Abstract
AIM
To study eyes with extraocular dissemination (EORB), with the following aims: first to establish the mean lag period and to understand various reasons for delayed presentation, second to study their imaging profiles and third to analyze histopathological features of eyes enucleated after neoadjuvant chemotherapy.
METHODS
Prospective study of clinical and imaging features of EORBs (stage III and IV International Retinoblastoma Staging System) presenting to a tertiary eye care centre. Histopathological features of eyes enucleated after receiving neoadjuvant chemotherapy were analyzed. A pictorial illustration of the varied imaging profile of EORB was also presented.
RESULTS
Over a period of one year, 97 eyes were diagnosed with retinoblastoma; 32 children (36 eyes) (37.1%) had EORB. Mean age 3.6±1.9 years, 71.9% males, 71.9% unilateral, 3.1% with positive family history and 40.6% with metastasis. On imaging, there was extrascleral involvement in 22.2%, involvement of orbital part of optic nerve in 33.3%, involvement of central nervous system in 27.8% and orbital wall involvement in 2.9% eyes. On histopathological analysis of eyes enucleated after neoadjuvant chemotherapy, 25.0% had no residual viable tumour tissue and rest all tumours were poorly differentiated.
CONCLUSION
There are very few human malignancies where definitive treatment is started without any confirmed histopathological diagnosis and imaging plays an important role in diagnosis and appropriate staging of the disease. Chemotherapy has a variable effect on EORB, 75.0% of eyes with EORB had residual viable tumour tissue when enucleated after receiving neoadjuvant chemotherapy.
Keywords: extraocular retinoblastoma, imaging, histopathological features
Introduction
Retinoblastoma is the most common primary intraocular malignancy in children[1]. The tumour is highly curable when confined within coats of the eye, either by globe preserving methods [early intraocular; group A-C, international classification of retinoblastoma (ICRB)] or by enucleation (advanced intraocular; group D-E, ICRB); thus leading to a high probability of disease free survival. Once the tumour extends through the coats of the eye, it has access to the vascular channels of the orbit and to the central nervous system through the optic nerve[2]. In developing countries, rates of ocular salvage and patient survival are low since the diagnosis is frequently made at a later stage, when extraocular dissemination (EORB) has already occurred[3]-[5]. Ignorance on behalf of the parents, delay in referral on behalf of the general practitioner/pediatrician and refusal of medical advice have been associated with delayed diagnosis and treatment[6]-[9].
Herein we describe clinical, imaging and histopathological features of eyes with EORB at a major tertiary referral hospital of North India. The aims of the study were; firstly to establish the mean lag period between the first symptom and initiation of treatment and to understand various reasons for the delayed presentation; since imaging is a vital tool for diagnosis and appropriate staging of patients with EORB, our second aim was to study their imaging profile at presentation; thirdly we have analyzed histopathological features of eyes which were enucleated after neoadjuvant chemotherapy in order to understand their behavior after chemoreduction.
SUBJECTS AND METHODS
Subjects
We conducted a prospective analysis of clinical, imaging and histopathology features of children with EORB (stage III and IV; international retinoblastoma staging system) who presented at our hospital from May 2008 to April 2009. EORB was defined as tumor extending beyond the globe, clinically as well as on imaging and lag period was defined as lag time between noticing the first symptom and the initiation of treatment (in months). Demographic data included age, sex, laterality (unilateral vs bilateral) and family history of retinoblastoma, which was collected at the time of presentation.
Methods
Parents were enquired about the duration of lag period, any consultation (with general practitioner, pediatrician, ophthalmologist or others) taken in this period and reasons for the delayed presentation. The imaging features on computed tomography (CT scan) and/or magnetic resonance imaging (MRI) were reviewed by the radiologist with special reference to the involvement of optic nerve, extrascleral and central nervous system involvement. Further evaluation for systemic metastasis (CSF and bone marrow) was undertaken by the pediatric oncologist. Staging was done as per the current International Retinoblastoma Staging System (IRSS) proposed by Chantada in 2006[10]. The recommended protocol of 12 cycles of VEC (Vincristine, Etopside, Carboplatin), with enucleation after completion of 3-6 cycles along with adjuvant external beam radiotherapy, was followed. Histopathological features of eyes which were enucleated after receiving neo-adjuvant chemotherapy were also studied. A pictorial illustration of the varied imaging profile of EORB is also presented. Information regarding subsequent treatment and long term survival of these children is not a part of the study.
RESULTS
During the study period, a total of 97 eyes were diagnosed with retinoblastoma at our center; 32 children (36 eyes) (37.1%) had EORB. There were 71.9% (23/32) males; 1 patient (3.1%) had positive family history. There was bilateral involvement in 28.1% children (9/32); 6.2% (2 patients) had early intraocular retinoblastoma (ICRB group A-C) of the other eye, 6.2% (2 patients) had advanced intraocular tumor (ICRB group D-E) involving the other eye, 12.5% (4 patients) had bilateral EORB (IRSS stage III, IV) at presentation, and 3.1% (1 patient) had other eye enucleated owing to advanced intraocular retinoblastoma. The overall mean age of presentation was 3.6±1.9 years (range: 11 months to 8 years); in children with unilateral retinoblastoma, the mean age was 3.6±1.04 years (range: 11 months to 8 years) and in bilateral cases, the mean age was 3.3±1.06 years (range: 2 to 5 years); the difference in age between the unilateral and bilateral group was not statistically significant.
The initial mode of presentation was varied, there was large extraocular mass in 41.7% (15/36) eyes, proptosis in 41.7% (15/36), staphyloma in 11.1% (4/36) and orbital cellulites in 5.5% (2/36) eyes (Figure 1). About 87.0% of parents had noticed leukokoria at one or the other time before presentation to us. The mean lag period in different groups is summarized in Table 1. The various reasons given by parents for delayed presentation was unawareness about the seriousness of the disease (75.0%), fear of enucleation (6.2%), lack of appropriate finances to arrange for their travel (6.2%) and lack of timely referral by the general practioner / pediatrician with whom the parents had taken an earlier consultation (12.5%). During the lag period, 68.8% (22/32) had not taken any other consultation, 25.0% (8/32) had consulted general practitioner (5 of them had been advised further referral to an ophthalmologist but none of them had been told about the seriousness of the disease) and 6.2% (2/32) had consulted a general ophthalmologist nearby (2 of them had been advised enucleation).
Figure 1. Clinical photographs of children with EORB.
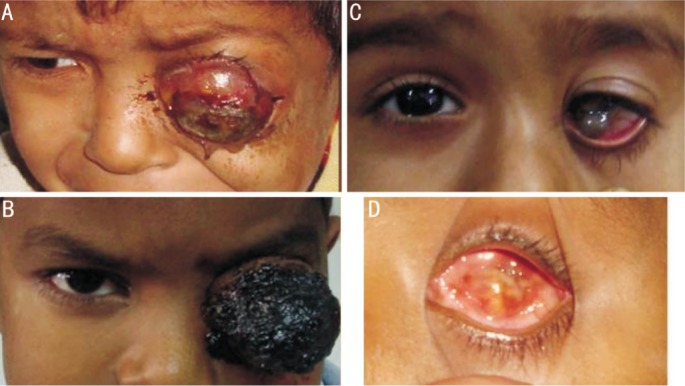
A: A 5 years old male child presenting with left eye severe proptosis; the parents had been noticing leukokoria since the last 18 months but had not considered significant enough to warrant consultation; B: A 5 years old male child presenting with a large fungating mass left eye since the last 2 months, the history of leukokoria dated back to the preceding year; C: A 7 years old female child presented with complaint of something protruding out of the left eye; on examination there was a large limbal staphyloma. Imaging revealed presence of a large retinoblastoma extending upto optic chiasma. Note that this presentation of retinoblastoma is extremely rare; D: Appearance of a phthisical eye post neoadjuvant chemotherapy.
Table 1. Lag period between first symptom and initiation of treatment in children presenting as EORB (months).
| Parameters | Lag period | Range |
| Lag period | 8.5±2.17 | 3-14 |
| Lag period according to laterality | ||
| Unilateral | 8.12±1.97 | 3-12 |
| Bilateral | 9.70±3.34 | 4-14 |
| P value not significant | ||
| Lag period according to age | ||
| 0-2 years | 7.75±2.77 | 4-14 |
| 2-4 years | 9.47±3.20 | 4-12 |
| More than 4 years | 7.67±2.24 | 3-12 |
| P value not significant |
The imaging features are summarized in Table 2 (Figures 2–7). Direct extension of the tumor to the central nervous system was found in 28.1% (9/32) cases (prechiasmatic and suprasellar involvement) and distant metastasis in 12.5% cases at presentation (2/32 with lymph node metastasis, 1/32 with leptomeningeal and 1/32 with bone metastasis).
Table 2. Imaging features of 36 eyes with EORB.
| Imaging features | n(%) |
| Isolated extrascleral involvement | 8 (22.2) |
| Isolated involvement of orbital part of optic nerve | 12(33.3) |
| Involvement of both 1 and 2 | 5(13.9) |
| Involvement of the central nervous system | 10(27.8) |
| Prechiasmatic | 6 (16.7) |
| Suprasellar mass | 3 (8.3) |
| Leptomeningeal metastasis | 1 (2.8) |
| Orbital wall involvement | 1(2.8) |
Figure 2. Clinical and imaging features of a 7 years old child with EORB.
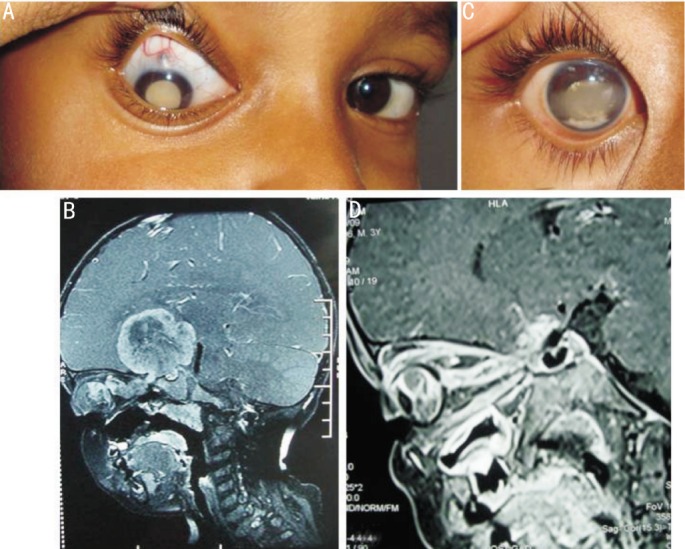
A: He presented with leukokoria and thinning of sclera; B: T1W sagittal post contrast MR image of the patient showing heterogeneously enhancing right intraocular mass having contagious giant intracranial extension along the optic nerve sheath complex into the suprasellar region; C: Appearance after 3 cycles of chemotherapy; D: T1W oblique saggital post contrast MR imaging of the same patient after 5 cycles of chemotherapy showing marked reduction of the intraorbital mass, resolution of optic nerve thickening with marked tram-track enhancement up to the chiasma. Note that there is near total resolution of the suprasellar mass (post-chemotherapy).
Figure 7. Coronal reconstructed computed tomography image showing a contiguous destruction of the right zygomatic bone because of the adjacent extraocular retinoblastoma.
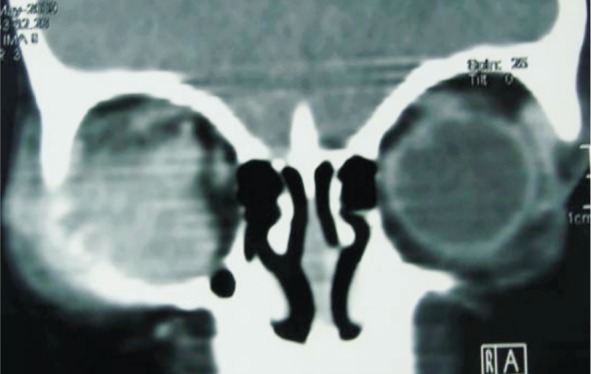
Figure 3. Imaging features of EORB.
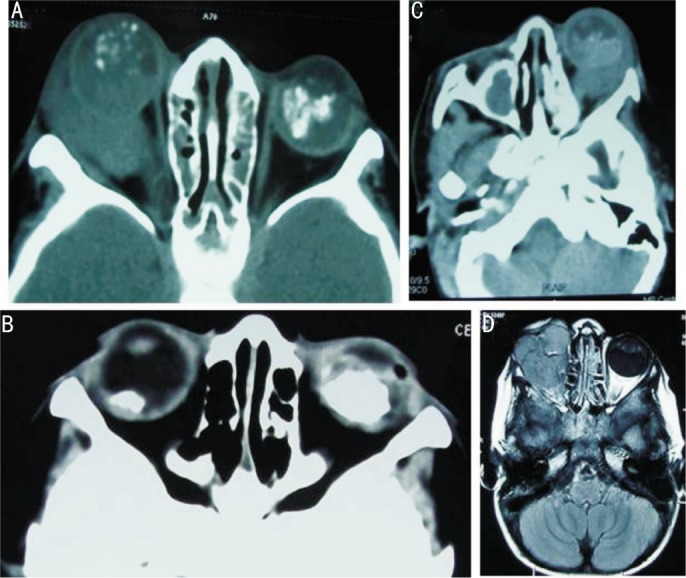
A: Wide window setting right intraocular mass with speckled foci of calcification with perforation of globe posteriorly and a large mass causing proptosis with optic nerve extension reaching up to the orbital apex. There is a soft tissue mass in left eye with dense calcification with no extraocular extension; B: Bilateral post chemo computed tomography images showing calcified mass in both globes, much larger on left side. The left globe is smaller in size; there is a small soft tissue irregularity of left globe on the temporal aspect, a telltale evidence of a previous extraocular extension at this end; C: Axial CT wide window setting showing the left globe with a large mass posteriorly with retrobulbar extension and proptosis with right side post enucleation with bony remodeling. There is an incidental opacification of right maxillary antrum; D: T1W axial post contrast image showing large lobulated mildly enhancing soft tissue mass in a patient with previous history of enucleation for advanced intraocular retinoblastoma. This is a sign of a tumor recurrence.
Figure 4. Mass seen completely filling left globe with extraocular extension seen in form of staphyloma at and temporal to the optic nerve.
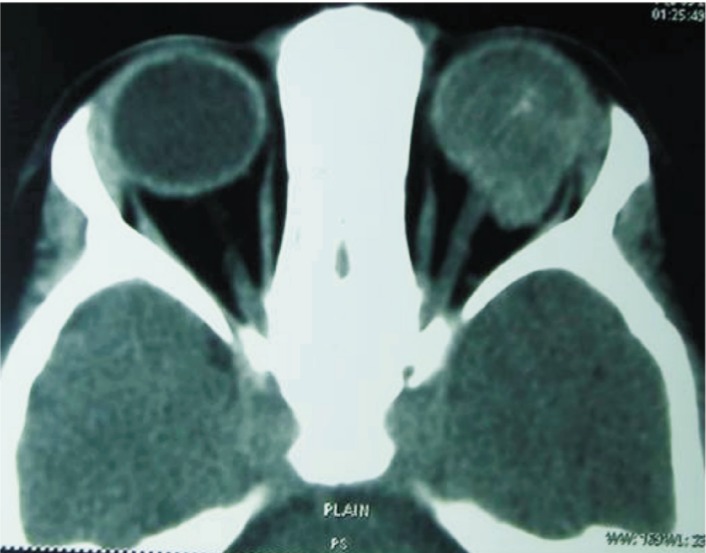
Figure 5. Imaging features of EORB.
A: T1W saggital post contrast magnetic resonance imaging showing optic nerve extension of an extraocular retinoblastoma and reaching the pre-chaismatic location; B: Oblique reconstructed image of right globe (non-contrast) showing marked optic nerve thickening and large suprasellar mass; C: Axial brain CT image of the same patient showing densely enhancing suprasellar mass as a result of contiguous optic nerve extension; D: Axial post contrast computed tomography scan showing a calcified mass completely filling the globe with marked optic nerve thickening. Note the rare occurrence of cystic optic nerve enlargement reaching up to the orbital apex.
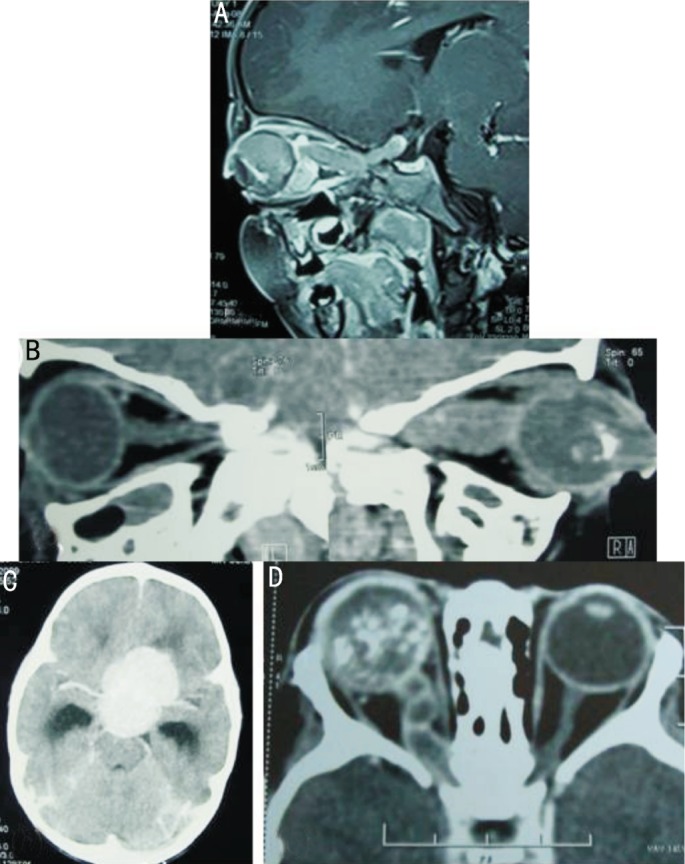
Figure 6. Imaging features of CNS involvement in EORB.
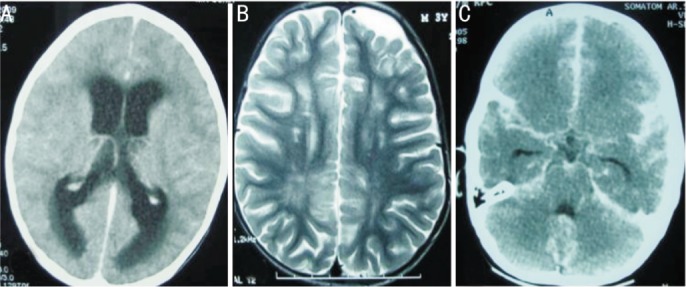
A: Axial post contrast CT showing symmetrical obstructive dilatation of bilateral lateral ventricles and periventricular ooze, especially noticed in peri-trigonal region, due to large suprasellar mass (not shown in this section) causing an obstruction to the CSF outflow; B: Axial spin echo T2W magnetic resonance imaging showing ex-vacuo dilatation of left frontal subarachnoid space secondary to underlying brain atrophy as a result of post readiotherapy changes. Ill defined hyperintense signals in the deep white matter, especially prominent in right posterior parietal location is presumably in keeping with periventricular leukomalacia changes as a result of hypoxic ischaemic encephalopathy (HIE); C: Axial post contrast computed tomography scan of the brain showing marked enhancement of the basal cisterns, sylvian fissures and subarachnoid space along the convexities consistent with extensive CSF pathway metastasis.
After staging the disease, all the parents were counseled about the treatment regimen. Parents of 3 children refused treatment, 2 children abandoned treatment after 1 chemotherapy cycle and 4 children were planned for palliative chemotherapy in lieu of massive central nervous system involvement. Rest 28 eyes (of 24 children) were enucleated after 3-6 cycles of neoadjuvant chemotherapy. Twenty-five percent (7/28) eyes had become phthsical with grossly distorted ocular structures and thickened coats of the eye with no viable tumour cells (Figure 8). Rest 21 eyes (75%) had presence of poorly differentiated tumor. Eleven eyes had invasion of cut section of optic nerve, 13 had massive choroidal invasion of which 7 had scleral / extrascleral involvement as well, 5 eyes had anterior segment invasion. There was presence of extensive necrosis in 35.7% (10/28), severe calcification in 92.8% (26/28), severe fibrosis in 39.3% (11/28) and foamy macrophages in 85.7% (24/28) eyes. Parents of all the 24 children who were enucleated were counseled for the need to complete the recommended 12 cycles of chemotherapy along with external beam radiotherapy; of them 13 children completed the recommended protocol (rest 11 children were lost to follow up and no information regarding their survival is available).
Figure 8. Histopathological features of EORB post chemotherapy.
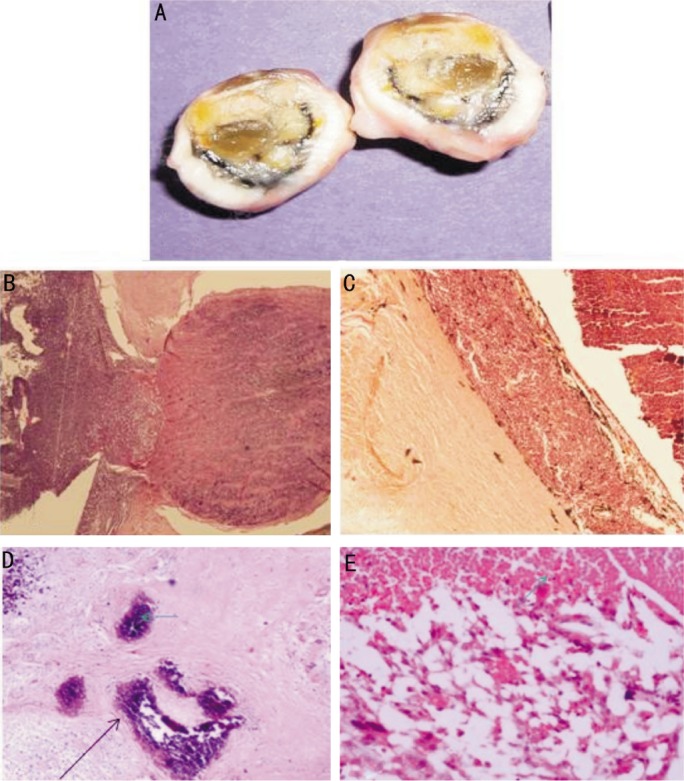
A: Cut section of a phthsical eye with extraocular retinoblastoma post-chemotherapy showing thickened sclera; B: Hematoxylin and Eosin stained sections showing invasion of the optic nerve head and retrolaminar optic nerve by the tumor (magnification ×40); C: Hematoxylin and Eosin stained sections showing massive choroidal invasion (magnification ×100); D: Extensive hyalinization (green arrow) and calcification (black arrow) after chemoreduction in the vitreous cavity (H&E ×200); E: Liquifactive necrosis seen after chemoreduction in the vitreous cavity (H&E ×200).
DISCUSSION
In developed countries, retinoblastoma is usually diagnosed in its early intraocular stages leading to high chances for preservation of vision, globe and disease free survival of the patient. However, in developing countries like ours, retinoblastoma is often diagnosed at a later stage with extraocular dissemination, thus leading to much lower rates of ocular salvage and patient survival[3], [9], [11]-[13]. We prospectively studied 32 consecutive children (36 eyes) diagnosed with extraocular retinoblastoma at our centre with respect to their clinical, imaging and histopathological features.
In our series, 37.1% eyes had extraocular disease (mean age: 43.2±22.8 months), with regional and distant metastasis in 40.6%. A major Brazilian series had reported EORBs in 20% of all new retinoblastoma patients (mean age: 32.9 months)[13]. A comparatively larger percentage of EORBs (52%) have been reported in a Malaysian study[14].
The mean lag period in our patients was 8.5±2.17 months, with no statistically significant difference as per laterality and age. Other studies from the developing world have also described a long lag period and have analyzed various reasons for the same[15],[16]. Erwenne and Franco[9] concluded that delayed presentation with extraocular invasion is strongly associated with age at diagnosis and lateness of referral. Antoneli et al[3] analyzed the lag time and concluded that longer lag time is associated with disseminated disease and this period, if shortened may lead to decrease in number of cases with extraocular dissemination. Another study concluded that it is not only longer lag time but also denial of management by parents that are responsible for the delayed diagnosis. The various reasons reported for parents opting out against the recommended treatment are limited access to ophthalmologist, lack of finances and health insurance and denial of enucleation[5].
Various methods have been suggested for education of the public thus leading to early detection and a better prognosis for vision and life[16]. Chantada et al[3] in his recent review on strategies to manage retinoblastoma in developing countries concluded that the priorities for management of retinoblastoma in developing countries should be opposite to that in developed nations i.e. treatment of overt extraocular retinoblastoma, that with high risk histopathological features and eye preserving conservative therapy, in decreasing order of priority. Use of media campaigns to increase public awareness, incorporation of education program linked with national vaccination campaign, appropriate conselling of parents as not to abondon treatment and education programmes for pediatricians and general ophthalmologists have all been suggested to avoid delays in diagnosis and management[3], [4], [7], [15], [16].
There are very few human malignancies where definitive treatment is started without any confirmed histopathological diagnosis and imaging plays an important role in diagnosis and staging of the disease. Imaging (preferably magnetic resonance imaging) is required to confirm the diagnosis, access for local spread into the orbit through the sclera or into the optic nerve, metastasis into the central nervous system and for trilateral retinoblastoma[17].
Since there is not much literature on behavior of these tumors after neoadjuvant chemotherapy, we also studied the histopathological features of enucleated eyes and found presence of residual viable tumour tissue in 75% of eyes enucleated after receiving neo-adjuvant chemotherapy. In a recent Indian study on orbital retinoblastoma patients, the authors concluded the presence of residual viable tissue in 95% of enucleated eyes[18]. Longer lag period was found to be associated with occurance of histopathological high risk factors in eyes undergone primary enucleation in another study from our centre[19]. In yet another study from our centre, we had studied histopathological profile of primarily enucleated eyes and concluded that poorly differentiated tumour present at later age and is a more aggressive form of presentation[20]. Occurrance of all PD tumours in EORBs further confirm the theory that in due course of time, the less severe well differentiated ones progress to the more aggressive poorly differentiated ones. Chemotherapy has a variable effect on retinoblastoma with extra ocular invasion; 25% of eyes enucleated after receiving neoadjuvant chemotherapy had no residual viable tumour tissue when enucleated after receiving neoadjuvant chemotherapy. Since viable tumour cells remain in most of the eyes post chemotherapy, the eye has to be enucleated. It's thus very important to counsel the parents in advance about the fact that the eye will need to be removed despite completion of systemic treatment.
To conclude, as in other developing countries, EORB is a common mode of presentation in Indian children as well. Since a long lag period is associated with greater risk for disseminated disease, a trial for reducing this lag period can probably reduce the number of extraocular cases. Firstly, it needs awareness on behalf of parents not only to know the importance of early detection of the signs but also to realize the seriousness of the disease and completion of treatment. Secondly, there is need for awareness on part of the pediatrician as well as ophthalmologist to detect the early signs and a timely referral even when they find the initial ophthalmic examination normal. Thirdly, a good accessibility to health care will help in timely initiation of treatment in children with retinoblastoma. EORBs have a varied imaging profile on presentation and neoadjuvant chemotherapy has a variable effect on children with extraocular dissemination of retinoblastoma.
Acknowledgments
Council of Scientific and Industrial research (CSIR), New Delhi, India.
REFERENCES
- 1.Kivelä T. The epidemiological challenge of the most frequent eye cancer: retinoblastoma, an issue of birth and death. Br J Ophthalmol. 2009;93(9):1129–1131. doi: 10.1136/bjo.2008.150292. [DOI] [PubMed] [Google Scholar]
- 2.Finger PT, Harbour JW, Karcioglu ZA. Risk factors for metastasis in retinoblastoma. Surv Ophthalmol. 2002;47(1):1–16. doi: 10.1016/s0039-6257(01)00279-x. [DOI] [PubMed] [Google Scholar]
- 3.Chantada GL, Qaddoumi I, Canturk S, Khetan V, Ma Z, Kimani K, Yeniad B, Sultan I, Sitorus RS, Tacyildiz N, Abramson DH. Strategies to manage retinoblastoma in developing countries. Pediatr Blood Cancer. 2011;56(3):341–348. doi: 10.1002/pbc.22843. [DOI] [PubMed] [Google Scholar]
- 4.Canturk S, Qaddoumi I, Khetan V, Ma Z, Furmanchuk A, Antoneli CB, Sultan I, Kebudi R, Sharma T, Rodriguez-Galindo C, Abramson DH, Chantada GL. Survival of retinoblastoma in less-developed countries impact of socioeconomic and health-related indicators. Br J Ophthalmol. 2010;94(11):1432–1436. doi: 10.1136/bjo.2009.168062. [DOI] [PubMed] [Google Scholar]
- 5.Navo E, Teplisky D, Albero R, Fandino AC, Demirdjian G, Chantada GL. Clinical presentation of retinoblastoma in a middle-income country. J Pediatr Hematol Oncol. 2012;34(3):e97–101. doi: 10.1097/MPH.0b013e31821d18f9. [DOI] [PubMed] [Google Scholar]
- 6.Chantada GL. Retinoblastoma: lessons and challenges from developing countries. Ellsworth Lecture 2011. Ophthalmic Genet. 2011;32(4):196–203. doi: 10.3109/13816810.2011.592173. [DOI] [PubMed] [Google Scholar]
- 7.Sitorus RS, Moll AC, Suhardjono S, Simangunsong LS, Riono P, Imhof S, Völker-Dieben HJ. The effect of therapy refusal against medical advice in retinoblastoma patients in a setting where treatment delays are common. Ophthalmic Genet. 2009;30(1):31–36. doi: 10.1080/13816810802464320. [DOI] [PubMed] [Google Scholar]
- 8.Chantada G, Fandiño A, Manzitti J, Urrutia L, Schvartzman E. Late diagnosis of retinoblastoma in a developing country. Arch Dis Child. 1999;80(2):171–174. doi: 10.1136/adc.80.2.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Erwenne CM, Franco EL. Age and lateness of referral as determinants of extra-ocular retinoblastoma. Ophthalmic Paediatr Genet. 1989;10(3):179–184. doi: 10.3109/13816818909009874. [DOI] [PubMed] [Google Scholar]
- 10.Chantada G, Doz F, Antoneli CB, Grundy R, Clare Stannard FF, Dunkel IJ, Grabowski E, Leal-Leal C, Rodríguez-Galindo C, Schvartzman E, Popovic MB, Kremens B, Meadows AT, Zucker JM. A proposal for an international retinoblastoma staging system. Pediatr Blood Cancer. 2006;47(6):801–805. doi: 10.1002/pbc.20606. [DOI] [PubMed] [Google Scholar]
- 11.Goddard AG, Kingston JE, Hungerford JL. Delay in diagnosis of retinoblastoma: risk factors and treatment outcome. Br J Ophthalmol. 1999;83(12):1320–1323. doi: 10.1136/bjo.83.12.1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rodrigues KE, Latorre Mdo R, de Camargo B. Delayed diagnosis in retinoblastoma. J Pediatr (Rio J) 2004;80(6):511–516. [PubMed] [Google Scholar]
- 13.Antoneli CB, Steinhorst F, de Cássia Braga Ribeiro K, Novaes PE, Chojniak MM, Arias V, de Camargo B. Extraocular retinoblastoma: a 13-year experience. Cancer. 2003;98(6):1292–1298. doi: 10.1002/cncr.11647. [DOI] [PubMed] [Google Scholar]
- 14.Menon BS, Alagaratnam J, Juraida E, Mohamed M, Ibrahim H, Naing NN. Late presentation of retinoblastoma in Malaysia. Pediatr Blood Cancer. 2009;52(2):215–217. doi: 10.1002/pbc.21791. [DOI] [PubMed] [Google Scholar]
- 15.Leander C, Fu LC, Peña A, Howard SC, Rodriguez-Galindo C, Wilimas JA, Ribeiro RC, Haik B. Impact of an education program on late diagnosis of retinoblastoma in Honduras. Pediatr Blood Cancer. 2007;49(6):817–819. doi: 10.1002/pbc.21052. [DOI] [PubMed] [Google Scholar]
- 16.Rodriguez-Galindo C, Wilson MW, Chantada G, Fu L, Qaddoumi I, Antoneli C, Leal-Leal C, Sharma T, Barnoya M, Epelman S, Pizzarello L, Kane JR, Barfield R, Merchant TE, Robison LL, Murphree AL, Chevez-Barrios P, Dyer MA, O'Brien J, Ribeiro RC, Hungerford J, Helveston EM, Haik BG, Wilimas J. Retinoblastoma: one world, one vision. Pediatrics. 2008;122(3):e763–770. doi: 10.1542/peds.2008-0518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schueler AO, Hosten N, Bechrakis NE, Lemke AJ, Foerster P, Felix R, Foerster MH, Bornfeld N. High resolution magnetic resonance imaging of retinoblastoma. Br J Ophthalmol. 2003;87(3):330–335. doi: 10.1136/bjo.87.3.330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Radhakrishnan V, Kashyap S, Pushker N, Sharma S, Pathy S, Mohanti BK, Vishnubhatla S, Ghose S, Bakhshi S. Outcome, pathologic findings, and compliance in orbital retinoblastoma (international retinoblastoma staging system stage III) treated with neoadjuvant chemotherapy: a prospective study. Ophthalmology. 2012;119(7):1470–1477. doi: 10.1016/j.ophtha.2012.01.029. [DOI] [PubMed] [Google Scholar]
- 19.Kashyap S, Meel R, Pushker N, Sen S, Bakhshi S, Sreenivas V, Sethi S, Chawla B, Ghose S. Clinical predictors of high risk histopathology in retinoblastoma. Pediatr Blood Cancer. 2012;58(3):356–361. doi: 10.1002/pbc.23239. [DOI] [PubMed] [Google Scholar]
- 20.Kashyap S, Sethi S, Meel R, Pushker N, Sen S, Bajaj MS, Chandra M, Ghose S. A histopathologic analysis of eyes primarily enucleated for advanced intraocular retinoblastoma from a developing country. Arch Pathol Lab Med. 2012;136(2):190–193. doi: 10.5858/arpa.2010-0759-OA. [DOI] [PubMed] [Google Scholar]


