Abstract
AIM
To investigate the clinical characteristics and prognosis of patients with malignant eyelid tumors.
METHODS
This was a retrospective, non-randomized, clinical reviews. Between January, 2002 and December, 2011, 75 cases with histologically confirmed malignant eyelid tumors were evaluated. Patients' charts were reviewed for clinical information, treatment procedure, and disease course. Survival analysis in terms of recurrence-free survival was performed using age, sex, location of tumor and histopathological type. The follow-up ranged from 1 to 78 months (mean=21 months).
RESULTS
The 75 eyelid tumors included 35 basal cell carcinoma (BCC, 46.7%), 22 sebaceous gland carcinoma (SGC, 29.3%), 7 squamous cell carcinoma (SCC, 9.3%), 10 malignant melanoma (MM, 13.3%), and 1 Merkel cell carcinoma (MCC, 1.3%). Recurrence developed in 17 cases (22.7%). The recurrence rate of BCC (4/35, 11.4%) was significant lower than MM (6/10, 60.0%, P<0.001). The mean interval of recurrence was 21 months (range 3-62) for all eyelid tumors. Tumor located at canthus had higher recurrence rate (50%) compared with those located at eyelid (19%, P<0.05). Histological type was independent variable for recurrence by Cox regression analysis.
CONCLUSION
It is important to achieve a negative tumor margin in canthus located malignant eyelid tumor. Clinicians should have a high level of suspicion for recurrence according to histological type when treating patients with eyelid tumor.
Keywords: eyelid tumors, histological type, location, recurrence
INTRODUCTION
Malignant eyelid tumors are rare, but they are challenging to recognize and treat, even for experienced ophthalmologists. A wide range of clinical presentations contributes to a high risk of misdiagnosis. In a review of the literature on periocular skin cancers, Lober and Fenske[1] found that a substantial proportion of malignant eyelid tumors are misdiagnosed. The main malignant tumors affecting the eyelid are basal cell carcinoma (BCC), sebaceous gland carcinoma (SGC), squamous cell carcinoma (SCC), and malignant melanoma (MM)[2]. BCC is the most common malignant eyelid tumor, especially in Western countries[3]-[5], and SGC occurs less frequently in the west than it does in China[6]. The influencing factors such as larger lesions, incompletely excision, histopathologic features such as poor differentiation, multicentric origin, pagetoid spread and delayed diagnosis were known or thought to associated with poor prognosis[7]-[10].
The Western and Asian data have considerable variations in case distribution and presentation. In the present study, we performed a retrospective study on cases of malignant eyelid tumors registered at both the Department of Ophthalmology, Yonsei University in Korea and the Zhejiang University in China. The purpose of this study was to characterize clinicopathologic features of these tumors and to define prognostic factors for disease-free intervals following initial treatment in Chinese and Korean population.
SUBJECTS AND METHODS
Subjects
Seventy-five cases with histopathologically confirmed malignant eyelid tumors were retrieved through a detailed retrospective review of charts covering the period January, 2002 to December, 2011. Ethics Committee approval was obtained and informed consent was obtained from patients.
Methods
Data were recorded for each patient including general and demographic information, exact anatomic site of lesion, time to presentation, treatment procedure, and disease course. Follow-up data included length of follow-up, incidence of tumor recurrence, time between treatment and recurrence, and management of recurrence.
Statistical Analysis
All statistical analysis was performed on computer (SPSS 13 for Windows; SPSS, Inc.). A P value less than 0.05 was considered statistically significant.
RESULTS
Pertinent demographic data and clinical and histologic features of the 75 patients included in the study are summarized in Table 1. None of the patients had specific systemic diseases related to their eyelid tumor.
Table 1. Summary of clinical and histopathologic data on patients with malignant eyelid tumor.
| Parameters | n(%) | Recurrence n(%) | Mean recurrence time (months) |
| Histopathologic type | |||
| BCC | 35(46.7) | 4(11.4) | 74 |
| SGC | 22(29.3) | 3(13.7) | 47 |
| SCC | 7(9.3) | 4(57.1) | 30 |
| MM | 10(13.3) | 6(60.0) | 22 |
| Gender | |||
| Female | 34(45.3) | 6(17.6) | 61 |
| Male | 41(54.7) | 11(26.8) | 55 |
| Age (a) | |||
| ≤65 | 39(52.0) | 10(25.6) | 59 |
| >65 | 36(48.0) | 7(19.4) | 49 |
| Eye | |||
| Right eye | 45(60.0) | 12(26.7) | 45 |
| Left eye | 30(40.0) | 5(16.7) | 75 |
| Location | |||
| Upper lid | 35(46.7) | 7(20.0) | 46 |
| Lower lid | 32(42.7) | 6(18.7) | 68 |
| Canthus | 8(10.7) | 4(50.0) | 22 |
| Medial | 22(29.3) | 4(18.2) | 43 |
| Central | 24(32.0) | 4(16.7) | 72 |
| Lateral | 29(38.7) | 9(31.0) | 47 |
BCC: Basal cell carcinoma; SGC: Sebaceous gland carcinoma; SCC: Squamous cell carcinoma; MM: Malignant melanoma.
Clinical Features
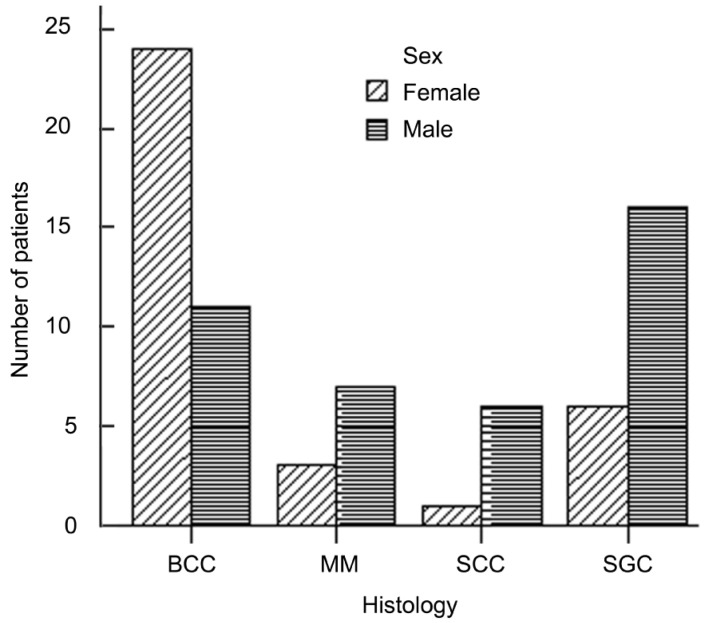
A total of 75 patients were analyzed of which 41 were males and 34 were females. The age of the patients ranged from 13 to 92. Thirty of the carcinomas were found on the left, 45 on the right side (P>0.05; binominal test). The tumor was localized in the upper eyelid in 35 patients, in the lower eyelid in 32 patients, and less frequently in the canthus (both medial canthus and lateral canthus) in 8 patients. Thirty-five tumors (46.7%) were diagnosed as BCC, 22 (29.3%) were SGC, 7 (9.3%) were SCC, 10 (13.3%) were MM, and 1 was Merkel cell carcinoma. The mean age at diagnosis was 62.4 years (range, 13-92); 39 patients (48.0%) were over 65 years old, and 36 (52.0%) were younger than 65 years old[11]. The mean size of tumor at diagnosis was (2.24±0.5)cm for BCC, (2.35±0.4)cm for SGC, (2.09±0.6)cm for SCC and (2.06±0.4)cm for MM. There was no statistically significant difference between the histological subtypes in terms of size of tumor (P>0.05). Of all patients, 41 were men and 34 were women. Female predominance was seen in patients with BCC (24 females, 11 males), whereas SGC, SCC, MM occurred more frequently in men (Figure 1).
Figure 1. Sex distribution of malignant eyelid tumors.
Female predominance was seen in patients with BCC (24 females, 11 males), whereas SGC, SCC, MM occurred more frequently in men.
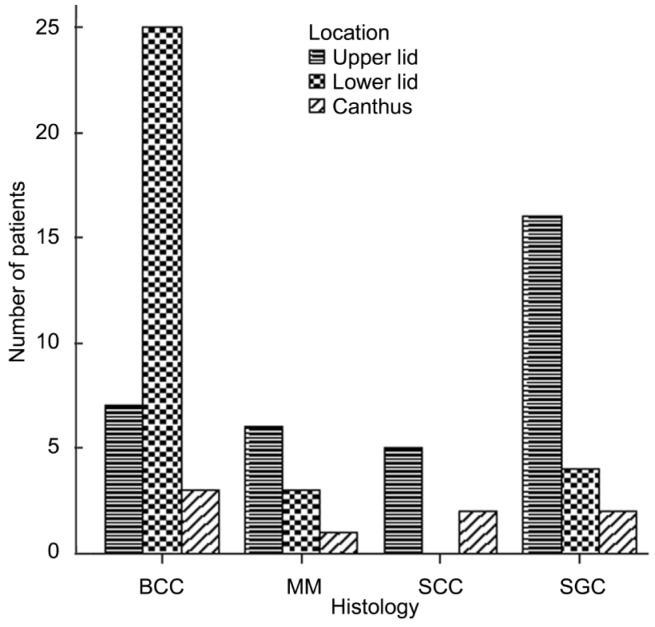
BCC lesions presented on the lower eyelids in 25/35 patients (71.4%). In contrast, SGC, SCC, and MM occurred more commonly on the upper eyelid (SGC, 72.7%; SCC, 71.4%; MM, 60.0%) (Figure 2).
Figure 2. Anatomic locations of malignant eyelid tumors.
BCC lesions presented on the lower eyelids in 25/35 patients (71.4%). In contrast, SGC, SCC, and MM occurred more commonly on the upper eyelid (SGC, 72.7%; SCC, 71.4%; MM, 60.0%).
Treatment and Disease Course
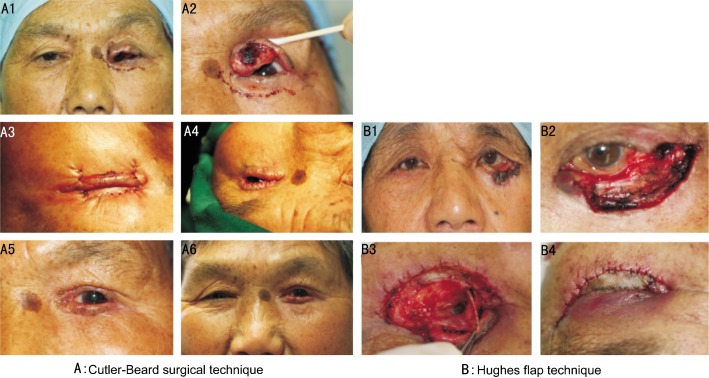
The most common primary treatment for tumors was histological controlled excision with eyelid reconstruction (60/75, 80.0%). Eyelid reconstructive technique included direct closure for defects measuring less than 25%, direct closure with lateral canthotomy and cantholysis for defects measuring slightly larger than 25%, Tenzel semicircular rotational flap for defects up to 2/3's of upper or lower lid width. Lid-sharing procedure was used when the defect is too large to be closed by Tenzel semicircular rotational flap, such as Hughes flap technique for lower eyelid defect, reverse Hughes flap or Cutler-Beard surgical technique for upper eyelid defect (Figure 3)[12],[13].
Figure 3. Eyelid reconstruction for defects using Cutler-Beard surgical technique (Figure 3A) and Hughes flap technique (Figure 3B) .
A1 and A2: Preoperative photo of sebaceous gland carcinoma of left upper eyelid; A3: Immediate postoperative figure after Cutler-Beard surgical technique; A4: Secondary separation procedure of the bridge flap; A5 and A6: Photograph at 2 weeks of separation procedure. B1: Preoperative photo of basal cell carcinoma of left lower eyelid; B2: 80% defect of lower eyelid after complete excision of basal cell carcinoma; B3: Tarsoconjunctival flap from upper eyelid sutured to the edge of lower eyelid defect; B4: Full thickness skin graft for reconstruction of anterior lamella and the immediate postoperative photo after Hughes flap technique.
Adjuvant radiotherapy was given after surgical treatment in 7 cases (7/75, 9.3%). Indications for adjuvant radiotherapy were unclear tumor margins in SGC, SCC or MM, nodal distant metastatic disease in MM or SGC, and patient with MCC. Exenteration was performed in 6 patients (6/75, 8.0%); the exenteration rate was significantly higher for MM (4/10, 40.0%) than for other tumor types [SGC (1/22, 4.5%); SCC (1/7, 14.2%)].
Ten patients (10/75, 13.3%) had less than 1 year of follow-up, and 12 patients (12/75, 16.0%) had more than 5 years of follow-up. The median follow-up was 21 months (range, 1-78) at the time of data collection. Local recurrence had developed in 17 cases (17/75, 22.7%). Management of recurrence included excisional biopsy (11/17, 64.7%), excisional biopsy and cryotherapy (4/17, 23.5%), chemotherapy (1/17, 5.9%), and orbital exenteration (1/17, 5.9%).
The mean recurrence-free time was 21 months (range, 3-72) after the initial surgery. Tumors located at the canthus had a higher recurrence rate (50.0%) than those located on the eyelid (19.4%, P<0.05). The recurrence rate was 11.4% for BBC, 13.7% for SGC, 57.1% for SCC, and 60.0% for MM. The difference in recurrence-free interval was statistically significant between BCC and SCC (P=0.005); BCC and MM (P=0.000); SGC and SCC (P=0.044), and SGC and MM (P=0.009) by Kaplan-Meier analysis. However, the difference in recurrence-free interval between BCC and SGC (P=0.463) and between SCC and MM (P=0.506) was not statistically significant. Histopathologic features comprised the only independent factor of tumor recurrence by Cox regression analysis (P=0.001, Table 2).
Table 2. Independent risk factors according to recurrence by Cox regression analysis.
| Features | Groups | RR | 95% CI | P | |
| Histology | BCC+SGC | 6.201 | 2.216 | 17.347 | 0.001 |
| SCC+MM | |||||
| Location | UL+LL | 2.843 | 0.859 | 9.409 | 0.087 |
| Canthus | |||||
RR: Relative risk; CI: Confidence interval.
DISCUSSION
Only a few large studies of malignant eyelid tumors in Asian population have been reported[14],[15]. Our series focuses on clinicopathologic features of malignant eyelid tumors and prognostic factors for recurrence.
Basal-cell carcinoma is the most common type of skin cancer. In the largest series of excised benign and malignant eyelid lesions (5 504 specimens), BCC comprised 14% of all lesions and 86% of malignancies[16]. Statistically, approximately 3 out of 10 Caucasians may develop a basal-cell cancer within their lifetime[17]. The classical constituent “basaloid” cells of BCC are small and ovoid or spindled, with a high nuclear-tocytoplasmic ratio, thereby creating the microscopic appearance of a hematoxylophilic or “blue” tumor. The tumors are characteristically organized into variably sized lobules exhibiting peripheral palisading and clefting artifact[18]. It rarely metastasizes or kills. However, it can cause significant destruction and disfigurement by invading surrounding tissues[17]. Sebaceous carcinoma is a malignant neoplasm that arises from the sebaceous glands and occurs most often in the eyelids[19],[20]. Owing to its rarity and its ability to masquerade as other periocular benign conditions such as chalazion, conjunctivitis, blepharitis, and keratoconjunctivitis, diagnosis of the disease might be difficult. Prognosis is still regarded as being poor compared with most other malignant eyelid tumors with a mortality second only to malignant melanoma. A delay in diagnosis is a common factor contributing to increased morbidity and mortality[21]. In America, the tumor is rare with an incidence ranging from 0.2% to 4.7% of malignant epithelial eyelid tumors[10],[22],[23]. While in China, the tumor is reported to occur in 28% of the lid cancers [24]. Squamous cell carcinoma and melanoma, although much less common than basal cell carcinoma, behave more aggressively. Palpation of preauricular and submandibular lymph nodes is crucial to detect potential metastatic spread. During examination of the melanoma, the physician should always evert the eyelid to look for conjunctival involvement. Systemic evaluation for regional or distant metastasis is necessary with the diagnosis of squamous cell carcinoma and melanoma[25]. Merkel cell carcinoma (MCC) is a rare and aggressive neuroendocrine skin malignancy that occasionally affects the eyelid, with the potential for regional and distant metastasis[26]. MCC tends to occur in elderly Caucasians, it has been reported that more than 90% of patients with MCC are Caucasian. Ultraviolet exposure and the Merkel cell polyomavirus appear pathogenic for MCC. There are few reports of MCC in Asian patients[27]. The number of cases in our present study was only one. Wide excision is the primary modality of treatment. Consideration should be given to the use of prophylactic adjunctive therapies beyond wide surgical excision. As MCC is known to be highly radiosensitive, radiotherapy plays an important role in the management of patients with MCC. Adjuvant radiotherapy could be positively considered if the tumor is large and the lesion is not confined to the dermis[27].
In agreement with prior reports, BCC is the most common eyelid malignancy. BCC comprised 46.7% of the lesions in our study. Although SGC seems to be rare among Caucasians [28],[29], it was much more common than SCC and MM in our study, ranking second and constituting 29.3% of the lesions in our study. MM ranked third (13.3%) and SCC ranked fourth, comprising 9.3% of tumors in our series (Table 3).
Table 3. Incidence of malignant eyelid tumors reported in different studies from Asia, NA, and Europe.
| Region | BCC | SGC | SCC | MM |
| Asia | ||||
| China & Korea (this study) | 46.7 | 29.3 | 9.3 | 13.3 |
| Taiwan[14] | 62.2 | 23.6 | 8.7 | 3.9 |
| Japan[15] | 39.5 | 28.9 | 10.5 | 7.9 |
| India[35] | 48.2 | 31.2 | 13.7 | 4.7 |
| NA | ||||
| Minnesota[28] | 90.8 | - | 8.6 | 0.6 |
| Alabama[29] | 83.7 | 1.7 | 10.3 | 2.6 |
| Europe | ||||
| Switzerland[16] | 86 | 3 | 7 | 0.2 |
| Romania[2] | 72.5 | 2 | 19.6 | 5.9 |
BCC: Basal cell carcinoma; SGC: Sebaceous gland carcinoma; SCC: Squamous cell carcinoma; MM: Malignant melanoma.
(%)
The mean age at diagnosis of all eyelid malignancies in our study was 62.4 years, similar to that found in studies in Singapore[5] and Taiwan[14], suggesting that eyelid malignancy tends to be a disease of older adults. We found that 68.6% of BCC cases occurred in females, a finding consistent with some reports that females are more prone to BCC than males[30],[31]. This differs from gender distribution of SGC, SCC, and MM, which show greater predilection for males: SGC, 72.7%; SCC, 85.7%; and MM, 70.0%.
In our study, BCC affected the lower eyelid more frequently than the upper eyelid or canthus. The predominance of BCC in the lower eyelid has been shown in various studies[32],[33]. Among whites, light pigmentation is a risk factor for BCC; conversely, the disease is rare among blacks. These evidences suggest that BCC is related to chronic and cumulative solar damage, and it is more common in fair-skinned and elderly adults[32]. The relation between BCC and sun exposure had been proposed by various researchers. Up to 30% of Caucasians develop basal-cell carcinomas in their lifetime[17]. Paul et al[34] reported that BCC comprised 71.8% of malignancies and sebaceous cell carcinoma with 7.3%. In our study, BCC comprised 46.7% of the lesions. As most people in China and South Korea tend to be yellow skin with more pigmentation than white in general, the incidence for BCC is correspondingly lower than that reported in the West. The incidence of SGC in the eyelid is subject to considerable geographical variation. Among white people the tumor is rare and according to reports from the USA, represents between 0.2% to 4.7% of malignant epithelial eyelid tumors[22],[23]. In China and other Asian countries forming the western Pacific seaboard, the incidence appears to be much higher; one study from gave an incidence of 28% of all eyelid malignancies in China[24]. In agreement with the published studies [15],[35], we found an incidence of 29.3% of the lesions in our study. SGC is most common in the upper eyelid and second most common in the lower eyelid. This is as expected, because most SGC lesions originate in the meibomian glands of the tarsus, which are much more abundant in the upper eyelids[20].
The recurrence rate of BCC was 11.4% in our series. The mean time to recurrence (recurrence-free time) was 74 months for BCC. The mean time to recurrence or metastasis after primary treatment was longer for BCC than for SGC (47 months), SCC (30 months), or MM (22 months). Thus, it is reasonable to suggest that BCC should be followed up for a longer period.
It was not the purpose of this article to compare outcomes of various treatment modalities. There is a general consensus that complete surgical excision is the essential element in the treatment of primary malignant eyelid tumors. If an eyelid lesion is localized and seems to be well circumscribed, it can be managed by excision and eyelid reconstruction with pathological section control of surgical margins. An evidence-based review reported that for basal cell carcinoma, squamous cell carcinoma and sebaceous gland carcinoma, the published evidence supporting two recommendations (Moh's micrographic surgery or excision with frozen-section control) were graded as I (providing strong evidence in support of a recommendation). For sebaceous gland carcinoma, the recommendations also included conjunctival map biopsies. It concluded that the strongest evidence favors complete surgical removal using histologic controls for verifying tumor-free margins of excision[36]. Supplemental cryotherapy, topical chemotherapy, and irradiation should be applied if the tumor margin is unclear or if there is residual involvement of bulbar conjunctiva[37]-[39].
The results of our retrospective study indicate that histologic type is the most important prognostic indicator for recurrence of malignant eyelid tumors. In our cohort, SCC and MM gain statistical significance as a prognostic factor for high recurrence rate and relatively short recurrence-free interval compared to BCC and SGC. Although location of the tumor in the canthus seemed to be associated with high recurrence (50.0%) than those located on the eyelid (19.4%) (P<0.05) and short recurrence-free interval (mean 22 months), when it comes to independent factor of tumor recurrence by Cox regression analysis, this difference was not statistically significant. Similarly, sex, age were not important prognostic indicators.
In summary, we have reviewed our experience with 75 patients with malignant eyelid tumors. Although most of these tumors have a relatively favorable course, early diagnosis remains essential for adequate functional and cosmetic lid reconstruction. It is especially important to achieve a negative tumor margin in canthus-located tumors. As Cox regression analysis showed that histologic type is the only significant independent prognostic indicator for recurrence of malignant eyelid tumors in our study, clinicians should have a high level of suspicion of recurrence according to histopathological type when treating patients with eyelid tumor. Since our present study is a retrospective record based study, it does have a few limitations. Therefore, much more prospective randomised, case-controlled studies with close and long-term follow-up should be conducted to identify the clinicopathologic features and prognosis of malignant eyelid tumors.
Footnotes
Foundation items: National Natural Science Foundation of China (No. 81070756); National “Twelfth Five-Year” Plan for Science and Technology Support Program (No. 2012BAI08B01); Zhejiang Key Innovation Team Project of China (No. 2009R50039); Zhejiang Key Laboratory Fund of China (No. 2011E10006)
REFERENCES
- 1.Lober CW, Fenske NA. Basal cell, squamous cell, and sebaceous gland carcinomas of the periorbital region. J Am Acad Dermatol. 1991;25(4):685–690. doi: 10.1016/0190-9622(91)70254-y. [DOI] [PubMed] [Google Scholar]
- 2.Coroi MC, Rosca E, Mutiu G, Coroi T, Bonta M. Eyelid tumors: histopathological and clinical study performed in County Hospital of Oradea between 2000–2007. Rom J Morphol Embryo. 2010;51(1):111–115. [PubMed] [Google Scholar]
- 3.Tesluk GC. Eyelid lesions: incidence and comparison of benign and malignant lesions. Ann Ophthalmol. 1985;17(11):704–707. [PubMed] [Google Scholar]
- 4.Margo CE, Waltz K. Basal cell carcinoma of the eyelid and periocular skin. Surv Ophthalmol. 1993;38(2):169–192. doi: 10.1016/0039-6257(93)90100-l. [DOI] [PubMed] [Google Scholar]
- 5.Lee SB, Saw SM, Au Eong KG, Chan TK, Lee HP. Incidence of eyelid cancers in Singapore from 1968 to 1995. Br J Ophthalmol. 1999;83(5):595–597. doi: 10.1136/bjo.83.5.595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ni C, Searl SS, Kuo PK, Chu FR, Chong CS, Albert DM. Sebaceous cell carcinomas of the ocular adnexa. Int Ophthalmol Clin. 1982;22(1):23–61. doi: 10.1097/00004397-198202210-00006. [DOI] [PubMed] [Google Scholar]
- 7.Nelson BR, Hamlet KR, Gillard M, Railan D, Johnson TM. Sebaceous carcinoma. J Am Acad Dermatol. 1995;33(1):1–15. doi: 10.1016/0190-9622(95)90001-2. [DOI] [PubMed] [Google Scholar]
- 8.Rao NA, Hidayat AA, McLean IW, Zimmerman LE. Sebaceous carcinomas of the ocular adnexa: a clinicopathologic study of 104 cases, with five-year follow-up data. Hum Pathol. 1982;13(2):113–122. doi: 10.1016/s0046-8177(82)80115-9. [DOI] [PubMed] [Google Scholar]
- 9.Burns SJ, Foss AJ, Butler TK. Outcome of periocular sebaceous gland carcinoma. Ophthal Plast Reconstr Surg. 2005;21(5):353–355. doi: 10.1097/01.iop.0000176273.32152.70. [DOI] [PubMed] [Google Scholar]
- 10.Doxanas MT, Green WR. Sebaceous gland carcinoma: review of 40 cases. Arch Ophthalmol. 1984;102(2):245–249. doi: 10.1001/archopht.1984.01040030195025. [DOI] [PubMed] [Google Scholar]
- 11.Esmaeli B, Wang B, Deavers M, Gillenwater A, Goepfert H, Diaz E, Eicher S. Prognostic factors for survival in malignant melanoma of the eyelid skin. Ophthal Plast Reconstr Surg. 2000;16(4):250–257. doi: 10.1097/00002341-200007000-00002. [DOI] [PubMed] [Google Scholar]
- 12.Gündüz K, Demirel S, Günalp I, Pola B. Surgical approaches used in the reconstruction of the eyelids after excision of malignant tumors. Ann Ophthalmol. 2006;38(3):207–212. doi: 10.1007/s12009-006-0006-1. [DOI] [PubMed] [Google Scholar]
- 13.Lee SY, Kim YD, Khwarg SI, Kim S. Atlas of eyelid plastic surgery. Chapter 14, Korea. 2009:261–303. [Google Scholar]
- 14.Wang JK, Liao SL, Jou JR, Lai PC, Kao SC, Hou PK, Chen MS. Malignant eyelid tumours in Taiwan. Eye (Lond) 2003;17(2):216–220. doi: 10.1038/sj.eye.6700231. [DOI] [PubMed] [Google Scholar]
- 15.Takamura H, Yamashita H. Clinicopathological analysis of malignant eyelid tumor cases at Yamagata University Hospital: statistical comparison of tumor incidence in Japan and in other countries. Jpn J Ophthalmol. 2005;49(5):349–354. doi: 10.1007/s10384-005-0229-5. [DOI] [PubMed] [Google Scholar]
- 16.Deprez M, Uffer S. Clinicopathological features of eyelid skin tumors: a retrospective study of 5 504 cases and review of literature. Am J Dermatopathol. 2009;31(3):256–262. doi: 10.1097/DAD.0b013e3181961861. [DOI] [PubMed] [Google Scholar]
- 17.Wong CS, Strange RC, Lear JT. Basal cell carcinoma. BMJ. 2003;327(7418):794–798. doi: 10.1136/bmj.327.7418.794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kirzhner M, Jakobiec FA. Clinicopathologic and immunohistochemical features of pigmented basal cell carcinomas of the eyelids. Am J Ophthalmol. 2012;153(2):242–252. doi: 10.1016/j.ajo.2011.07.008. [DOI] [PubMed] [Google Scholar]
- 19.Shields JA, Demirci H, Marr BP, Eagle RC, Jr, Shields CL. Sebaceous carcinoma of the ocular region: a review. Surv Ophthalmol. 2005;50(2):103–122. doi: 10.1016/j.survophthal.2004.12.008. [DOI] [PubMed] [Google Scholar]
- 20.Shields JA, Demirci H, Marr BP, Eagle RC, Jr, Shields CL. Sebaceous carcinoma of the eyelids: personal experience with 60 cases. Ophthalmology. 2004;111(12):2151–2157. doi: 10.1016/j.ophtha.2004.07.031. [DOI] [PubMed] [Google Scholar]
- 21.Zürcher M, Hintschich CR, Garner A, Bunce C, Collin JR. Sebaceous carcinoma of the eyelid: a clinicopathological study. Br J Ophthalmol. 1998;82(9):1049–1055. doi: 10.1136/bjo.82.9.1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Boniuk M, Zimmerman LE. Sebaceous carcinoma of the eyelid, eyebrow, caruncle, and orbit. Trans Am Acad Ophthalmol Otolaryngol. 1968;72(4):619–642. [PubMed] [Google Scholar]
- 23.Ginsberg J. Present status of meibomian gland carcinoma. Arch Ophthalmol. 1965;73:271–277. doi: 10.1001/archopht.1965.00970030273022. [DOI] [PubMed] [Google Scholar]
- 24.Ni C, Kuo PK. Meibomian gland carcinoma: a clinicopathological study of 156 cases with long-period follow-up of 100 cases. Jpn J Ophthalmol. 1979;23:388–401. [Google Scholar]
- 25.Carter SR. Eyelid disorders: diagnosis and management. Am Fam Physician. 1998;57(11):2695–2702. [PubMed] [Google Scholar]
- 26.Tarantola TI, Vallow LA, Halyard MY, Weenig RH, Warschaw KE, Grotz TE, Jakub JW, Roenigk RK, Brewer JD, Weaver AL, Otley CC. Prognostic factors in Merkel cell carcinoma: analysis of 240 cases. J Am Acad Dermatol. 2013;68(3):425–432. doi: 10.1016/j.jaad.2012.09.036. [DOI] [PubMed] [Google Scholar]
- 27.Woo KJ, Choi YL, Jung HS, Jung G, Shin YK, Jang KT, Han J, Pyon JK. Merkel cell carcinoma: our experience with seven patients in Korea and a literature review. J Plast Reconstr Aesthet Surg. 2010;63(12):2064–2070. doi: 10.1016/j.bjps.2010.01.020. [DOI] [PubMed] [Google Scholar]
- 28.Cook BE, Jr, Bartley GB. Epidemiologic characteristics and clinical course of patients with malignant eyelid tumors in an incidence cohort in Olmsted County, Minnesota. Ophthalmology. 1999;106(4):746–750. doi: 10.1016/S0161-6420(99)90161-6. [DOI] [PubMed] [Google Scholar]
- 29.Swanson MW, Cloud G. A retrospective analysis of primary eye cancer at the University of Alabama 1958–1988. Part 2: Eyelid tumors. J Am Optom Assoc. 1991;62(11):820–823. [PubMed] [Google Scholar]
- 30.Pieh S, Kuchar A, Novak P, Kunstfeld R, Nagel G, Steinkogler FJ. Long-term results after surgical basal cell carcinoma excision in the eyelid region. Br J Ophthalmol. 1999;83(1):85–88. doi: 10.1136/bjo.83.1.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Steinkogler FJ, Scholda CD. The necessity of long-term follow up after surgery for basal cell carcinomas of the eyelid. Ophthalmic Surg. 1993;24(11):755–758. [PubMed] [Google Scholar]
- 32.Vaziri M, Buffam FV, Martinka M, Oryschak A, Dhaliwal H, White VA. Clinicopathologic features and behavior of cutaneous eyelid melanoma. Ophthalmology. 2002;109(5):901–908. doi: 10.1016/s0161-6420(02)00962-4. [DOI] [PubMed] [Google Scholar]
- 33.Mamalis N, White GL, Jr, Pedersen DM, Holds J, Anderson RL. Malignant lesions of the eyelid. Am Fam Physician. 1989;39(1):95–102. [PubMed] [Google Scholar]
- 34.Paul S, Vo DT, Silkiss RZ. Malignant and benign eyelid lesions in San Francisco: study of a diverse urban population. Am J Clin Med. 2011;8(1):40–46. [Google Scholar]
- 35.Kale SM, Patil SB, Khare N, Math M, Jai A, Jaiswal S. Clinicopathological analysis of eyelid malignancies - A review of 85 cases. Indian J Plast Surg. 2012;45(1):22–28. doi: 10.4103/0970-0358.96572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cook BE, Jr, Bartley GB. Treatment options and future prospects for the management of eyelid malignancies: an evidence-based update. Ophthalmology. 2001;108(11):2088–2098. doi: 10.1016/s0161-6420(01)00796-5. [DOI] [PubMed] [Google Scholar]
- 37.Yoon JS, Kim SH, Lee CS, Lew H, Lee SY. Clinicopathological analysis of periocular sebaceous gland carcinoma. Ophthalmologica. 2007;221(5):331–339. doi: 10.1159/000104764. [DOI] [PubMed] [Google Scholar]
- 38.Lisman RD, Jakobiec FA, Small P. Sebaceous carcinoma of the eyelids. The role of adjunctive cryotherapy in the management of conjunctival pagetoid spread. Ophthalmology. 1989;96(7):1021–1026. doi: 10.1016/s0161-6420(89)32798-9. [DOI] [PubMed] [Google Scholar]
- 39.Shields CL, Naseripour M, Shields JA, Eagle RC., Jr Topical mitomycin-C for pagetoid invasion of the conjunctiva by eyelid sebaceous gland carcinoma. Ophthalmology. 2002;109(11):2129–2133. doi: 10.1016/s0161-6420(02)01239-3. [DOI] [PubMed] [Google Scholar]