981), which is sensitive to individual differences (Ehlers & Breuer, 1992; Domschke et al., 2010; Dunn et al., 2010). Self versus non-self observation was investigated by requiring participants to look into a mirror or at a non-reflective screen. Reported confounds of heartbeat detection tasks were recorded - gender, change in heart rate, age, body mass index (BMI) and level of exercise (Cameron, 2001).
Methods
Participants
Data for 129 visitors at the Science Museum, London was analyzed (aged 10 to 74 years, Table 1) after excluding 10 for not following the instructions and 14 for incomplete data. The study was approved by the Department of Psychology Ethics Committee, Royal Holloway, University of London. Written consent was obtained for all participants, including parental consent for those under 18 years of age.
Table 1.
Descriptive statistics for all recorded variables
| Variable | All participants (n=129) | High interoception group (n=65) | Low interoception group (n=64) |
|---|---|---|---|
| Mean IS1 baseline (SD) | 0.64(0.19) [skewness=−.35, kurtosis=−.27] |
0.80(0.10) | 0.49(0.13) |
| Mean IS1 mirror (SD) | 0.66(0.19) [skewness=-.25, kurtosis=-.53] |
0.79(0.12) | 0.52(0.15) |
| Mean HR2 Baseline (SD) | 75.8(10.5) | 72.0(9.7) | 79.6(10) |
| Mean HR2 Mirror (SD) | 75.6(10.8) | 71.9(10.2) | 79.4(10.1) |
| % who performed the Baseline Condition first |
52% | 49% | 55% |
| % Male | 43% | 48% | 38% |
| Mean age yrs (SD) | 28.7(13.5) | 29.6(13.5) | 27.8(13.6) |
| Mean BMI3 (SD) | 23.1(4.3) (for n=119) | 23.6(4.0) (for n=59) | 22.5(4.5) (for n=60) |
| Mean level of exercise hrs/week (SD) |
3.4(4.3) | 3.7(3.8) | 3.1(4.8) |
Interoceptive Sensitivity (Standard Deviation)
Heart Rate
Body Mass Index
Procedure
All instructions were delivered, and behavioral responses recorded, using Presentation software (Neurobehavioral Systems, Albany, CA) on a standard desktop PC. After giving informed consent, participants’ gender, age, height, weight and their level of habitual exercise (hours/week) were recorded. Heartbeat signals were acquired with a piezo-electric pulse transducer, fitted to the participant’s left index finger and connected to a physiological data unit (26T PowerLab, AD Instruments), sampling at 1 kHz, which recorded the derived electrical signal onto a second PC running LabChart6 software (AD Instruments). Instructions for the Mental Tracking Method (Schandry, 1981) were presented over noise-attenuating headphones. The onset and offset of each heartbeat counting trial were cued by the words “go” and “stop”, presented audiovisually. We used a standard instruction (Ehlers & Breuer, 1992) whereby participants were asked to concentrate hard and try to silently count their own heartbeats, simply by “listening” to their bodies, without taking their pulse. In the baseline condition they were required to gaze at a black screen (30cm by 50cm) placed on an easel at eye level and at a distance of 40cm. In the mirror condition they were explicitly instructed to gaze at the reflection of their own face in a similarly sized, and positioned, mirror. Each condition consisted of three intervals (25s, 35s and 45s), presented in random order, after one training interval. No feedback was given. The order of conditions was counterbalanced.
Data Reduction
LabChart6 was employed to identify and count the number of R-wave peaks on the heart trace recorded for each participant in each trial, as well as to calculate the average heart rates for each trial (Jennings, et al. 1981). Every heart trace was visually inspected for artefacts and the number of R-wave peaks was recounted manually if necessary. Participants (n=14) were excluded where artefacts created uncertainty about the number of recorded beats. Interoceptive sensitivity was calculated for baseline and mirror conditions as {1/n Σ [1 − (|recorded heartbeats − counted heartbeats|/recorded heartbeats)]} where n is the number of trials (Schandry, 1981). Higher scores indicate higher interoceptive sensitivity.
Results
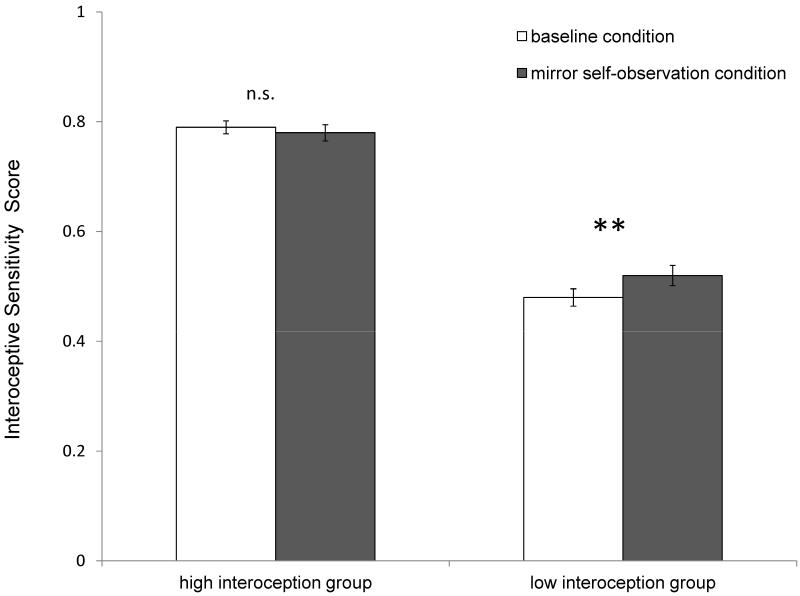
We performed a median split analysis of the interoceptive sensitivity scores (median = 0.66) to directly contrast performance of the groups with low and high interoceptive sensitivity (see Table 1). We analysed using a mixed-design ANOVA, with (baseline vs. mirror) as the within-subjects factor and the order of conditions (baseline, followed by mirror, or the reverse), gender, and interoception group as between-subjects factors. The change in heart rate between conditions, age, level of habitual exercise and BMI, for each individual, were entered as covariates. Levene’s test of equality of error variances and Box’s test of equality of covariance matrices were non-significant. The main effect on interoceptive sensitivity of the two conditions (baseline vs. mirror) was not significant F(1, 107) = 0.00, p = .96,. However, the interaction of experimental condition by interoception group was significant F(1, 107) =6.70, p = .01, η2 = 0.06 (Figure 1) indicating that self-observation significantly improved interoceptive sensitivity for the low interoception group t(63) = −3.46, p = .001, but not for the high interoception group t(64) = 0.64, p = .52. There were no significant interactions between the experimental condition and gender F(1, 107) = 1.63, p = .21, order of presentation of the two conditions F(1, 107) = 0.68, p = .41, change in heart rate between conditions F(1, 107) = 0.15, p = .70, age F(1, 107) = 0.00, p = .98, exercise F(1, 107) = 0.54, p = .46, or BMI F(1, 107) = 0.16, p = .90. The main effects of gender F(1, 107) = 0.17, p = .68, and of order of conditions F(1, 107) = 3.82, p = .05, were not significant
Figure 1.
Mean Interoceptive Sensitivity across conditions for the high and low interoceptive sensitivity groups. Error bars represent S.E.M.
To investigate possible differences in arousal between the baseline and mirror conditions, the same ANOVA design (minus the change in HR) was used with mean heart rate as the dependent variable. Levene’s test of equality of error variances and Box’s test of equality of covariance matrices were non-significant. The main effect of condition on heart rate was non-significant F(1, 108) = 0.02, p = .90, showing that heart rates did not change significantly between the two conditions. There were no significant interactions of condition with interoception group F(1, 108) = 0.42, p = .52, gender F(1, 108) = 0.07, p = .79, order of conditions (F(1, 108) = 1.13, p = .29, exercise F(1, 108) = 0.00, p = 0.99, or BMI F(1, 108) = 0.58, p = .45, or age F(1, 108) = 3.88, p = .05. The main effect of gender F(1, 108) = 1.24, p = .27, and order of conditions F(1, 108) = 2.55, p = .11 were both non-significant. We did observe, as expected, a main effect of interoception group F(1, 108) = 21.3, p < .001, η2 = 0.17. Mean heart rate was significantly lower in the high interoception group because heart rate was negatively correlated with interoceptive sensitivity r = −.28, p = .001, in the baseline, a result which has been reported previously (Cameron, 2001; Fairclough & Goodwin, 2007; Knapp-Kline & Kline, 2005; Stevens et al., 2011).
Discussion
We compared interoceptive sensitivity measured during mirror self-observation and at baseline. Individuals with above median interoceptive awareness showed no improvement while looking into a mirror but those with poorer accuracy at baseline showed a significant improvement in interoceptive sensitivity during self-observation. This effect was independent of the order in which the conditions were presented, gender, age, body mass index, the participant’s habitual level of exercise, or change in heart rate between the two conditions. Our results contrast with Weisz et al. (1988) who found a learning effect between conditions. Given that self-focus decreases available processing resources (Panayiotou & Vrana, 1998), it seems improbable that the improvement we found during self-observation can be explained by reduced task demands. The result is also unlikely to be attributable to higher arousal in the mirror condition (Van der Does, Van Dyk & Spinhoven, 1997) because heart rates did not change significantly, for either group, between the two conditions.
It is possible that our analysis has uncovered an effect that was not identified in previous studies. Past research has focused on the effects of experimental treatments on the mean interoceptive sensitivity of the particular populations tested, without considering the potentially different effects of the experimental manipulation on participants with high and low interoceptive sensitivity. For example, attempts to enhance bodily self-focus, e.g. using a yogic breathing pattern (Khalsa et al., 2008; Fairclough & Goodwin, 2007) or a mirror (Weisz et al., 1988), reported interoceptive sensitivity means for the whole group, but not did not examine differential effects for individuals with low or high interoceptive sensitivity at baseline. In common with Weisz et al. (1988) we found no significant effect of the mirror vs. baseline condition, in heartbeat tracking, for our participants taken as a whole. However, we demonstrate a significant effect of self-observation for those participants with low baseline interoceptive sensitivity.
Our results suggest there is scope for experimental manipulations of interoceptive sensitivity. While our manipulation resulted in improved awareness only for those with low baseline interoceptive sensitivity, manipulating interoceptive awareness in general might have important clinical applications for patients whose conditions are associated with abnormal interoceptive sensitivity, as both low and high interoceptive sensitivity are associated with different clinical conditions.
The present study shows how exteroception (the perception of one’s body from the outside, such as when viewing one’s face) may interact with interoception. This finding extends recent results on the interaction between interoception and exteroception, which showed that interoceptive sensitivity plays an active modulatory role in weighting and integrating exteroceptive percepts relating to the body (Tsakiris, Tajadura-Jiménez, & Costantini, 2011). Here, mirror self-observation, which relies on exteroception, enhanced low interoceptive sensitivity. Taken together, these interactions between awareness of the self from within and from the outside point to the integrative role of brain structures such as the right anterior insula, which is thought of as a convergence zone where interoceptive and exteroceptive signals are integrated, underpinning the awareness of the sentient self (Craig, 2010). Activity in this area correlates with interoceptive sensitivity (Critchley, Wiens, Rotshtein, Öhman & Dolan, 2004) but is also engaged during self-face recognition (Devue & Bredart, 2011). The use of mirror self-observation while people are performing the heartbeat detection task might result in enhanced activity in the insula. Such enhancement can, in turn, facilitate self-processing. It is possible, for example, that increased activity in the insula as a result of our experimental manipulation of mirror observation could have the effect of top-down gating of attention to other aspects of self-processing, resulting in the individual’s improved sensitivity to his/her own interoceptive signals, as found in our study. Top-down gating of attention could also explain why the effect was not significant in individuals with high baseline interoceptive sensitivity, who are presumably better able to attend to internal states of their bodies, even in the absence of any externally-driven focus of attention to the self.
Our study has several limitations as we did not screen for medical conditions (Cameron, 2001) nor for anxiety (Domschke et al., 2010). We did not take account of participants’ possible use of time-estimation strategies (Dunn et al., 2010; Ehlers & Breuer, 1992) or respiratory manoeuvers (Weisz et al., 1988). However, it is unlikely that either of the two latter potential confounds could account for a change in heartbeat detection between conditions, as they would apply equally in both. Further research is required to discover whether the effect of self-focus we discovered is specific to focusing on physical as opposed to more abstract dimensions of oneself (such as self-relevant words) and whether it depends on looking at one’s own, as opposed to another person’s face.
Overall, our results provide additional evidence that the sense of bodily self results from the integration of both interoceptive and exteroceptive sensory inputs (Craig, 2010). That low interoceptive sensitivity can be enhanced by mirror self-observation, complements other recent findings that accuracy in perception of our external bodies interacts with our awareness of the body from within (Fotopoulou et al., 2012; Tsakiris et al., 2011), showing that the ‘self’ is a complex result of interoceptive and exteroceptive percepts, acting upon and reinforcing each other.
Acknowledgments
ESRC First Grant RES-061-25-0233, and the European Research Council (ERC-2010-StG-262853) under the FP7 (to M.T.).Volkswagen Foundation ‘European Platform for Life Sciences, Mind Sciences and the Humanities’ grant for the ‘Body-Project’ (to A.K. and M.T.). We would like to thank the Science Museum, London, for hosting our experiment at the “Who am I?” Gallery.
Footnotes
The Authors declare no conflict of interest.
References
- Cameron OG. Interoception: The inside story – A model for psychosomatic processes. Psychosomatic Medicine. 2001;63:697–710. doi: 10.1097/00006842-200109000-00001. [DOI] [PubMed] [Google Scholar]
- Craig AD. The sentient self. Brain Structure and Function. 2010;214:563–577. doi: 10.1007/s00429-010-0248-y. [DOI] [PubMed] [Google Scholar]
- Critchley HD, Wiens S, Rotshtein P, Öhman A, Dolan RJ. Neural systems supporting interoceptive awareness. Nature Neuroscience. 2004;7:189–195. doi: 10.1038/nn1176. [DOI] [PubMed] [Google Scholar]
- Damasio AR. Self comes to mind. Constructing the conscious brain. Heineman; London: 2010. [Google Scholar]
- Devue C, Bredart S. The neural correlates of visual self-recognition. Consciousness and Cognition. 2011;20:40–51. doi: 10.1016/j.concog.2010.09.007. [DOI] [PubMed] [Google Scholar]
- Domschke K, Stevens S, Pfleiderer B, Gerlach AL. Interoceptive sensitivity in anxiety and anxiety disorders: an overview and integration of neurobiological findings. Clinical Psychology Review. 2010;30:1–11. doi: 10.1016/j.cpr.2009.08.008. [DOI] [PubMed] [Google Scholar]
- Dunn BD, Dalgleish T, Ogilvie AD, Lawrence A. Heartbeat perception in depression. Behaviour Research and Therapy. 2007;45:1921–1930. doi: 10.1016/j.brat.2006.09.008. [DOI] [PubMed] [Google Scholar]
- Dunn BD, Galton HC, Morgan R, Evans D, Oliver C, Meyer M, Dalgleish T. Listening to your heart: How interoception shapes emotion experience and intuitive decision making. Psychological Science. 2010;21:1835–1844. doi: 10.1177/0956797610389191. [DOI] [PubMed] [Google Scholar]
- Ehlers A, Breuer B. Increased cardiac awareness in panic disorder. Journal of Abnormal Psychology. 1992;101:371–382. doi: 10.1037//0021-843x.101.3.371. [DOI] [PubMed] [Google Scholar]
- Fairclough SH, Goodwin L. The effect of psychological stress and relaxation on interoceptive accuracy: Implications for symptom perception. Journal of Psychosomatic Research. 2007;62:289–295. doi: 10.1016/j.jpsychores.2006.10.017. [DOI] [PubMed] [Google Scholar]
- Fejfar MC, Hoyle RH. Effect of private self-awareness on negative affect and self-referent attribution: A quantitative review. Personality and Social Psychology Review. 2000;4:132–142. [Google Scholar]
- Fotopoulou A, Jenkinson P, Tsakiris M, Haggard P, Rudd T, Kopelman M. Mirror-view reverses somatoparaphrenia: Dissociation between first- and third-person perspectives on body ownership. Neurophyschologia. 2012;49:3946–3955. doi: 10.1016/j.neuropsychologia.2011.10.011. [DOI] [PubMed] [Google Scholar]
- Gibbons FX, Carver CS, Scheier MF. Self-focused attention and the placebo effect: Fooling some of the people some of the time. Journal of Experimental Social Psychology. 1979;15:263–274. [Google Scholar]
- Herbert BM, Herbert C, Pollatos O. On the relationship between interoceptive awareness and alexithymia: Is interoceptive awareness related to emotional awareness? Journal of Personality. 2011;79:1149–1175. doi: 10.1111/j.1467-6494.2011.00717.x. [DOI] [PubMed] [Google Scholar]
- Jennings JR, Berg WK, Hutcheson JS, Obrist P, Porges S, Turpin G. Publication guidelines for heart rate studies in man. Psychophysiology. 1981;18:226–231. doi: 10.1111/j.1469-8986.1981.tb03023.x. [DOI] [PubMed] [Google Scholar]
- Katkin ES, Wiens S, Ohman A. Nonconscious fear conditioning, visceral perception, and the development of gut feelings. Psychological Science. 2001;12:366–370. doi: 10.1111/1467-9280.00368. [DOI] [PubMed] [Google Scholar]
- Khalsa SS, Rudrauf D, Damasio AR, Davidson RJ, Lutz A, Tranel D. Interoceptive awareness in experienced meditators. Psychophysiology. 2008;45:671–677. doi: 10.1111/j.1469-8986.2008.00666.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knapp-Kline K, Kline JP. Heart rate, heart rate variability, and heartbeat detection with the method of constant stimuli: slow and steady wins the race. Biological Psychology. 2005;69(3):387–396. doi: 10.1016/j.biopsycho.2004.09.002. [DOI] [PubMed] [Google Scholar]
- Mussgay L, Klinkenberg N, Ruddel H. Heart beat perception in patients with depressive, somatoform and personality disorders. Journal of Psychophysiology. 1999;13:27–36. [Google Scholar]
- Panayiotou G, Vrana SR. Effect of self-focused attention on the startle reflex, heart rate, and memory performance among socially anxious and nonanxious individuals. Psychophysiology. 1998;35:328–336. doi: 10.1017/s0048577298960875. [DOI] [PubMed] [Google Scholar]
- Pollatos O, Kurtz A, Albrecht J, Schreder T, Kleemann A, Schopf V, Schandry R. Reduced perception of bodily signals in anorexia nervosa. Eating Behaviors. 2008;9:381–388. doi: 10.1016/j.eatbeh.2008.02.001. [DOI] [PubMed] [Google Scholar]
- Schandry R. Heart beat perception and emotional experience. Psychophysiology. 1981;18:483–488. doi: 10.1111/j.1469-8986.1981.tb02486.x. [DOI] [PubMed] [Google Scholar]
- Scheier MF, Carver CS, Gibbons FX. Self-directed attention, awareness of bodily states and suggestibility. Journal of Personality and Social Psychology. 1979;37:1576–1588. doi: 10.1037//0022-3514.37.9.1576. [DOI] [PubMed] [Google Scholar]
- Stevens S, Gerlach AL, Cludius B, Silkens A, Craske MG, Hermann C. Heartbeat perception in social anxiety before and during speech anticipation. Behaviour Research and Therapy. 2011;49:138–143. doi: 10.1016/j.brat.2010.11.009. [DOI] [PubMed] [Google Scholar]
- Tsakiris M, Tajadura-Jiménez A, Costantini C. Just a heartbeat away from one’s body: Interoceptive sensitivity predicts malleability of body-representations. Proceedings of the Royal Society B Series. 2011;278:2470–2476. doi: 10.1098/rspb.2010.2547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van der Does AJW, Van Dyk R, Spinhoven P. Accurate heartbeat perception in panic disorder: fact and artefact. Journal of Affective Disorders. 1997;43:121–130. doi: 10.1016/s0165-0327(96)01414-0. [DOI] [PubMed] [Google Scholar]
- Weisz J, Balazs L, Adam G. The influence of self-focused attention on heartbeat perception. Psychophysiology. 1988;25:193–199. doi: 10.1111/j.1469-8986.1988.tb00987.x. [DOI] [PubMed] [Google Scholar]
- Werner NS, Jung K, Duschek S, Schandry R. Enhanced cardiac perception is associated with benefits in decision-making. Psychophysiology. 2009;46:1123–1129. doi: 10.1111/j.1469-8986.2009.00855.x. [DOI] [PubMed] [Google Scholar]
- Werner NS, Peres I, Duschek S, Schandry R. Implicit memory for emotional words is modulated by cardiac perception. Biological Psychology. 2010;85:370–376. doi: 10.1016/j.biopsycho.2010.08.008. [DOI] [PubMed] [Google Scholar]
- Whitehead WE, Drescher VM. Perception of gastric contractions and self-control of gastric motility. Psychophysiology. 1980;17:552–558. doi: 10.1111/j.1469-8986.1980.tb02296.x. [DOI] [PubMed] [Google Scholar]
- Wiens S, Mezzacappa S, Katkin ES. Heartbeat detection and the experience of emotions. Cognition and Emotion. 2000;14:417–427. [Google Scholar]