Abstract
AIM
To compare the efficacy of the sole intravitreal triamcinolone (IVT) versus intravitreal bevacizumab (IVB) alone or IVB combined with IVT in the treatment of diabetic macular edema (DME).
METHODS
Pertinent publications were identified through systematic searches of database and manually searching. Methodological quality of the literatures was valuated according to the Jadad Score. RevMan 5.1.0 was used to do the meta-analysis. Heterogeneity was determined and sensitivity was conducted.
RESULTS
Six studies were ultimately included in the meta-analysis. The results of our analysis showed IVT had a statistically significant improvement in vision over the IVB at 1 month and 3 months (P<0.01). However, the reduction was not significant regarding central macular thickness (CMT) during the earlier (1 month and 3 months) follow-up period (P=0.12, P=0.41, respectively). At later visit (6 months), IVT had a significant decrease in CMT when compared to IVB (P<0.01) while no significant improvement in visual acuity (VA) was observed (P=0.14). The incidence of intraocular hypertension was 13/102 in IVT group during follow-up period while 0/103 in IVB group. The difference was significant (P<0.01). With regards to IVT versus IVB combined with IVT, there were no significant differences in CMT at 1 month (P=0.86) and 3 months (P=0.06). The incidence of intraocular hypertension was 6/67 in IVT group during follow-up period while 4/66 in IVB+IVT group. But the difference was not significant (P=0.53).
CONCLUSION
Current evidence shows IVT is superior in improving VA at earlier follow-up (1 month and 3 months) and in reducing CMT at later follow-up (6 months) for DME. At other time, it is in favor of IVT treatment but there are no statistically significances. However, IVT has the side-effect of ocular hypertension. There is no adequate evidence of the benefit adding IVB to IVT in contrast to IVT alone.
Keywords: triamcinolone, bevacizumab, diabetic macular edema, meta-analysis
Introduction
Diabetic macular edema (DME) is one of the most common causes of visual loss as a complication of diabetes in the working population[1]. Besides, the worldwide prevalence of diabetes is estimated to rise to 366 millions in 2030[2]. The 10 years incidence of macular edema in patients with type 2 diabetes was 14% and 29% of type 1 developed DME over a 25-year period[3],[4]. Hence, finding safe and effective treatment of DME becomes so important.
At present, there have been many therapies for the treatment of DME including laser photocoagulation, intravitreal injection of anti-vascular endothelial growth factor (VEGF) drug, ocular corticosteroids and pars plana vitrectomy. Laser photocoagulation was proved to be useful in limiting vision loss in the past three decades and is still considered a gold standard therapy for the treatment of diabetic retinopathy[5],[6]. There is growing evidence that intravitreal available agents in combination with laser photocoagulation is more advantageous than laser alone in reducing maculae edema[7],[8]. Base on our knowledge, intravitreal corticosteroids and anti-VEGF are being widely used as pharmacotherapy for DME. Ranibizumab and bevacizumab are two main anti-VEGF agents for DME. Although ranibizumab has been recently approved by the United States Food and Drug Administration for the treatment of DME, its cost is immense. Bevacizumab, which costs much less than ranibizumab, is commonly used as an off-label therapeutic option in treating DME. Many studies have indicated intravitreal bevacizumab (IVB) was effective for reducing DME[9]-[14]. Triamcinolone, one of corticosteroids, has the effect of anti-inflammatory and anti-angiogenic. Many reports have demonstrated the usefulness of intravitreal triamcinolone (IVT) in patients with DME[15]-[18]. With the increasing use of IVB and IVT, it is of interest to confirm which agent is more effective and safe.
This meta-analysis was performed to compare the efficacy of the sole IVT versus IVB alone or IVB combined with IVT in patients with DME. The results could be important to choose the better drug as adjunctive treatment to laser photocoagulation and also help to clarify the pathogenesis of DME.
MATERIALS AND METHODS
We searched Medline, Embase, Web of science, and the Cochrane library from inception until January 2013. There were no language or date restrictions on the publications. The search strategy was based on combinations of medical subject headings and free text word. Search terms used were “diabetic macular edema”, “bevacizumab”, “avastin”, “triamcinolone”, “randomized controlled trials”. The searches were supplemented by manually searching the bibliographies of included studies and reviews.
Inclusion and Exclusion Criteria
Inclusions for analysis were restricted to: 1) Study design and intervention: randomized clinical controlled trials (RCCTs) which compared IVB of any dose with or without IVT of any dose versus IVT of any dose in the treatment of DME; 2) Population: trials that enrolled participants of any age and sex with any type of DME (focal or diffuse, primary or refractory); 3) Outcome measurement: studies that have indicated visual acuity (VA) and central macular thickness (CMT) were the main outcome measures and reported as mean±SD. Exclusion criteria were: 1) Studies of macular edema secondary to causes other than diabetic retinopathy (DR); 2) Studies of DR without macular edema; 3) Studies that were not randomized controlled trials.
Data Extraction and Quality Assessment
For each study, the following data were extracted: 1) General data: name of first author, the year of publication and location of the study, major inclusion criteria, various intervention groups, number of subjects, age and gender and duration of follow-up; 2) Outcomes: means and standard deviations (SDs) of final value after treatment in CMT and VA of logarithm of the minimum angle of resolution (logMAR) units, the number of cases which had IOP greater than 21mmHg. The methodological quality of the included trials were assessed using the Jadad scale[19]. Studies were scored according to three main study characteristics: randomization, blinding and participant withdrawals dropouts. A study was recognized as high quality if it had a Jadad score (with a score range of 0-5) of three points or greater.
Assessment of Risk of Bias
The following parameters were assessed: random sequence generation (selection bias); allocation concealment (selection bias); blinding of participants and personnel (performance bias); blinding of outcome assessment (detection bias); incomplete outcome data (attrition bias); selective reporting (reporting bias) and other biases that included: an extreme baseline imbalance, risk of bias related to the specific study design used, trial stopped early due to some data-dependent process. For the above questions, a judgment of “Yes” of each parameter indicated low risk of bias, “No” indicated high risk of bias, and “unclear” indicated unclear or unknown risk of bias.
Statistical Analysis
Statistical analysis was performed using the Review Manager 5.1.0 software from the Cochrane Collaboration. Continuous data were expressed as means and standard deviations, and weighted mean differences (WMD) were calculated while dichotomous data were calculated as relative risk (RR). Hence, the mean WMD (VA and CMT) and RR (IOP) between groups (IVT versus IVB or IVT versus IVT/IVB) were analyzed and provided a 95% confidence interval (CI). A Chi-square test and an I2 test were used to test for statistical heterogeneity between trials. We used the fixed effects model in the meta-analysis if there was no statistical heterogeneity(P>0.1, I2<50%). However, when there was statistical heterogeneity(P≤0.1, I2≥50%), sources of heterogeneity should be examined to make sure if a random-effect model could be applied.
RESULTS
Results of Research
Figure 1 shows a flow diagram depicting the selection process of articles. A total of 80 articles that were potentially relevant were identified through database searches, and 69 of these studies were eliminated after finding duplicates and reviewing the title and abstract. Eleven studies were retrieved, of which six were excluded based on comprehensive full-text review[20]-[25]. An additional one study was supplemented by searching references[26]. Six studies were ultimately included in the meta-analysis[26]-[31].
Figure 1. Flow diagram of literatures screening.
Characteristics of Included Studies
The characteristics of the six included studies are shown in Table 1. Two of the trials were performed in South America, two in Asia, and the other were in Africa. Mean ages of patients ranged from 52.70 to 67.08 years and both male and female participants were involved in almost equal proportion. All trials reported balanced baselined characteristics between comparison groups. The outcome measures of Shahin and El-lakkany[31] were not reported as mean±SD; Marey and Ellakwa[30] did not present VA in logMAR units so that the data could not be pooled in the Meta-analysis. Clinical heterogeneity was seen in several areas such as dosage of drugs or treatment protocols. For example, the intervention of the five studies were a single injection of bevacizumab vs one injection of triamcinolone except the study by Lim et al[28], whose IVB group was 2 injections of bevacizumab with 6-week intervals.
Table 1. Study characteristics of the included six randomized controlled trials.
| Study | Country of publication | Major inclusion criteria | Mean age (a) | M/F | No. of eyes | Intervention groups | Durationof follow-up (weeks) | Jadad Score |
| Paccola 2008 | Brazil | Refractory DME and diffuse fluorescein leakage involving the foveal centre and most of the macular area on FFA; BCVA (logMAR) ≥0.3; CMT>300µm | Group 1 65.58±8.44 | Group 1 7/6 | Group 1 n=13 | Group 1: IVB (1.5mg) | 1, 4, 8, 12, 24 | 5 |
| Group 2 67.08±4.67 | Group 2 8/5 | Group 2 n=13 | Group 2: IVT (4mg) | |||||
| Shimura 2008 | Japan | CMT>400µm; VA (logMAR)>0.3 | 65.70±5.30 | 8/6 | Group 1 n=14 | Group 1: IVT (4mg) | 1,24 | 3 |
| Group 2 n=14 | Group 2: IVB(1.25mg) | |||||||
| Shahin 2010 | Egypt | Diffuse macular edema | 52.70 | 12/20 | Group 1 n=24 | Group1: IVT (4mg) | 1,4 | 2 |
| Group 2 n=24 | Group 2: IVB (1.25mg) | |||||||
| Marey 2011 | Egypt | NA | Group 1 57.66±7.19 | Group 1 18/12 | Group 1 n=30 | Group1: IVT (4mg) | 1,6,12 | 1 |
| Group 2 57.66±7.44 | Group 2 19/11 | Group 2 n=30 | Group 2: IVT/IVB (1.25mg/2mg) | |||||
| Group 3 57.60±7.30 | Group 3 16/14 | Group 3 n=30 | Group3: IVB(1.25mg) | |||||
| Lim 2012 | Korea | Eyes with clinically significant DME; CMT ≥300µm | Group 1 61.40±6.70 | Group 1 19/19 | Group 1 n=38 | Group 1: IVB(2 injections of 1.25mg) | 6,12,24,48 | 5 |
| Group 2 58.40±5.90 | Group 2 16/18 | Group 2 n=36 | Group 2: IVB/IVT (1.25mg/2mg) | |||||
| Group 3 59.80±7.90 | Group 3 15/18 | Group 3 n=37 | Group 3: IVT (2mg) | |||||
| Isaac 2012 | Brazil | CMT >300µm in both eyes, HbA1c of up to 1% above the reference value and BP < 160/90mmHg measured at initial visit | 64.60±9.75 | 6/5 | Group1n=11 | Group 1: IVB (1.25mg) | 4,12,24 | 5 |
| Group2 n=11 | Group2: IVT (4.0mg) |
DME: Diabetic macular edema; BCVA: Best corrected visual acuity; CMT: Central macular thickness; HbA1c: glycosylated haemoglobin; MPC: Macular laser photocoagulation; PDR: Proliferative diabetic retinopathy; PRP: Panretinal photocoagulation; FFA: Fluorescein angiography; DR: Diabetic retinopathy.
Methodological Quality of Included Studies
All the included studies were assessed for methodological quality according to the Jadad score. A risk of bias summary for publication is shown in Figure 2.
Figure 2. Risk of bias summary for each included study (+: low risk of bias; ?: unclear risk of bias; −: high risk of bias).
Comparing Bevacizumab to Triamcinolone
As functional outcome measure, VA was most important for evaluating efficacy. The pooled results revealed that IVT significantly improved VA compared with IVB at 1 month (WMD, -0.10logMAR; 95% CI, -0.14 to -0.06, P<0.01) and 3 months (WMD, -0.12 logMAR; 95% CI, -0.16 to -0.08, P<0.01). At 6 months, IVT group tended to have more improvement, but the difference was not significant (WMD, -0.08 logMAR; 95% CI, -0.18 to 0.03, P=0.14) (Figure 3).
Figure 3. Forest plot showing mean difference in VA (logMAR) along with the associated 95%CI in the IVT group versus IVB group.
A: VA at 1 month; B: VA at 3 months; C: VA at 6 months.
CMT represented the anatomic change after treatment. No significant CMT reduction was detected in the IVT group in comparison with IVB group at 1 month follow-up, although IVT group tended to have greater reduction (WMD, -47.24µm; CI, -106.79 to 12.31, P=0.12). There was significant heterogeneity among trials for this measure of effect (I2=88%, P<0.01). At 3 months follow-up, the reduction in CMT was still no statistically significant between the two groups and IVT group still tended to have greater reduction (WMD, -36.63µm; CI, -123.28 to 50.02, P=0.41). There was substantial heterogeneity among trials for this measure of effect (I2=93%, P<0.01). However, at 6 months follow-up, IVT significantly reduced CMT compared with IVB (WMD, -52.02µm; CI, -70.71 to -33.33, P<0.01) (Figure 4).
Figure 4. Forest plot showing mean difference in CMT along with the associated 95%CI in the IVT group versus IVB group.
A: CMT at 1 month; B: CMT at 3 months; C: CMT at 6 months.
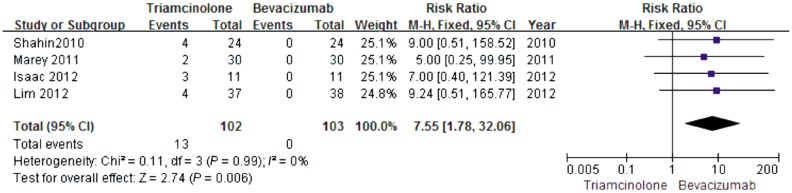
Intraocular hypertension was reported in IVT group in four studies. The incidence of intraocular hypertension was 13/102 in IVT group during follow-up period while 0/103 in IVB group. The difference was significant (RR, 7.55; CI, 1.78 to 32.06, P=0.006) (Figure 5). The intraocular hypertension could be managed with glaucoma medications. No other systematic or intraocular side-effects were noted in either group.
Figure 5. Forest plot showing mean difference in intraocularhypertension along with the associated 95%CI in the IVT group versus IVB group.
Comparing Bevacizumab+Triamcinolone to Triamcinolone
Due to the inadequate data of VA, the meta-analysis could not be assessed. With regards to CMT, no significant difference among groups receiving IVT versus IVB combined with IVT at 1 month (WMD, 3.04µm; CI, -31.29 to 37.36, P=0.86) and 3 months (WMD, 76.39µm; CI, -4.09 to 156.88, P=0.06) (Figure 6).
Figure 6. Forest plot showing mean difference in CMT along with the associated 95%CI in the IVT group versus IVB+IVT group.
A: CMT at 1 month; B: CMT at 3 months.
The incidence of intraocular hypertension was 6/67 in IVT group during follow-up period while 4/66 in IVB+IVT group. But the difference was not significant (RR, 1.47; CI, 0.44 to 4.95, P=0.53) (Figure 7).
Figure 7. Forest plot showing mean difference in intraocularhypertension along with the associated 95%CI in the IVT group versus IVB+IVT group.

Sensitivity Analysis
The study by Lim et al[28] compared 2 injections of bevacizumab with 6-week intervals and a single triamcinolone, which differed from other studies. Removaling of this study has not changed the results.
DISCUSSION
In the studies by Chakrabarti et al[25] and Marey and Ellakwa[30], the response to therapy with bevacizumab showed superiority compared with triamcinolone for DME. However, these studies differed from that of Shimura et al[26], Paccola et al[29], Isaac et al[27] and Lim et al[28], who demonstrated that intravitreal triamcinolone was more efficient in reducing DME relative to bevacizumab. And in the other study by Rensch et al[32], IVT and IVB did not differ markedly in term of their effects in improving VA and reducing macular thickness. Which treatment is more effective remains controversial. Thus, we conducted this meta-analysis to compare the efficacy of intravitreal triamcinolone and intravitral bevacizumab in DME. As far as we know, this is the first systematic review comparing these two drugs.
In our analysis, we found that the group that received IVT had a statistically significant improvement in vision over the IVB group, and this difference persisted to 3 months. However, the reduction was not significant regarding CMT during the earlier follow-up period (1 month and 3 months). At later visit (6 months), the triamcinolone-treated eyes had a significant decrease in CMT while no significant improvement in VA was observed. These results showed that there was no absolute correlation between anatomic change (CMT) and functional change (VA). This relation between CMT and VA in DME was discussed by previous studies, which reported a subset of eyes that showed paradoxical increases in CMT with increases in VA or paradoxical decreases in CMT with decreases in VA[33],[34]. Browning et al[34] pointed out that not only CMT, but age, hemoglobin A1C, and severity of fluorescein leakage in the center and inner subfields were responsible for the change in VA. In another study conducted by Jonas et al[35], they concluded the varying degree of macular ischemia may explain why some patients do not show a marked improvement in vision despite a regression of the thickness.
From our results, we showed a favorable response to IVT compared with IVB in improvement of VA at 1 and 3 months. The reason why the difference was not observed in VA at 6 months may be the limited effective time of these two drugs. Many studies have indicated that IVT treatment improved VA at three months but treatment was no longer effective at six months[18],[36]. And pharmacokinetic data suggest a single intravitreal injection of 1.25mg bevacizumab is effective for 6-7 weeks[37]. Thus, a single IVT did not keep its effect to 6 months, so did IVB. There is small number of study comparing the effect of repeating injection of these two drugs and keeping the level of medications in vitreous cavity. Kreutzer et al[23] suggested that a single triamcinolone injection may be as effective as a 3 injections of bevacizumab for the treatment of DME. Less number of injections of triamcinolne reduces injection-related complications such as endophthalmitis, decrease burden to the patient and improve the patient compliance. In addition, the cost of treatment with IVT is much lower so IVT seems to be more cost-effective option.
On the other hand, our results demonstrated that the intraocular higher level of VEGF is just one of the pathogenesis of DME. Other mechanisms suppressed by corticosteroid also contribute to it. The conclusion is consistent with previous studies, which indicated various inflammatory mediators that are up-regulated in DME including Tumor Necrosis Fator-α (TNF-α), interleukin-1β (IL-1β) and VEGF play a important role in the pathogenesis of DME[6]. The inflammatory mediators can be modulated with steroids. The angiostatic through inhibition of VEGF and anti-inflammation properties of steroid may make its superiority for DME. Even so, intravitral triamcinolone is gradually losing its leading position as the drug most often used in the treatment of DME since the emerging of anti-VEGF agents. The most important reason is the high rate of complications of IVT including marked elevation of IOP and cataract formation even though no other side-effects were reported in the included studies other than ocular hypertension. To achieve long-lasting concentrations and reduce associated adverse events at high doses of triamcinolone, a novel intravitreal steroid sustained-release device is being introduced into clinic.
We also compared the effect of combination of IVB and IVT versus IVT. The combination of different treatments interest ophthalmologists. The study[38] conducted by the DRCR.net indicated that intravitreal ranibizumab (IVR) or steroid combined with laser photocoagulation produced a rapid and sustained improvement in VA compared with laser alone for DME. It has remained unclear the efficacy of laser photocoagulation combined with anti-VEGF agent versus laser combined with TA. Further clinical trials are needed.
The quality of a systematic review lies on the qualities of included studies. The qualities of the included studies of our analysis are relative high. The results can provide certain reference significance for clinical selection. Even so, there were some limitations of our study. The results in CMT at 1 and 3 months were limited by heterogeneity of the included trials. The differences in the dose of intervention and type of DME may contribute to the clinical heterogeneity. Besides, the included studies all had the characteristics of relative small sample sizes and short duration of follow-up. Several prospective, randomized, blinded and large-sample clinical trials that have been conducted to demonstrate the efficacy and safety of anti-VEGF agents in the management of DME were worth mentioning. The BOLT study[12],[39] focused on IVB. The RIDE, RISE[40] and RESTORE studies[41] focused on IVR. The DA VINCI study[42],[43] tested the efficacy of VEGF Trap-Eye, which is a VEGF inhibitor whose binding affinity to VEGF is greater than that of bevacizumab and ranibizumab. These studies were well designed.The comparison of repeat injection of IVT and IVB in long duration of follow-up needs multi-center, large sample RCTs.
Acknowledgments
We would like to give thanks to Qing-Shan Chen, Professor of the Department of Epidemiology, Medical College, Jinan University for guidance.
Footnotes
Foundation items: National Natural Science Foundation of China (No.81100637); Jinan University Scientific Research Creativeness Cultivation Project for Outstanding Undergraduates Recommended for Postgraduate Study (No.50503592)
REFERENCES
- 1.Bunce C, Xing W, Wormald R. Causes of blind and partial sight certifications in England and Wales: April 2007-March 2008. Eye. 2010;24(11):1692–1699. doi: 10.1038/eye.2010.122. [DOI] [PubMed] [Google Scholar]
- 2.Habib SL, Rojna M. Diabetes and risk of cancer. ISRN Oncol. 2013;2013:583786. doi: 10.1155/2013/583786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Klein R, Klein BE, Moss SE, Cruickshanks KJ. The wisconsin epidemiologic study of diabetic retinopathy. XV. The long-term incidence of macular edema. Ophthalmology. 1995;102(1):7–16. doi: 10.1016/s0161-6420(95)31052-4. [DOI] [PubMed] [Google Scholar]
- 4.Klein R, Knudtson MD, Lee KE, Gangnon R, Klein BE. The wisconsin epidemiologic study of diabetic retinopathy XXIII: the twenty-five-year incidence of macular edema in persons with type 1 diabetes. Ophthalmology. 2009;116(3):497–503. doi: 10.1016/j.ophtha.2008.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nicholson BP, Schachat AP. A review of clinical trials of anti-VEGF agents for diabetic retinopathy. Graefes Arch Clin Exp Ophthalmol. 2010;248(7):915–930. doi: 10.1007/s00417-010-1315-z. [DOI] [PubMed] [Google Scholar]
- 6.Kumar B, Gupta SK, Saxena R, Srivastava S. Current trends in the pharmacotherapy of diabetic retinopathy. J Postgrad Med. 2012;58(2):132–139. doi: 10.4103/0022-3859.97176. [DOI] [PubMed] [Google Scholar]
- 7.Wang H, Sun X, Liu K, Xu X. Intravitreal Ranibizumab (Lucentis) for the Treatment of Diabetic Macular Edema: a systematic review and meta-analysis of randomized clinical control trials. Curr Eye Res. 2012;37(8):661–670. doi: 10.3109/02713683.2012.675616. [DOI] [PubMed] [Google Scholar]
- 8.Liu L, Wu X, Geng J, Yuan Z, Chen L. IVTA as adjunctive treatment to PRP and MPC for PDR and macular edema: a meta-analysis. Plos One. 2012;7(9):e44683. doi: 10.1371/journal.pone.0044683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Soheilian M, Ramezani A, Obudi A, Bijanzadeh B, Salehipour M, Yaseri M, Ahmadieh H, Dehghan MH, Azarmina M, Moradian S, Peyman GA. Randomized trial of intravitreal bevacizumab alone or combined with triamcinolone versus macular photocoagulation in diabetic macular edema. Ophthalmology. 2009;116(6):1142–1150. doi: 10.1016/j.ophtha.2009.01.011. [DOI] [PubMed] [Google Scholar]
- 10.Lam DS, Lai TY, Lee VY, Chan CK, Liu DT, Mohamed S, Li CL. Efficacy of 1.25mg versus 2.5mg intravitreal bevacizumab for diabetic macular edema six-month results of a randomized controlled trial. Retina. 2009;29(3):292–299. doi: 10.1097/IAE.0b013e31819a2d61. [DOI] [PubMed] [Google Scholar]
- 11.Scott IU, Edwards AR, Beck RW, Bressler NM, Chan CK, Elman MJ, Friedman SM, Greven CM, Maturi RK, Pieramici DJ, Shami M, Singerman LJ, Stockdale CR. A phase II randomized clinical trial of intravitreal bevacizumab for diabetic macular edema. Ophthalmology. 2007;114(10):1860–1867. doi: 10.1016/j.ophtha.2007.05.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rajendram R, Fraser-Bell S, Kaines A, Michaelides M, Hamilton RD, Esposti SD, Peto T, Egan C, Bunce C, Leslie RD, Hykin PG. A 2-Year Prospective Randomized Controlled Trial of Intravitreal Bevacizumab or Laser Therapy (BOLT) in the Management of Diabetic Macular Edema 24-Month Data: Report 3. Arch Ophthalmol. 2012;130(8):972–979. doi: 10.1001/archophthalmol.2012.393. [DOI] [PubMed] [Google Scholar]
- 13.Yilmaz T, Cordero-Coma M, Gallagher MJ, Teasley LA. Systematic review of intravitreal bevacizumab injection for treatment of primary diabetic macular oedema. Acta Ophthalmol. 2011;89(8):709–717. doi: 10.1111/j.1755-3768.2010.01918.x. [DOI] [PubMed] [Google Scholar]
- 14.Goyal S, LaValley M, Subramanian ML. Meta-analysis and review on the effect of bevacizumab in diabetic macular edema. Graefes Arch Clin Exp Ophthalmol. 2011;249(1):15–27. doi: 10.1007/s00417-010-1452-4. [DOI] [PubMed] [Google Scholar]
- 15.Sutter FK, Simpson JM, Gillies MC. Intravitreal triamcinolone for diabetic macular edema that persists after laser treatment:Three-month efficacy and safety results of a prospective, randomized, double-masked, placebo-controlled clinical trial. Ophthalmology. 2004;111(11):2044–2049. doi: 10.1016/j.ophtha.2004.05.025. [DOI] [PubMed] [Google Scholar]
- 16.Karacorlu M, Ozdemir H, Karacorlu S, Alacali N, Mudun B, Burumcek E. Intravitreal triamcinolone as a primary therapy in diabetic macular oedema. Eye. 2005;19(4):382–386. doi: 10.1038/sj.eye.6701512. [DOI] [PubMed] [Google Scholar]
- 17.Negi AK, Vernon S, Lim CS, Owen-Armstrong K. Intravitreal triamcinolone improves vision in eyes with chronic diabetic macular oedema refractory to laser photocoagulation. Eye. 2005;19(7):747–751. doi: 10.1038/sj.eye.6701636. [DOI] [PubMed] [Google Scholar]
- 18.Yilmaz T, Weaver CD, Gallagher MJ, Cordero-Coma M, Cervantes-Castaneda RA, Klisovic D, Lavaque AJ, Larson RJ. Intravitreal triamcinolone acetonide injection for treatment of refractory diabetic macular edema: a systematic review. Ophthalmology. 2009;116(5):902–911; quiz 912–913. doi: 10.1016/j.ophtha.2009.02.002. [DOI] [PubMed] [Google Scholar]
- 19.Jadad AR, Moore RA, Carroll D, Jenkinson C, Reynolds DJ, Gavaghan DJ, McQuay HJ. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials. 1996;17(1):1–12. doi: 10.1016/0197-2456(95)00134-4. [DOI] [PubMed] [Google Scholar]
- 20.Sobaci G, Ozge G, Erdurman C, Durukan HA, Bayraktar ZM. Comparison of grid laser, intravitreal triamcinolone, and intravitreal bevacizumab in the treatment of diffuse diabetic macular edema. Ophthalmologica. 2012;227(2):95–99. doi: 10.1159/000331322. [DOI] [PubMed] [Google Scholar]
- 21.Azad R, Sain S, Sharma YR, Mahajan D. Comparison of intravitreal bevacizumab, intravitreal triamcinolone acetonide, and macular grid augmentation in refractory diffuse diabetic macular edema: A prospective, randomized study. Oman J Ophthalmol. 2012;5(3):166–170. doi: 10.4103/0974-620X.106100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Penha FM, Maia M, Cardillo JA, Arevalo JF, Wu L, Rodriguez FJ, Berrocal MH, Farah ME. Pan-American Collaborative Retina Study. Comparison of a single intravitreal injection of bevacizumab versus triamcinolone acetonide as primary treatment for diffuse diabetic macular oedema. Acta Ophthalmol. 2012;90(2):e160–161. doi: 10.1111/j.1755-3768.2010.02098.x. [DOI] [PubMed] [Google Scholar]
- 23.Kreutzer TC, Al Saeidi R, Kook D, Wolf A, Ulbig MW, Neubauer AS, Haritoglou C. Comparison of intravitreal bevacizumab versus triamcinolone for the treatment of diffuse diabetic macular edema. Ophthalmologica. 2010;224(4):258–264. doi: 10.1159/000284466. [DOI] [PubMed] [Google Scholar]
- 24.Zhou YY, Zhang RJ. Avastin combined with vitreous cavity injection of triamcinolone acetonide in treatment of diabetic retinopathy with macular edema. Int J Ophthalmol. 2010;10(3):475–476. [Google Scholar]
- 25.Chakrabarti M, John SR, Chakrabarti A. Intravitreal monotherapy with bevacizumab (IVB) and triamcinolone acetonide (IVTA) versus combination therapy (IVB and IVTA) for recalcitrant diabetic macular edema. Kerala Journal of Ophthalmology. 2009;21(2):139–148. [Google Scholar]
- 26.Shimura M, Nakazawa T, Yasuda K, Shiono T, Iida T, Sakamoto T, Nishida K. Comparative therapy evaluation of intravitreal bevacizumab and triamcinolone acetonide on persistent diffuse diabetic macular edema. Am J Ophthalmol. 2008;145(5):854–861. doi: 10.1016/j.ajo.2007.12.031. [DOI] [PubMed] [Google Scholar]
- 27.Isaac DL, Abud MB, Frantz KA, Rassi AR, Avila M. Comparing intravitreal triamcinolone acetonide and bevacizumab injections for the treatment of diabetic macular oedema: a randomized double-blind study. Acta Ophthalmol. 2012;90(1):56–60. doi: 10.1111/j.1755-3768.2009.01817.x. [DOI] [PubMed] [Google Scholar]
- 28.Lim JW, Lee HK, Shin MC. Comparison of intravitreal bevacizumab alone or combined with triamcinolone versus triamcinolone in diabetic macular edema: a randomized clinical trial. Ophthalmologica. 2012;227(2):100–106. doi: 10.1159/000331935. [DOI] [PubMed] [Google Scholar]
- 29.Paccola L, Costa RA, Folgosa MS, Barbosa JC, Scott IU, Jorge R. Intravitreal triamcinolone versus bevacizumab for treatment of refractory diabetic macular oedema (IBEME study) Br J Ophthalmol. 2008;92(1):76–80. doi: 10.1136/bjo.2007.129122. [DOI] [PubMed] [Google Scholar]
- 30.Marey HM, Ellakwa AF. Intravitreal bevacizumab alone or combined with triamcinolone acetonide as the primary treatment for diabetic macular edema. Clin Ophthalmol. 2011;5:1011–1016. doi: 10.2147/OPTH.S22103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shahin MM, El-Lakkany RS. A prospective, randomized comparison of intravitreal triamcinolone acetonide versus intravitreal bevacizumab (avastin) in diffuse diabetic macular edema. Middle East Afr J Ophthalmol. 2010;17(3):250–253. doi: 10.4103/0974-9233.65496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rensch F, Spandau UH, Wickenhauser A, Jonas JB. Diffuse diabetic macular oedema treated with intravitreal bevacizumab or triamcinolone acetonide. Acta Ophthalmol. 2010;88(2):e36–37. doi: 10.1111/j.1755-3768.2008.01443.x. [DOI] [PubMed] [Google Scholar]
- 33.Larsson J, Zhu MD, Sutter F, Gillies MC. Relation between reduction of foveal thickness and visual acuity in diabetic macular edema treated with intravitreal triamcinolone. Am J Ophthalmol. 2005;139(5):802–806. doi: 10.1016/j.ajo.2004.12.054. [DOI] [PubMed] [Google Scholar]
- 34.Browning DJ, Glassman AR, Aiello LP, Beck RW, Brown DM, Fong DS, Bressler NM, Danis RP, Kinyoun JL, Nguyen QD, Bhavsar AR, Gottlieb J, Pieramici DJ, Rauser ME, Apte RS, Lim JI, Miskala PH. Relationship between optical coherence tomography-measured central retinal thickness and visual acuity in diabetic macular edema. Ophthalmology. 2007;114(3):525–536. doi: 10.1016/j.ophtha.2006.06.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jonas JB, Martus P, Degenring RF, Kreissig I, Akkoyun I. Predictive factors for visual acuity after intravitreal triamcinolone treatment for diabetic macular edema. Arch Ophthalmol. 2005;123(10):1338–1343. doi: 10.1001/archopht.123.10.1338. [DOI] [PubMed] [Google Scholar]
- 36.Qi HP, Bi S, Wei SQ, Cui H, Zhao JB. Intravitreal versus subtenon triamcinolone acetonide injection for diabetic macular edema: a systematic review and meta-analysis. Curr Eye Res. 2012;37(12):1136–1147. doi: 10.3109/02713683.2012.705412. [DOI] [PubMed] [Google Scholar]
- 37.Zhu Q, Ziemssen F, Henke-Fahle S, Tatar O, Szurman P, Aisenbrey S, Schneiderhan-Marra N, Xu X, Grisanti S. Vitreous levels of bevacizumab and vascular endothelial growth factor-A in patients with choroidal neovascularization. Ophthalmology. 2008;115(10):1750–1755, 1755 e1751. doi: 10.1016/j.ophtha.2008.04.023. [DOI] [PubMed] [Google Scholar]
- 38.Elman MJ, Bressler NM, Qin HJ, Beck RW, Ferris FL, 3rd, Friedman SM, Glassman AR, Scott IU, Stockdale CR, Sun JK. Expanded 2-Year Follow-up of Ranibizumab Plus Prompt or Deferred Laser or Triamcinolone Plus Prompt Laser for Diabetic Macular Edema. Ophthalmology. 2011;118(4):609–614. doi: 10.1016/j.ophtha.2010.12.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Michaelides M, Kaines A, Hamilton RD, Fraser-Bell S, Rajendram R, Quhill F, Boos CJ, Xing W, Egan C, Peto T, Bunce C, Leslie RD, Hykin PG. A prospective randomized trial of intravitreal bevacizumab or laser therapy in the management of diabetic macular edema (BOLT Study) 12-month data: report 2. Ophthalmology. 2010;117(6):1078–1086. doi: 10.1016/j.ophtha.2010.03.045. [DOI] [PubMed] [Google Scholar]
- 40.Nguyen QD, Brown DM, Marcus DM, Boyer DS, Patel S, Feiner L, Gibson A, Sy J, Rundle AC, Hopkins JJ, Rubio RG, Ehrlich JS, RISE and RIDE Research Group Ranibizumab for diabetic macular edema results from 2 phase III randomized trials: RISE and RIDE. Ophthalmology. 2012;119(4):789–801. doi: 10.1016/j.ophtha.2011.12.039. [DOI] [PubMed] [Google Scholar]
- 41.Mitchell P, Bandello F, Schmidt-Erfurth U, Lang GE, Massin P, Schlingemann RO, Sutter F, Simader C, Burian G, Gerstner O, Weichselberger A, RESTORE study group The RESTORE study ranibizumab monotherapy or combined with laser versus laser monotherapy for diabetic macular edema. Ophthalmology. 2011;118(4):615–625. doi: 10.1016/j.ophtha.2011.01.031. [DOI] [PubMed] [Google Scholar]
- 42.Do DV, Schmidt-Erfurth U, Gonzalez VH, Gordon CM, Tolentino M, Berliner AJ, Vitti R, Ruckert R, Sandbrink R, Stein D, Yang K, Beckmann K, Heier JS. The DA VINCI Study: phase 2 primary results of VEGF Trap-Eye in patients with diabetic macular edema. Ophthalmology. 2011;118(9):1819–1826. doi: 10.1016/j.ophtha.2011.02.018. [DOI] [PubMed] [Google Scholar]
- 43.Do DV, Nguyen QD, Boyer D, Schmidt-Erfurth U, Brown DM, Vitti R, Berliner AJ, Gao B, Zeitz O, Ruckert R, Schmelter T, Sandbrink R, Heier JS. One-year outcomes of the DA VINCI Study of VEGF Trap-Eye in eyes with diabetic macular edema. Ophthalmology. 2012;119(8):1658–1665. doi: 10.1016/j.ophtha.2012.02.010. [DOI] [PubMed] [Google Scholar]