Abstract
AIM
To explore the effects and potential mechanisms of curcumin on retinal Müller cell in early diabetic rats.
METHODS
Diabetic rats were induced by a single intraperitoneal injection of streptozotocin (STZ). Male Sprague-Dawley (SD) rats were randomly assigned into 4 groups: control group (naïve SD rats administered with a single intraperitoneal injection of citric buffer), diabetic group (STZ-diabetic rats), dimethyl sulfoxide (DMSO) group (diabetic rats intraperitoneally administered with mixture of DMSO and normal saline, once a day) and curcumin group (diabetic rats intraperitoneally administered with curcumin, 80mg/kg, once a day). Three months after diabetes onset, malondialdehyde (MDA, indication of oxidative stress level) and reduced glutathione (GSH) in retina were detected with kits, glial fibrillary acidic protein (GFAP) in retina was revealed by immunohistochemistry and Western blot, and retinal glutamine synthetase (GS) were observed by Western blot.
RESULTS
Compared with control group, retinal MDA was increased, and GSH was decreased in diabetic and DMSO groups (P<0.05, respectively). While, retinal MDA and GSH in curcumin group showed no difference compared with control group (P>0.05). Furthermore, up-regulation of retinal GFAP and down-regulation of retinal GS were detected in diabetic and DMSO groups, and no alteration could be observed in curcumin group revealed with Western blot. Compared with control group, retinal Müller cells showed significant increase in GFAP immunochemistry staining in diabetic and DMSO groups. Moreover, GFAP-positive staining was decreased in curcumin group compared with diabetic group.
CONCLUSION
Curcumin inhibits diabetic retinal oxidative stress, protects Müller cell, and prevents the down-regulation of GS in diabetic retina. Therefore, curcumin has a therapeutic potential in the treatment of diabetic retinopathy (DR).
Keywords: diabetic retinopathy, curcumin, oxidative stress, Müller cell
INTRODUCTION
Müller cells, the principal glial cells in vertebrate retina, play a vital role in regulating the function of retinal photoceptors and neurons, and in maintaining integrality of blood-retinal barrier. Oxidative stress in diabetic Müller cell alters the expressions of glial fibrillary acidic protein (GFAP) and glutamine synthetase (GS, detoxicating accumulated glutamate into glutamine), thus contributing to diabetic retinopathy (DR)[1]-[3]. Oxidative stress is usually associated with reduced glutathione (GSH) and shows alteration of malondialdehyde (MDA), an indication of oxidative stress level[4]. Furthermore, previous study revealed that curcumin inhabits oxidative stress in SH-SY5Y cells and brain neurons[5],[6]. However, effects of curcumin on oxidative stress in diabetic Müller cell remain unclear. Therefore, effects and potential mechanisms of curcumin on retinal Müller cell oxidative stress in early diabetic rats were detected in our study.
MATERIALS AND METHODS
Materials
Male Sprague-Dawley(SD) rats (220g–250g) were obtained from Liaoning Medical University; MDA and GSH assay kit were got from Beyotime Institute of Biotechnology (Beijing, China); curcumin, streptozotocin (STZ), and antibodies for detecting GFAP and GS were from Sigma (USA).
Methods
Diabetic models and drug administration
Diabetic rats were induced by a single intraperitoneal injection of STZ in a 1% solution of citrate buffer at dose of 55mg/kg. Forty-eight hours after STZ administration, rats with blood glucose concentration>15.0mmol/L were considered diabetic and used in our experiments. In reference to other studies[3],[4], curcumin was firstly dissolved in dimethyl sulfoxide (DMSO), then in normal saline to 1mg/mL (0.5‰ for curcumin). Animals were randomly assigned into 4 groups: control group (naïve SD rats received injection of citrate buffer), diabetic group (STZ-induced diabetic rats), DMSO group (STZ-diabetic rats intraperitoneally administrated with mixture of DMSO and normal saline, once a day), and curcumin group (STZ-diabetic rats intraperitoneally administrated with curcumin at dose of 80mg/kg, once a day). Three months after drug administration, rats were sacrificed for biochemical studies.
Detection of retinal MDA and GSH
After anaesthetization with 10% chloral hydrate, rats were sacrificed and retinas were detached for homogenate to concentration of 5%, and then centrifuged at 4 000r/min. Next procedures for detecting retinal MDA and GSH were performed with the supernatant according to protocols and formulas provided by the kits.
Immunohistochemistry staining for retinal GFAP
Rats were perfused with 4% paraformaldehyde, and retinal paraffin sections were performed at 5µm. After dewaxed to water, retinal paraffin sections were incubated with 3% H2O2 for 10min, then with rabbit anti-GFAP serum (1:2000, 4°C, overnight), biotinylated goat anti-rabbit serum (1:200, room temperature, 2h), and incubated with mixture of DAB for 20min. Hematoxylin-eosin was used for after staining.
Western blot for detecting retinal GFAP and GS
Rats were sacrificed, and retinas were prepared for homogenate with lysis buffer, then centrifugalized 15min at 12 000r/min. Proteins in supernate were separated by SDS polyacrylamide gels, then transferred to polyvinylidene fluoride membranes, which were incubated with antibodies to GFAP, GS or β-actin respectively at 37°C for 2h, and then with corresponding horseradish peroxidase (HRP)-conjugated antibodies at 37°C for 1h. Immune complexes were detected with enhanced chemiluminescence Western Blot substrate kit.
Statistical Analysis
All data were presented as mean±SD, one-way ANOVA followed by LSD-t test was employed. P<0.05 was considered statistically significant.
RESULTS
Levels of Retinal MDA and GSH
Compared with control group, level of retinal MDA was significantly increased, while, GSH was obviously decreased in diabetic group and DMSO group (P<0.05, respectively). However, no difference of retinal MDA or GSH could be detected between control group and curcumin group (P>0.05) (Table 1).
Table 1. Levels of retinal MDA and GSH.
| Parameters | Control group | Diabetic group | DMSO group | Curcumin group |
| MDA (nmol/mg prot) | 3.47±0.51 | 7.21±0.83b | 7.13±0.74b | 3.91±0.43 |
| GSH (mg/g prot) | 98.61±9.82 | 54.86±3.47b | 59.61±5.38b | 86.93±7.84 |
bP<0.01 vs control group.
(n=6 per group)
Immunohistochemistry Staining for Retinal GFAP
Immunohistochemistry staining was performed in present study (n=4 per group), which revealed that retinal GFAP-positive staining locates in nerve fiber layer in control group. Inner nuclear layer, characterized by accumulation of Müller cell somata, shows GFAP-negative in control group. Müller cells were obviously GFAP-positive in diabetic and DMSO groups. Furthermore, GFAP staining was decreased in curcumin group compared with diabetic group (Figure 1).
Figure 1. Immunohistochemistry staining for GFAP (×400). GFAP-positive like staining is labeled with arrow.
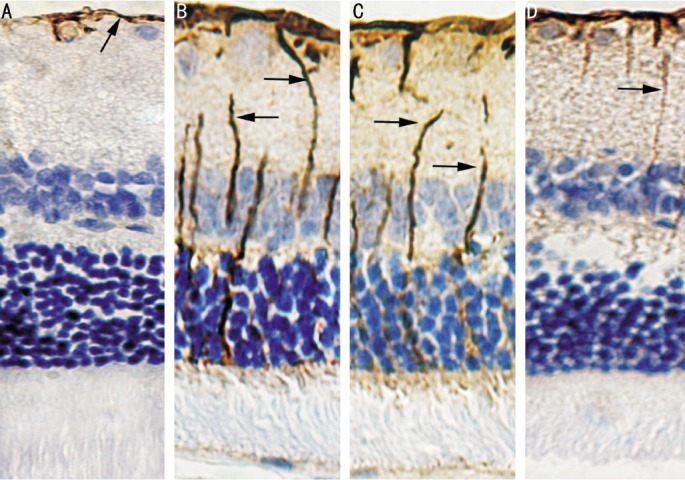
A: Control group; B: Diabetic group; C: DMSO group; D: Curcumin group
Expression of Retinal GFAP and GS
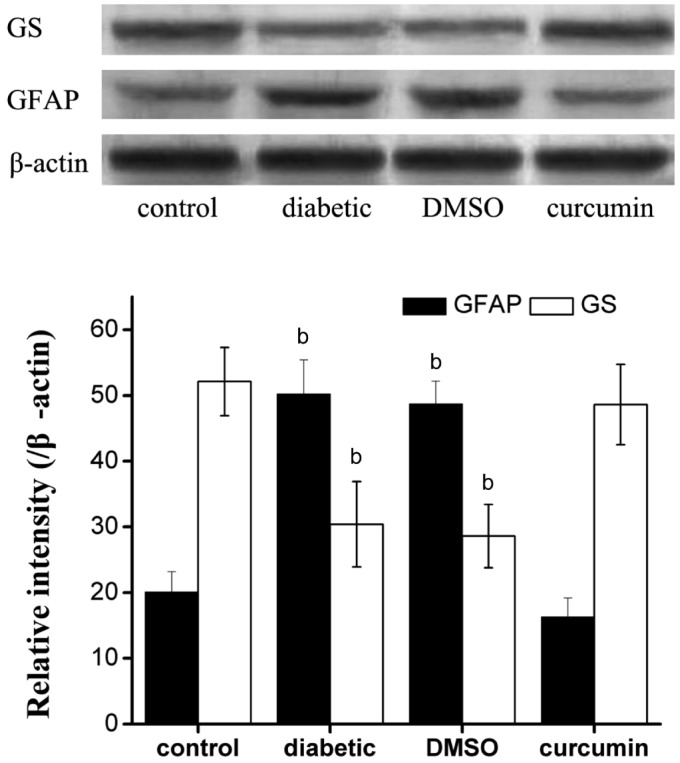
The bands density of GFAP and GS were normalized to that of β-actin (n=6 per group). The relative densities of GFAP were 20.1±3.1%, 50.2±5.2%, 48.7±3.5%, 16.3±2.9%, and GS were 52.1±5.2%, 30.4±6.5%, 28.6±4.8%, 48.6±6.1% in control, diabetic, DMSO and curcumin group respectively. GFAP was significantly up-regulated, and GS was obviously down-regulated in diabetic and DMSO groups compared with control group (P<0.01). However, no difference of GFAP or GS could be detected between control group and curcumin group (P>0.05, Figure 2).
Figure 2. Western blot for GS, GFAP and β-actin.
GFAP was up-regulated, while GS was down-regulated in diabetic and DMSO groups compared with control group. bP < 0.01 vs control group.
DISCUSSION
Here we demonstrated protective effects of curcumin on retinal Müller cells in early STZ-diabetic rats. Briefly, Three months after diabetes onset, diabetic retina shows obviously oxidative stress, and curcumin inhibits diabetic retinal oxidative stress, reversing diabetes-induced up-regulation of retinal GFAP and down-regulation of retinal GS.
In addition to support and insulation, Müller cell plays an important role in surviving neurons and transmitting excitation. Oxidative stress occurred in diabetes mellitus is involved in dysfunction and structural abnormalities of Müller cell, showing up-regulation of GFAP[1],[7]. Müller cell somata mainly accumulate in inner nuclear layer, and processes spread between internal limiting membrane and external limiting membrane. We revealed that 3 months after diabetes onset the level of retinal MDA is increased, the level of retinal GSH is decreased in addition to up-regulation of GFAP in Müller cell. It was reported that curcumin inhibits multifactor induced oxidative stress in SH-SY5Y cell, and decreases ischemia induced oxidative stress in brain neurons[5], [8]. In present study, curcumin decreased the level of diabetic retinal MDA, increasing the level of diabetic retinal GSH, and down-regulating diabetic retinal GFAP. Therefore, our study indicated that curcumin inhibits diabetic retinal oxidative stress, thus protecting Müller cell.
Glutamate is the major excitatory neurotransmitter in retina and plays an important role in neurotransmission. However, elevated glutamate is neurotoxic, resulting in neurodegeneration in retina[7]. Glutamate-aspartate transporter (GLAST) transfers extracellular glutamate into Müller cell, and then detoxicated into glutamine via GS. It was reported that oxidative stress in diabetic Müller cell, resulting in decreased activity of GLAST and down-regulation of GS, is involved in accumulation of glutamate in retina[7]. We found that retinal oxidative stress is accompanied with GS down-regulation in diabetic retina. While, curcumin inhibits diabetes oxidative stress, and reverses down-regulation of GS in diabetic retina. Therefore, based on the vital role of GS in metabolism of retinal glutamate, we postulated down-regulation of GS may be accompanied with neurotoxicity of accumulated glutamate in diabetic retina. While curcumin up-regulating GS may result in detoxication by catalyzing glutamate into glutamine in diabetic retina.
Our current study indicates a therapeutic potential for curcumin in the treatment of DR by protecting Müller cells. However, we recognize the limitations of our present study. Effects of curcumin on the activity and function of GLAST in diabetic Müller cell remains unclear, and the effects of curcumin on the level of glutamate and neurons in diabetic retina needs to be further elucidated.
Footnotes
Foundation items: National Natural Science Foundation of China (No. 31140072); Doctoral Scientific Starting Foundation of Liaoning Medical University (No. Y2012B005)
REFERENCES
- 1.Lieth E, Barber AJ, Xu B, Dice C, Ratz MJ, Tanase D, Strother JM. Glial reactivity and impaired glutamate metabolism in short-term experimental diabetic retinopathy. Diabetes. 1998;47(5):815–820. doi: 10.2337/diabetes.47.5.815. [DOI] [PubMed] [Google Scholar]
- 2.Bringmann A, Pannicke T, Biedermann B, Francke M, Iandiev I, Grosche J, Wiedemann P, Albrecht J, Reichenbach A. Role of retinal glial cells in neurotransmitter uptake and metabolism. Neurochem Int. 2009;54(3–4):143–160. doi: 10.1016/j.neuint.2008.10.014. [DOI] [PubMed] [Google Scholar]
- 3.Li Q, Puro DG. Diabetes-induced dysfunction of the glutamate transporter in retinal Müller cells. Invest Ophthalmol Vis Sci. 2002;43(9):3109–3116. [PubMed] [Google Scholar]
- 4.Jomova K, Vondrakova D, Lawson M, Valko M. Metals, oxidative stress and neurodegenerative disorders. Mol Cell Biochem. 2010;345(1–2):91–104. doi: 10.1007/s11010-010-0563-x. [DOI] [PubMed] [Google Scholar]
- 5.Yin W, Zhang X, Shi X, Li Y. Curcumin protects SH-SY5Y cells from oxidative stress by up-regulating HO-1 via Phosphatidylinositol 3 Kinase/Akt/Nrf-2 and down-regulating HO-2. Mol Neurodegener. 2012;7(Suppl 1):S14. [Google Scholar]
- 6.Rajeswari A. Curcumin protects mouse brain from oxidative stress caused by 1-methyl-4-phenyl-1, 2, 3, 6-tetrahydropyridine. Eur Rev Med Pharmacol Sci. 2006;10(4):157–161. [PubMed] [Google Scholar]
- 7.Li Q, Puro DG. Diabetes-induced dysfunction of the glutamate transporter in retinal Müller cells. Invest Ophthalmol Vis Sci. 2002;43(9):3109–3116. [PubMed] [Google Scholar]
- 8.Liu L, Zhang P, Li Y, Yu G. Curcumin protects brain from oxidative stress through inducing expression of UCP2 in chronic cerebral hypoperfusion aging-rats. Mol Neurodegener. 2012;7(Suppl 1):S10. [Google Scholar]