Abstract
Two new tryptophan derivatives, N-sulfonyl-L-tryptophan (tryptorheedei A) (1) and 3-(N-sulfonylindolyl)-D-lactic acid (tryptorheedei B) (2) together with the known 5-O-β-D-glucopyranosyl-2-hydroxyphenylacetic acid (3), 1-O-methylglucopyranoside, entadamide A, homogentisic acid and 3-O-β-D-glucopyranosyl-β-sitosterol, were isolated from the seed kernels of Entada rheedei (Mimosaceae). Their structures were established using 1D and 2D NMR spectroscopy, mass spectrometry and by comparison with spectroscopic data reported in the literature. Compounds 1 and 2 showed no toxicity to TZM and Human PBMC cells. Both compounds 1 and 2 were found to promote early infection events in HIV, likely by inhibiting the enzyme indolamine 2,3-dioxygenase (IDO) and preventing tryptophan depletion. Inhibition of IDO acutely in HIV infection inhibits viral replication, but chronic activation of IDO leads to immune impairment in AIDS. IDO is also the gatekeeper enzyme for kynurenine metabolism, a pathway involved in serotonin and melatonin biosynthesis and the regulation of glutamate and dopamine levels in the brain. Therefore inhibition of IDO might explain both the reported medicinal and neuropsychiatric effects of E. rheedei.
Keywords: Entada rheedei, Mimosaceae, Tryptophan derivatives, HIV infection
1. Introduction
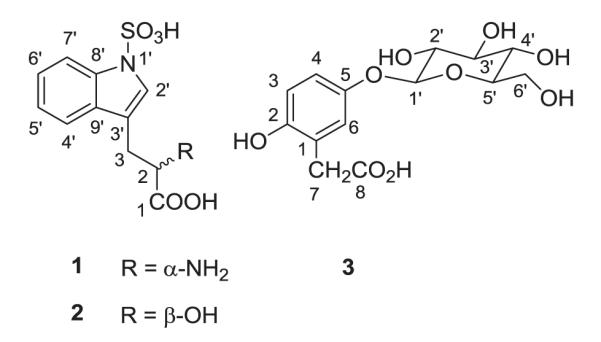
As part of our continuous investigation on Cameroonian medicinal plants [1], we have conducted a phytochemical study of the seed kernels of Entada rheedei Spreng (Mimosaceae), also known as E. rheedii [2], a large woody liana or climber growing naturally throughout tropical Africa and Southeastern Asia. Tobacco made from the seeds of this plant has been reported to cause vivid dreaming and, for this reason, the plant is commonly known as African dream herb or snuff box sea bean (entheology.org). E. rheedei has various medicinal uses, including treatment of jaundice, diarrhea [3], musculo-skeletal problems[4] and mumps [5]. Previous phytochemical studies on the genus Entada revealed the presence of saponins [6,7], thioamides [8–10] and phenylacetic acid derivatives [11,12]. More recently, investigation of the seed kernels of E. rheedei led to the isolation of oleanane-type saponins, thioamide glycosides [13], and phenylpropanoid glycosides [14]. In a recent paper some of us reported the isolation and characterization of two antiproliferative and antioxidant saponins from the n-butanol extract of the seed kernels of this plant [15]. In the present study, we describe the isolation and structure elucidation of two new tryptophan derivatives, N-sulfonyl-L-tryptophan (tryptorheedei A) (1) and 3-(N-sulfonylindolyl)-D-lactic acid (tryptorheedei B) (2), together with the known 5-O-β-D-glucopyranosyl-2-hydroxyphenylacetic acid (3) (Fig. 1), 1-O-methylglucopyranoside, entadamide A ((E)-N-(2-hydroxyethyl)-3-(methylthio)acrylamide), homogentisic acid (2-(2,5-dihydroxyphenyl)acetic acid) and 3-O-β-D-glucopyranosyl-β-sitosterol. Given the important role of tryptophan metabolism via the enzyme indolamine 2,3-dioxygenase 1 and 2 (IDO) in both HIV-1 pathogenesis and neurological diseases [16,17] and their structural similarities to an existing IDO inhibitor, 1-methyltryptophan (1-MT), compounds 1 and 2 were assayed on HIV infection and for their toxicity to TZM and Human PBMC (peripheral blood mononuclear) cells. Both compounds significantly enhanced HIV infection at concentrations above 40 μM. No significant toxicity of the compounds was observed on cells at concentrations of 80 μM and below. Since IDO inhibits HIV-1 replication, the loss of inhibition suggests that these compounds may be competitive inhibitors of IDO and have an effect at 5 fold less concentration of existing inhibitors of IDO (200 μM for 1-MT [18]).
Fig. 1.
2. Experimental
2.1. General experimental procedures
Optical rotations were measured on a PerkinElmer 241 MC Polarimeter. IR spectra were measured as a film on a KBr pellet using a FTIR-8400S Shimadzu spectrometer. ESIMS was carried out on a Hewlett-Packard HP-1100 series LC–MSD system while FABMS was recorded on a Joel-JMS-700 mass spectrometer using glycerol as matrix. NMR spectra were recorded in deuterated solvents (CD3OD, C5D5N) on a Bruker AM-500 and a Varian Mercury Plus 400 spectrometer at 500 or 400 MHz (1H) and 125 or 100 MHz (13C), respectively. All chemicals shifts (δ) are given in ppm units with reference to the residual solvent signal and the coupling constant (J) are in Hz. Column chromatography was performed using silica gel 60 Merck (63–200 μm and 32–63 μm). TLC was carried out on precoated silica gel 60 F254 (Merck) plates developed with EtOAc-MeOH-H2O (9:1:0.5 or 8:2:1). TLC plates were visualized by spraying with 50% H2SO4 and heating for 10 min at 110 C.
2.2. Plant material
The seeds of E. rheedei were collected in Konda village, Momo Division, North-West region of Cameroon, in August 2005, and authenticated by Dr. Gaston Achoundong, head of the National Herbarium of Cameroon. A voucher specimen (No. 19966/SRI/CAM) was deposited at the National Herbarium of Cameroon, Yaoundé.
2.3. Extraction and isolation
The dried and powdered seeds kernel (2.5 kg) of E. rheedei was extracted three times by maceration with 95% EtOH at room temperature for 24 h. The filtrate obtained was evaporated under reduced pressure to yield a brown residue (315 g). Part of this extract (300 g) was suspended in water (500 ml) and successively partitioned between EtOAc and n-BuOH, yielding 19.3 and 105.2 g of extracts after evaporation to dryness, respectively. Part of the EtOAc extract (17.5 g) was subjected to silica gel (63–200 μm) column chromatography, using a gradient of MeOH in EtOAc to give six main fractions (A–F). Fraction E (EtOAc-MeOH (7–3)) was rechromatographed using silica gel (32–63 μm) with EtOAc-MeOH-H2O (8–2–1) as eluent to afford compounds 1 (50 mg) and 2 (10 mg). Fraction D (EtOAc-MeOH (8–2)) was purified by column chromatography over silica gel (32–63 μm) using EtOAc-MeOH-H2O (10:2:1) as eluent to yield compound 3 (11 mg) and 1-O-methylglucopyranoside (50 mg). Fraction C (EtOAc-MeOH (9–1)) was purified by column chromatography over silica gel (32–63 μm) using EtOAc-MeOH-H2O (95–5–2) as eluent to yield entadamide A (25 mg) and 3-O-β-D-glucopyranosyl-β-sitosterol (150 mg) whereas fraction B (EtOAc) was rechromatographed using silica gel (32–63 μm) with hexane-EtOAc (3:7) as eluent to afforded homogentisic acid (17 mg).
N-sulfonyl-L-tryptophan (tryptorheedei A) (1): brown oil; [α]20D-12.0 (c 0.7, CH3OH); IR (KBr) νmax 3419, 3208, 1731, 1593 cm−1; 1H NMR and 13C NMR data, see Table 1; ESIMS m/z 283 [M–H]−, 224[M–CO2–NH2]−.
Table 1.
NMR spectroscopic data (400 MHz, CD3OD) for tryptorheedei A (1) and B (2), and L-tryptophan.
| Tryptorheedei A (1) |
Tryptorheedei B (2) |
L-tryptophan |
|||
|---|---|---|---|---|---|
| Position | δC, type | δH (J in Hz) | δ C | δH (J in Hz) | δ C |
| 1 | 174.3 | / | 181.3 | / | 174.5 |
| 2 | 56.2, CH | 3.84 dd (3.5, 10.6) | 73.8 | 4.20 dd (3.1, 8.8) | 56.9 |
| 3 | 28.6, CH2 | 3.05 dd (10.6, 14.1) | 32.4 | 2.88 dd (8.8, 14.7) | 28.9 |
| 3.55 dd (3.5, 14.1) | 3.36 ddd (1.0, 3.1, 14.7) | ||||
| 2′ | 127.5, CH | 7.50 s | 126.8 | 7.46 br, s | 126.8 |
| 3′ | 112.0, C | / | 114.9 | / | 109.7 |
| 4′ | 119.8, CH | 7.73 d (8.2) | 120.2 | 7.67 br, d (7.9) | 119.4 |
| 5′ | 122.1, CH | 7.17 t (8.2) | 121.5 | 7.08 ddd (1.2, 7.2, 7.9) | 120.2 |
| 6′ | 124.2, CH | 7.24 t (8.2) | 123.4 | 7.16 ddd (1.3, 7.2, 8.2) | 122.9 |
| 7′ | 115.1, CH | 7.94 d (8.2) | 114.6 | 7.87 br, d (8.2) | 112.6 |
| 8′ | 137.5, C | / | 137.0 | / | 138.8 |
| 9′ | 130.1, C | / | 131.5 | / | 128.8 |
3-(N-sulfonylindolyl)-D-lactic acid (tryptorheedei B) (2): brown oil; [α]20D +22 (c 1.7, CH3OH); 1H NMR and 13C NMR data, see Table 1; ESIMS m/z 284 [M–H]−, 286 [M + H]+, 224 [M + H–CO2–H2O]+, 142 [M–CO2–H2O–SO3H]−.
2.4. Acid hydrolysis of compound 3
A solution of compound 3 (3 mg) in water (1 mL) and 2 N aqueous CF3COOH (10 mL) was heated at reflux at 100 °C for 2 h. The mixture was then diluted in water (10 mL) and extracted with EtOAc (3 × 3 mL). The aqueous residue was concentrated to dryness by adding repeatedly MeOH to remove acid and analysed by TLC (silica gel) in comparison with standard sugars by using a mixture of CHCl3–MeOH–AcOH–H2O (60:32:12:8) as the eluant. The absolute configuration of the sugar residue was determined by GC analysis of its chiral derivative [19].
2.5. Bioassay procedure
TZM-bl cell line was obtained from The NIH AIDS Reagent Program, USA; LuSIV cells were obtained from J. Clements, and were maintained as previously described [20]; PE Annexin V Apoptosis Detection Kit was purchased from BD Biosciences; Vybrant MTT Cell Proliferation Assay Kit from Invitrogen; and One-Glo Luciferase Assay System from Promega.
2.5.1. HIV.RF virus preparation
The chronically infected H9 cell line (H9/HIV.RF) was maintained in cRPMI medium and used to produce infectious viral particles. H9/HIV.RF cells were cultured in a T-75 flask to reach a density of about 1 × 10−7 cells/ml. Culture supernatant was harvested and virus was pelleted at 100,000 g for 2 h. Pelleted virus was re-suspended in iRPMI medium and stored at −80 °C until use. p24 antigen concentration was quantified.
2.5.2. MTT assay
TZM-bl cells were seeded in a 96-well plate the day before experiment. After overnight culture, cell culture medium in the well was removed and replaced with fresh medium containing various concentrations of compounds 1 and 2. Cells were cultured overnight and then washed twice with serum-free medium. For each well, 100 μl of fresh medium containing MTT reagent was added and the cells were incubated at 37 °C for 4 h. After adding 100 μl/well of 10% SDS–HCl solution, the plate was incubated at 37 °C overnight and optical absorbance was read at 570 nm.
2.5.3. Flow cytometer assay
Human peripheral blood mononuclear cells were isolated in the laboratory by standard Ficoll protocol. Cells then were treated with various concentrations of compounds 1 and 2 overnight. After washing twice with 1×PBS, cells were stained with Annexin V kit and analyzed with BD LSRFortessa flow cytometer.
2.5.4. Luciferase assay
TZM-bl cells were seeded in a 96-well plate and cultured overnight. After washing once with 1×PBS, cells were treated with various concentrations of compounds 1 and 2 with or without HIV for 24 h. Luciferase assay then was performed according to manufacturer’s protocol. Briefly, reporter cells were washed with iRPMI before resuspension in cRPMI at a density of 4 × 10−6/ml. Cells then were seeded (100 μl/well) in a 96-well plate and mixed with HIV.RF virus at concentration of 200 ng/ml of p24. Then, compound 1 or 2 was added to the wells and the cells were incubated overnight (16 h) at 37 °C. After incubation, One-Glo luciferase assay reagent (Promega, WI, USA) was added (100 μl/well) and the plate was incubated at room temperature for 5 min and luciferase activity was measured on a Fluoroskan Ascent Luminometer (Vantaa, Finland).
3. Results and discussion
The EtOAc soluble portion of the ethanol extract from dried and powdered seed kernels of E. rheedei was subjected to multiple chromatographic steps to afford two new tryptophan derivatives, namely tryptorheedei A (1) and tryptorheedei B (2), together with 5-O-β-D-glucopyranosyl-2-hydroxyphenylacetic acid (3) [21], 1-O-methylglucopyranoside [22], entadamide A [8], homogentisic acid [11,12], and 3-O-β-D-glucopyranosyl-β-sitosterol [23].
Compound 1 was obtained as brown oil, [α]D-12.0° (c 0.7, CH3OH). Its IR absorptions indicated the presence of a hydroxyl group (ν 3419 cm−1 max ), a carbonyl (1731 cm−1), a double bond (1593 cm−1) and an amine group (3208 cm−1). The ESI–MS (negative-ion mode) showed a quasimolecular ion peak at m/z 283 [M–H]−, consistent with the molecular formula of C11H12O5N2S, and an intense peak at m/z 224 [M–CO2–NH ]−. The 1H NMR spectrum of 1 revealed signals for aromatic protons at δ 7.94 (d, J = 8.2 Hz, H-7′); 7.24 (t, J = 8.2 Hz, H-6′); 7.17 (t, J = 8.2 Hz, H-5′), 7.73 (d, J = 8.2 Hz, H-4′) and 7.50 (s, H-2′). Signals at δ 3.84 (dd, J = 3.5, 10.6 Hz, H-2), 3.55 (dd, J = 3.5, 14.1 Hz, H-3a) and 3.05 (dd, J = 10.6, 14.1 Hz, H-3b) suggested that compound 1 is a tryptophan derivative [24]. The 13C NMR signals of compound 1, compared to those of tryptophan (Table 1), show a slight downfield shift in the aromatic region, probably due to the presence of the sulfonyl group attached to the indolic nitrogen atom. Assignments of proton and carbon signals of 1 were achieved by 1H–1H COSY, HSQC and HMBC. The S configuration of carbon 2 was assigned by comparison of its optical rotation ([α]D = −12.0° (c 0.7, CH3OH) with that of L-tryptophan ([α]D = −55.9° (c 0.3, CH3OH). This stereochemistry of C-2 is in good agreement with the fact that naturally occurring amino acids have the L configuration at their α-carbon atom [25]. The structure of compound 1 was thus elucidated as N-sulfonyl-L-tryptophan, a new naturally occurring metabolite to which we gave the trivial name tryptorheedei A.
Compound 2 was also obtained as brown oil, [α]D + 22° (c 1.7, CH3OH). It was assigned the molecular formula C11H11O6NS as determined by the ESI–MS (negative-ion mode) which showed a quasimolecular ion peak at m/z 284 [M–H]− and an important ion fragment at m/z 142 [M–CO2–H2O–SO3H]−. This implies that compound 2 has one more atomic units mass than tryptorheedei A. The 1H and 13C NMR chemical shifts (Table 1) of compounds 2 and 1 were almost superimposable. The 1H NMR spectrum of 2 exhibited signals at δ 7.87 (brd, J = 8.2 Hz, H-7′); 7.16 (ddd, J = 1.3, 7.2, 8.2 Hz, H-6′); 7.08 (ddd, J = 1.2, 7.2, 7.9 Hz, H-5′), 7.67 (brd, J = 7.9 Hz, H-4′) amd 7.46 (brs, H-2′), characteristic of tryptophan derivatives [24,26,27]. The 13C NMR spectrum showed a downfield shift of C-2 in compound 2 with respect to compound 1 (73.8 ppm in 2 vs 56.2 ppm in 1), suggesting the presence of an OH group at C-2 in compound 2 instead of the NH2 group present in 1. Assignments of proton and carbon signals of 2 were completed by 1H–1H COSY, HSQC and HMBC. The R configuration of carbon C-2 was assigned by comparison of its optical rotation ([α]D + 22° (c 1.7, CH3OH)) with that of L-tryptophan ([α]D = −55.9° (c 0.3, CH3OH)). Thus, the structure of 2 was concluded to be 3-(N-sulfonylindolyl)-D-lactic acid, a new derivative to which we gave the trivial name tryptorheedei B.
Compound 3 was obtained as an amorphous solid. Its molecular formula was determined as C14H18O9 by the FAB–MS (negative-ion mode) which showed the quasimolecular ion peak at m/z 328 [M–2H]−. The 1H NMR spectrum of 3 showed signals for three aromatic protons at δ 7.03 (d, J = 8.6 Hz, H-3); 7.14 (dd, J = 8.6, 2.6 Hz, H-4) and 7.34 (d, J = 2.6 Hz, H-6). The two protons singlet for a methylene group observed at δ 3.88 was quite characteristic of phenylacetic acid derivatives[11,12]. In addition, this spectrum revealed the presence of an anomeric proton signal at δ 5.32 (d, J = 7.8 Hz, H-1′). In the 13C NMR spectrum, the characteristic signals of six aromatic carbons, a methylene group at δ 40.0 ppm, a carboxyl group at δ 176.8 ppm and an anomeric carbon at δ 102.8 (C-1′) confirmed that compound 3 was a phenylacetic acid glycoside (Table 2). The sugar was identified as β-D-glucopyranose by comparison of its 1H and 13C NMR spectral data (Table 2) with those of β-anomers of glucopyranose derivatives [22], and confirmed, after hydrolysis, by chromatographic methods. The complete assignments 1H and 13C resonances were achieved by 1H–1H COSY, HSQC and HMBC. In the HMBC spectrum, the correlation between the anomeric proton δ 5.32 (H-1′) and the carbon at δ 151.1 (C-5) allowed us to locate the sugar unit at C-5 of the aglycone rather than placing it at C-2 as found in phaseoloidin (2-(2-O-β-D-glucopyranosyl-5-hydroxyphenyl) acetic acid)), commonly isolated from related species [6,11]. Thus, compound 3 was elucidated as 5-O-β-D-glucopyranosyl-2-hydroxyphenylacetic acid, a glycoside of homogentisic acid. As far as we know the same structure was reported once in the literature, but its identification was only based on chemical transformations, and spectroscopic data were not reported [21]. The structures of the known 1-O-methylglucopyranoside, entadamide A, homogentisic acid and 3-O-β-D-glucopyranosyl-β-sitosterol were confirmed by comparison with data reported in the literature.
Table 2.
NMR spectroscopic data (500 MHz, C5D5N) for compound 3.
| Compound 3 | ||
|---|---|---|
| position | δC, type | δH (J in Hz) |
| 1 | 124.9, C | / |
| 2 | 152.1, C | / |
| 3 | 116.6, CH | 7.03 d (8.6) |
| 4 | 116.3, CH | 7.14 dd (8.6, 2.6) |
| 5 | 151.1, C | / |
| 6 | 120.2, C | 7.34 d (2.6) |
| 7 | 40.0, CH2 | 3.88 s |
| 8 | 176.5, C | / |
| 1′ | 102.8, CH | 5.32 d (7.8) |
| 2′ | 74.4, CH | 4.14 dd (8.9, 7.8) |
| 3′ | 77.6, CH | 4.28 bt (9.0) |
| 4′ | 70.8, CH | 4.17 nd |
| 5′ | 77.8, CH | 3.92 m |
| 6′ | 61.9, CH2 | 4.20 dd (12.0, 5.0), 4.38 dd (12.0, 2.4) |
Thioamides named entadamides A–C and entadamide A-β-D-glucopyranoside were previously isolated from Entada phaseoloides [8–11]. The phenylacetic acid derivative named phaseoloidin was isolated in a very large amount from the seed kernels of E. phaseoloides [11] and Entada pursaetha [6] and seems to be the chemotaxonomic marker of this genus. Although during our investigation we have not isolated phaseoloidin but the isomer 5-O-β-D-glucopyranosyl-2-hydroxyphenylacetic acid (3), its presence, together with the isolation of entadamide A, shows the chemical homogeneity, in terms of metabolite profile, of plant species belonging to the genus Entada. Furthermore, amino-acid derivatives such as dopamine 3-O-glucopyranoside and tyrosine O-glucopyranoside were isolated from E. pursaetha [28].
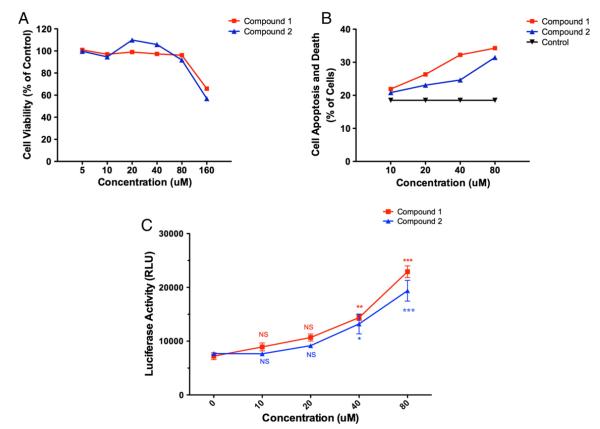
Large molecular weight polyanionic compounds such as dextran sulfate and suramin have previously been reported to be inhibitors of the HIV-1 integrase enzyme in vitro [29]. A series of smaller molecular weight mono, and di-sulfonated compounds with comparable potency was identified [30]. Furthermore, tryptophan is one of the essential amino acids implicated in the immune system defense [31,32]. Polyanions are also reported to interfere with the fusion process through charge-based interactions with the V3 loop of gp120 [33]. It was therefore quite obvious to assay the two sulfonated indoles on HIV infection. To this end we tested the effects of compounds 1 and 2 on HIV-1 infection using two different reporter cell lines, TZM-bl cells and LuSIV cells. TZM-bl cells are derived from HeLa cells, whereas LuSIV cells are derived from a B/T cell hybrid line that is routinely used to produce virus [34]. Although both lines showed a positive effect of compound 2, TZM-bl cells showed higher variability, thus we repeated our experiments using LuSIV cells, a more biologically relevant cell line in sixplicate. Both compounds 1 and 2 enhanced HIV-1 infection at concentrations of 40 and 80 μM (Fig. 2C). Experimental results were confirmed with three independent experiments. The compounds appeared to have minimal toxicity on both reporter cells and isolated peripheral blood mononuclear cells (PBMCs) at concentrations less than 40 μM (Fig. 2A and B), thus we believe the effect of compounds to be specific rather than due to toxicity. While the minimum effective concentration of compounds 1 and 2 are in the μM range, the effective concentration of 1-MT, the existing inhibitors of IDO is in the range of 200 μM [18].
Fig. 2.
Given the position of the sulfate group is in the active site of IDO [35], we posit that both compounds 1 and 2 are acting as competitive inhibitors of IDO in a mechanism similar to that observed for 1-MT, a drug being used in cancer therapy to prevent tumor associated suppression of the immune response [36]. Similar uses of 1-MT have been proposed for the treatment of patients with AIDS; however, preliminary studies in animals suggest that 1-MT may induce a fatal pancreatitis [37]. Thus these compounds may offer an alternative as potential therapeutic for cancer and AIDS. While our results may seem paradoxical as the compounds increase HIV-1 replication, the acute induction of IDO is beneficial as it prevents T cell activation required for HIV-1 replication. Whereas the same inhibition of T cell responses by IDO in chronic HIV-1 infection leads to immune impairment and AIDS [16].
IDO in addition to its immune regulatory functions is also the gatekeeper for kynurenine metabolism, which is a central metabolic pathway in the CNS and implicated in several neurological diseases [17]. Also kynurenic acid, a metabolite of the pathway has been shown to modulate extracellular dopamine and glutamate levels [17]. Tryptophan is also the precursor for both serotonin and melatonin [17]. If tryptophan is diverted to kynurenine metabolites, it would be unavailable for synthesis of serotonin and melatonin. We think that the observed effects of vivid dreaming and hallucinations offer more indirect support for the involvement of compounds 1 and 2 on IDO activity, and might shed light on the folklore associated with E. rheedei.
Supplementary Material
Acknowledgments
This research was supported by the International Foundation for Science (IFS, Stockholm, Sweden) through a grant to Prof. Tapondjou A. Léon (RGA No. F/3976-2). The authors would also like to thank MIUR for supporting this research through the COOPERLINK 2011 Prot. CII113PPUC “Tesi di Dottorato sullo studio di molecole biologicamente attive estratte da piante della medicina tradizionale del Camerun”.
Footnotes
Conflict of interest The authors declare no conflict of interest.
Appendix A. Supplementary data 1H and 13C NMR spectra for compounds 1–3 can be found online at http://dx.doi.org/10.1016/j.fitote.2013.03.017.
References
- [1].Tapondjou LA, Nyaa LBT, Tane P, Ricciutelli M, Quassinti L, Bramucci M, et al. Cytotoxic and antioxidant triterpene saponins from Butyrospermum parkii (Sapotaceae) Carbohydr Res. 2011;346:2699–704. doi: 10.1016/j.carres.2011.09.014. [DOI] [PubMed] [Google Scholar]
- [2]. [accessed 25 June 2012];The International Plant Names Index. 2008 Published on the Internet http://www.ipni.org.
- [3].Dash SK, Padhy S. Review on ethnomedicines for diarrhoea diseases from Orissa: prevalence versus culture. J Hum Ecol. 2006;20:59–64. [Google Scholar]
- [4].Uprety Y, Asselin H, Boon EK, Yadav S, Shrestha KKJ. Indigenous uses and bio-efficacy of medicinal plants in Rasuwa district, Central Nepal. Ethnobiol Ethnomed. 2010;6:3. doi: 10.1186/1746-4269-6-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Shivanna MB, Rajakumar N. Traditional medico-botanical knowledge of local communities in Hosanagara Taluk of Shimoga District in Karnataka, India. J Herbs Spices Med Plants. 2001;17:291–317. [Google Scholar]
- [6].Tapondjou AL, Miyamoto T, Mirjolet J-F, Guilbaud N, Lacaille-Dubois M-A. Pursaethosides A–E, triterpene saponins from Entada pursaetha. J Nat Prod. 2005;68:1185–90. doi: 10.1021/np0580311. [DOI] [PubMed] [Google Scholar]
- [7].Cioffi G, Dal PF, De Caprariis P, Sanego R, Marzocco S, Autore G, et al. Antiproliferative triterpene saponins from Entada africana. J Nat Prod. 2006;69:1323–9. doi: 10.1021/np060257w. [DOI] [PubMed] [Google Scholar]
- [8].Ikegami F, Shibasaki I, Ohmiya S, Ruangrungsi N, Murakoshi I. Entadamide A, a new sulphur-containing amide from Entada phaseoloides seeds. Chem Pharm Bull. 1985;33:5153–4. [Google Scholar]
- [9].Ikegami F, Ohmiya S, Ruangrungsi N, Sakai S-I, Murakoshi I. Entadamide B, a second new sulphur-containing amide from Entada phaseoloides. Phytochemistry. 1987;26:1525–6. [Google Scholar]
- [10].Ikegami F, Sekine T, Duangteraprecha S, Matsushita N, Matsuda N, Ruangrungsi N, et al. Entadamide C, a sulphur-containing amid from Entada phaseoloides. Phytochemistry. 1989;28:881–2. [Google Scholar]
- [11].Barua AK, Chakrabarty M, Datta PK, Ray S. Phaseoloidin, a homogentisic acid glucoside from Entada phaseoloides. Phytochemistry. 1988;27:3259–61. [Google Scholar]
- [12].Dai J, Kardono LBS, Tsauri S, Padmawinata K, Pezzuto JM, Kinghorn AD. Phenylacetic acid derivatives and a thioamide glycoside from Entada phaseoloides. Phytochemistry. 1991;30:3749–52. [Google Scholar]
- [13].Sugimoto S, Matsunami K, Otsuka H. Medicinal plants of Thailand. I: structures of rheedeiosides A–D and cis-entadamide A β-D-glucopyranoside from the seed kernels of Entada rheedei. Chem Pharm Bull. 2011;59:466–71. doi: 10.1248/cpb.59.466. [DOI] [PubMed] [Google Scholar]
- [14].Sugimoto S, Matsunami K, Otsuka H. Medicinal plants of Thailand. II: chemical studies on the seed kernels of Entada rheedei Sprengel. J Nat Med. 2012;66:552–7. doi: 10.1007/s11418-011-0608-9. [DOI] [PubMed] [Google Scholar]
- [15].Nzowa KL, Barboni L, Teponno RB, Ricciutelli M, Lupidi G, Quassinti L, et al. Rheediinosides A and B, two antoproliferative and antioxidant triterpene saponins from Entada rheedii. Phytochemistry. 2010;71:254–61. doi: 10.1016/j.phytochem.2009.10.004. [DOI] [PubMed] [Google Scholar]
- [16].Boasso A, Royle CM, Doumazos S, Aquino VN, Biasin M, Piacentini L, et al. Overactivation of plasmacytoid dendritic cells inhibits antiviral T-cell responses: a model for HIV immunopathogenesis. Blood. 2011;118:5152–62. doi: 10.1182/blood-2011-03-344218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Schwarcz R, Bruno JP, Muchowski PJ, Wu HQ. Kynurenines in the mammalian brain: when physiology meets pathology. Nat Rev Neurosci. 2012;13:465–77. doi: 10.1038/nrn3257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Boasso A, Herbeuval JP, Hardy AW, Anderson SA, Dolan MJ, Fuch D, et al. HIV inhibits CD4+ T-cell proliferation by inducing indoleamine 2,3-dioxygenase in plasmacytoid dendritic cells. Blood. 2007;109:3351–9. doi: 10.1182/blood-2006-07-034785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Elbandy M, Miyamoto T, Delaude C, Lacaille-Dubois M-A. Acylated preatroxigenin glycosides from Atroxima congolana. J Nat Prod. 2003;66:1154–8. doi: 10.1021/np030057+. [DOI] [PubMed] [Google Scholar]
- [20].Roos JW, Maughan MF, Liao Z, Hildreth JE, Clements JE. LuSIV cells: a reporter cell line for the detection and quantitation of a single cycle of HIV and SIV replication. Virology. 2000;273:307–15. doi: 10.1006/viro.2000.0431. [DOI] [PubMed] [Google Scholar]
- [21].Matsumura Y, Shibata Y, Mimura T. The isolation of homogentisic glucoside from bamboo shoot. Wakayama Igaku. 1962;13:71–6. [Google Scholar]
- [22].Agrawal PK. NMR spectroscopy in the structural elucidation of oligosaccharides and glycosides. Phytochemistry. 1992;31:3307–30. doi: 10.1016/0031-9422(92)83678-r. [DOI] [PubMed] [Google Scholar]
- [23].Teponno RB, Tapondjou AL, Gatsing D, Djoukeng JD, Abou-Mansour E, Tabacchi R, et al. Bafoudiosbulbins A, and B, two anti-salmonellal clerodane diterpenoids from Dioscorea bulbifera L. var sativa. Phytochemistry. 2006;67:1957–63. doi: 10.1016/j.phytochem.2006.06.019. [DOI] [PubMed] [Google Scholar]
- [24].Fattorusso E, Lanzotti V, Magno S, Novellino E. Trytophan derivatives from a Mediterranean anthozoan Astroides calycularis. J Nat Prod. 1985;48:924–7. [Google Scholar]
- [25].Carey AF. Organic Chemistry. 6th ed McGraw-Hill; New York: 2006. [Google Scholar]
- [26].Schnabel M, Rompp B, Ruckdeschel D, Unverzagt C. Synthesis of trytophan N-glucoside. Tetrahedron Lett. 2004;45:295–7. [Google Scholar]
- [27].Diem S, Bergmann J, Herderch M. Tryptophan-N-glucoside in fruits and fruit juices. J Agr Food Chem. 2000;48:4913–7. doi: 10.1021/jf0003146. [DOI] [PubMed] [Google Scholar]
- [28].Larsen PO, Pedersen E, Sorensen H, Sorup P. Tyrosine O-glucoside and dopamine 3-0-glucoside in seeds of Entada pursaetha. Phytochemistry. 1973;12:2243–7. [Google Scholar]
- [29].Carteau S, Mouscadet JF, Goulaouic H, Subra F, Auclair C. Inhibitory effect of the polyanionic drug suramin on the in vitro HIV DNA integration reaction. Arch Biochem Biophys. 1993;305:606–10. doi: 10.1006/abbi.1993.1468. [DOI] [PubMed] [Google Scholar]
- [30].Hazuda DJ, Wolfe AL, Felock PJ, Hastings JC, Uncapher BC, Schleif WA, et al. Inhibition of HIV-1 integrase by small molecular weight sulfonates. 3rd Conference on Retroviruses and Opportunistic Infections; Washington DC. 1996. [Google Scholar]
- [31].Favre D, Mold J, Hunt PW, Kanwar B, Loke P, Seu L, et al. Tryptophan catabolism by indoleamine 2,2-dioxygenase 1 alters the balance of TH17 to regulatory T cells in HIV disease. Sci Transl Med. 2010;2:1–12. doi: 10.1126/scitranslmed.3000632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Davies NWS, Guillemin G, Brew BJ. Tryptophan, neurodegeneration and HIV-associated neurocognitive disorder. Int J Trypt Res. 2010;3:121–40. doi: 10.4137/ijtr.s4321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Zeng XW, Huang N, Xu H, Yang WB, Yang LM, Qu H, et al. Anti human immunodeficiency virus type 1 (HIV-1) agents 4. Discovery of 5,5′-(p-phenylenebisazo)-8-hydroxyquinoline sulfonates as new HIV-1 inhibitors in vitro. Chem Pharm Bull. 2010;58:976–9. doi: 10.1248/cpb.58.976. [DOI] [PubMed] [Google Scholar]
- [34].Rudensey LM, Papenhausen MD, Overbaugh J. Replication and persistence of simian immunodeficiency virus variants after passage in macaque lymphocytes and established human cell lines. J Virol. 1993;67:1727–33. doi: 10.1128/jvi.67.3.1727-1733.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Capece L, Lewis-Ballester A, Marti MA, Estrin DA, Yeh SR. Molecular basis for the substrate stereoselectivity in tryptophan dioxygenase. Biochemistry. 2011;50:10910–8. doi: 10.1021/bi201439m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Zamanakou M, Germenis AE, Karanikas V. Tumor immune escape mediated by indoleamine 2,3-dioxygenase. Immunol Lett. 2007;111:69–75. doi: 10.1016/j.imlet.2007.06.001. [DOI] [PubMed] [Google Scholar]
- [37].Vaccari M, Boasso A, Fenizia C, Fuchs D, Hryniewicz A, Morgan T, et al. Fatal pancreatitis in simian immunodeficiency virus SIV(mac251)-infected macaques treated with 2′,3′-dideoxyinosine and stavudine following cytotoxic-T-lymphocyte-associated antigen 4 and indoleamine 2,3-dioxygenase blockade. J Virol. 2012;86:108–13. doi: 10.1128/JVI.05609-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.