Abstract
Background
Sedentary behavior and impaired cardiovascular reserve capacity are common late effects of cancer therapy emphasizing the need for effective strategies to increase physical activity (PA) in cancer survivors. We examined the efficacy of a 12-month exercise-based rehabilitation program on self-reported PA, cardiorespiratory fitness (VO2peak), strength, and patient-reported outcomes.
Patients and methods
Two hundred fourteen post-treatment cancer survivors were randomly assigned to a 12-month rehabilitation program consisting of individual (x3) and group-based (x6) counseling in combination with once weekly high-intensity group-based exercise training (the Copenhagen Physical Activity after Cancer Treatment, PACT; n = 108) or to a health evaluation program (HE, n = 106). Study outcomes were assessed at baseline, 6 months, and 12 months.
Results
After 12 months, the percentage of patients reporting meeting PA goal behavior (≥3 h/week) was significantly increased in the PACT group versus the HE group (70.4% versus 43.4%, P = 0.001). Repeated measures analyses indicated a statistically significant improvement in VO2peak (l min−1) in favour of PACT (treatment effect ratio = 1.04; 95% confidence interval 1.00–1.07; P = 0.032). Significant between group differences were also observed for strength (P < 0.001), depression (P = 0.020) and mental health (P = 0.040).
Conclusion
A 12-month exercise-based rehabilitation program is an effective strategy to promote PA and improve VO2peak in cancer survivors.
Keywords: cancer survivors, cardiorespiratory fitness, exercise, physical activity behavior, rct, rehabilitation
introduction
Early detection and advances in cancer therapy have resulted in steady increases in the overall cancer survival rate in developed countries [1]. However, the use of combination of anticancer therapy is associated with a diverse range of late effects, including marked impairments in cardiorespiratory fitness (peak oxygen consumption [VO2peak]), that may predispose cancer survivors to serious health conditions and risk of premature mortality [2].
Systematic reviews and meta-analyses indicate that structured exercise training following traditional exercise prescription guidelines (i.e. aerobic training 2–3 times/week for 12–15 weeks) is an effective strategy associated with significant improvements in VO2peak, strength, fatigue, and quality of life (QoL) in cancer survivors [3,4]. Moreover, prior work [5] has suggested that exercise at high relative intensity might be associated with larger beneficial adaption in the cardiovascular system in various patients groups without any negative outcomes.
To complement structured exercise training interventions, several research groups have investigated the efficacy of various behavioral intervention approaches to promote adoption of physical activity (PA) in cancer survivors. While these essentially have shown promising effects, the studies have relied predominantly on self-report measures to determine effectiveness with few exceptions [6–9]. Thus, evidence is still lacking as to whether it is possible to achieve sustainable increases in exercise behavior in cancer survivors, and whether such increases are associated with concomitant changes in an objective measure of cardiorespiratory fitness, such as VO2peak.
Against this background, we conducted the Copenhagen Physical Activity after Cancer Treatment (PACT) trial to determine the efficacy of a 12-month exercise-based rehabilitation program in cancer survivors after the completion of primary therapy. Our primary hypothesis was that PACT would cause significant improvements in self-reported PA and VO2peak compared with a health evaluation program (HE) including individual feedback following fitness testing. Secondary hypotheses were that PACT would be superior to HE for improvements in strength, health-related QOL, anxiety and depression, and general well-being.
patients and methods
settings and patient population
The PACT trial was conducted at Rigshospitalet and Herlev Hospital, Denmark. Inclusion eligibility were (i) histologically confirmed diagnosis of cancer, (ii) recent completion of chemotherapy for advanced disease or as adjuvant treatment (<6 months), and (iii) anticipated life expectancy >12 months. Exclusion criteria were (i) disease progression and/or terminal disease, (ii) WHO Performance status ≥2, (iii) contraindications to maximal exercise testing and/or exercise training, (iv) bone and brain metastases, and (v) symptomatic cardiac disease (<3 months).
study design and procedures
The study was a single-center, two-arm randomized, controlled trial. Ethical approval was obtained from The Scientific Committees of the Capital Region, Denmark (j.no. 01315155) and the Danish Data Protection Agency (j.no. 2006-41-6836). Following baseline assessments, patients were randomly allocated to one of the two experimental groups. All outcome data were entered and analysed by research assistants and a biostatistician (AT) blinded to participant randomization.
Randomization was carried out using a computer-based (Clinical Internet Trial Management System) with stratification by gender, cancer type (breast, bowel, haematological malignancies) and disease status (i.e. no evidence of residual disease [NED] versus evidence of residual or advanced disease [ED]).
the PACT intervention
The intervention consisted of a 12-month exercise-based rehabilitation program The overall goal of the program was for participants to exercise regularly ≥3 h/week.
‘Counseling component’ consisted of three individual sessions (1–2 h) delivered tri-monthly and six in-group sessions (2 h) delivered bi-monthly by a trained psychologist (JM). The counseling was based on principles from narrative therapy and social cognitive theory [10].
‘Supervised exercise training’ consisted of once weekly group-based session comprising of aerobic and resistance training for a total of ∼90 min/session. Aerobic training consisted of high-intensity interval training on stationary cycle ergometers. Intervals ranged from 30 s (maximum intensity) to 6 min (90%–95% of HRmax) at an exercise:to:recovery ratio of 1 : 2 to 3 : 1, respectively. Resistance training consisted of 3 sets of 8–10 repetitions at 70%–90% of one repetition maximum (1RM) involving leg press, knee extension, chest press, pull down, abdominal crunch, and lower back extension (Technogym, Gambettola, Italy). Intensity was increased when 12 repetitions could be carried out with proper form.
health evaluation group
The HE group received three individual health evaluation sessions (baseline, 6, and 12 months) that included feedback following fitness testing and education on the health benefits of regular exercise. Each session lasted ∼15 min and was delivered verbally and face-to-face by a member of the research team (JM, JFC, or JU).
study outcome measures
Demographic data were collected by self-report while medical data were abstracted from medical records. All outcome assessments were measured at baseline, 6 months (mid-intervention), and 12 months (post-intervention).
primary outcome measures
‘Self-reported PA’ was assessed by the Saltin and Grimby questionnaire [11]. Patients were asked to classify themselves into one of four categories: (I) sedentary; (II) low-to-moderate intensity walking or cycling for pleasure; (III) regular, moderate-to-high intensity physical exercise ≥3 h/week; or (IV) intense PA ≥4 h/week.
‘Cardiorespiratory fitness’ was assessed by an incremental exercise test on a stationary cycle ergometer (Monark Ergomedic 839E) [12]. The test consisted of a 4 min warm-up at a fixed load of 67 Watts and 4 min recovery, after which the incremental test was initiated at a baseline workload of 67 Watts followed by 20-Watts increments every minute until volitional exhaustion.
secondary outcome measures
‘Muscular strength’ was assessed using one repetition maximum (1RM) to evaluate upper (chest press) and lower (leg press) body strength [13]. ‘Health-related quality of life (HRQOL)’ was assessed using the European Organization for Research and Treatment of Cancer Quality of Life Questionnaire (EORTC QLQ-C30) [14]. All scale/single item measures range in score from 0 to 100. ‘Anxiety and depression’ was measured by the Hospital Anxiety and Depression Scale (HADS) [15].
‘General well-being’ was assessed by using the Medical Outcomes Study Short Form (MOS SF-36) [16] containing eight subscales measuring general health concepts in the range from 0 to 100.
statistical analysis
Fifty-three participants were needed in each arm to achieve a power of 80% to detect an increase in PA (ordinal scale) in 60% of the participants in the PACT group compared with 33% in the HE group. To account for an expected dropout rate of 50%, 106 patients were included in each group. The analyses were conducted on an intention to treat basis using all available data on all randomly assigned participants regardless of their adherence to the intervention. Fisher's exact test was used for examining dropout rates across groups and for comparing categorical variables at baseline. Fisher's exact test was used to compare the proportion of patients meeting PA goal (>3 h/week) whereas changes in PA from baseline to 6 and 12 months were compared across groups using a Mann–Whitney test. All continuous outcomes were analysed using a repeated measures analysis with treatment, assessment-points, and their interaction as independent variables. Physiological outcomes were log-transformed to meet the assumption of normality and homogeneity of variances. Psychometric outcomes (EORTC, SF-36) reported on bounded scales (0–100) were transformed using the Arcsine root transformation, whereas measures of anxiety and depression (HADS) were square root transformed. Estimates were back transformed to original scales to ease interpretation. The effect of the intervention was reported by treatment effect ratios (TER). In particular, the statistical analyses were adjusted for baseline values by evaluating the effect of the intervention through comparison of the relative changes between pairs of assessment-points in PACT versus HE. Exploratory analyses adjusting for main effects and effect modifications (interactions) with disease-related covariates (NED/ED, diagnosis) and demographic variables (sex, age) were conducted for physiological outcomes. Participants classified as lost to follow-up (N = 61) were compared with the study group (N = 153) for baseline demographic data using ordinary t test and χ2 test for homogeneity. Significance was set at 0.05 α level. Statistical analyses were carried out using ‘R: A Language and Environment for Statistical Computing’.
results
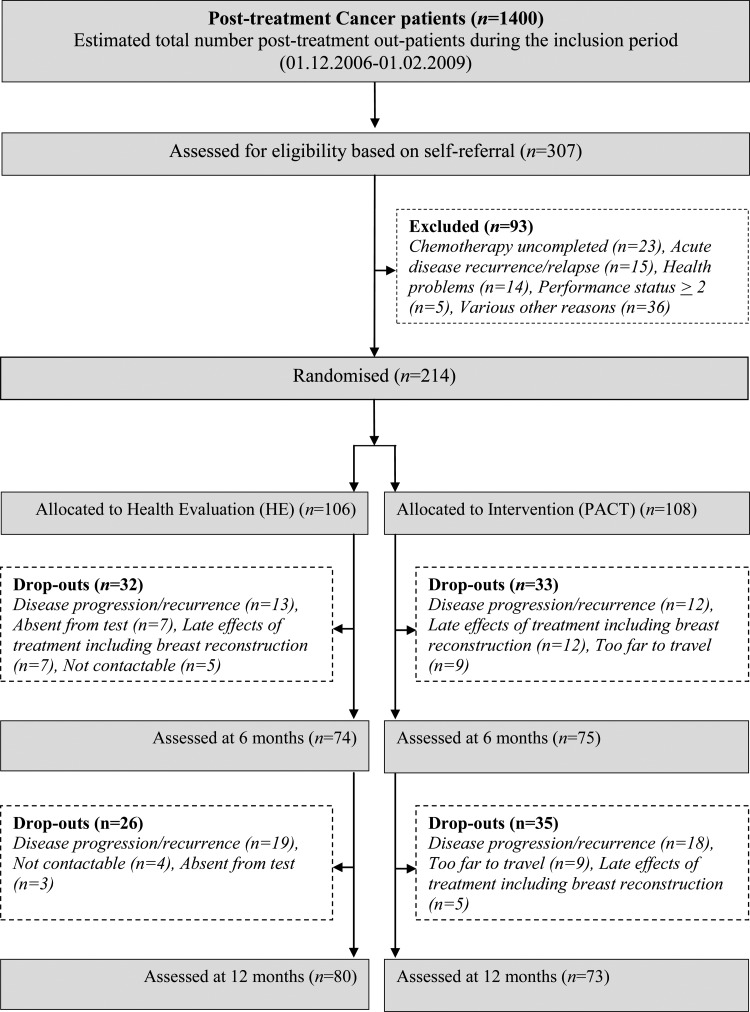
The study flow is presented in Figure 1, and participants' baseline characteristics are presented in Table 1.
Figure 1.
CONSORT diagram showing flow of participants through the trial
Table 1.
Demographic and medical characteristics for all participants and by group assignment
| Attention control group (HE) (n = 106) | Intervention group (PACT) (n = 108) | P-value | |
|---|---|---|---|
| Demographic characteristics | |||
| Age, years | |||
| Mean (SD) | 46.2 (11.6) | 48.2 (10.1) | 0.18 |
| Range | 25–74 | 25–71 | |
| Married, cohabiting, or in a relationship | 60.2 | 74.1 | 0.05 |
| Gender | |||
| Male | 18 (17.0) | 18 (16.7) | 0.95 |
| Female | 88 (83.0) | 90 (83.3) | |
| Sick leave (part time or full time) | 69 (66.3) | 75 (72.1) | 0.53 |
| Completed secondary school or higher | 76 (71.7) | 76 (71.7) | 1.00 |
| Health and medical characteristics | |||
| Body mass index, kg/m2 | |||
| Mean (SD) | 25.1 (3.8) | 24.6 (3.8) | 0.32 |
| Range | 18–36 | 17–37 | |
| <18.5 (underweight) | 4 | 8 | |
| 18.5–25 (healthy weight) | 59 | 58 | |
| 25–30 (overweight) | 28 | 30 | |
| >30 (obese) | 15 | 12 | |
| Disease statusa | |||
| No evidence of disease (NED) | 68 (65.4) | 72 (66.7) | 0.84 |
| Evidence of disease (ED) | 36 (34.6) | 36 (33.3) | |
| Time since chemotherapy completion (days)b | |||
| Mean (SD) | 80.9 (62.9) | 78 (63.8) | 0.69 |
| Treatment | |||
| Chemotherapy | 106 | 108 | |
| Surgery | 87 | 84 | |
| Radiation therapy including brachytherapy | 73 | 65 | |
| Hormonal | 50 | 49 | |
| Other (i.e. immune therapy, HSCT) | 9 | 12 | |
| Diagnosis | |||
| Cancer of breast | 65 (60.2) | 66 (61.1) | 0.83 |
| Cancer of bowel | 5 (5) | 6 (6) | |
| Cancer of ovaries | 2 (2) | 7 (6) | |
| Cancer of uterus | 2 (2) | 5 (5) | |
| Cancer of testes | 8 (8) | 3 (3) | |
| Other oncological diagnoses | 10 (9) | 10 (9) | |
| Haematological malignancies | 13 (12) | 12 (11) | |
| Physical activity level (self-reported) | |||
| Pre-illness | |||
| Sedentary | 5 (4.7) | 3 (2.8) | 0.35 |
| Walking or cycling for pleasure | 64 (60.4) | 64 (60.4) | |
| Regular physical exercise, at least 3 h/week | 35 (33.0) | 32 (30.2) | |
| Intense physical activity >4 h/week | 2 (1.9) | 7 (6.6) | |
| Baseline | |||
| Sedentary | 14 (13.0) | 11 (10.2) | 0.80 |
| Walking or cycling for pleasure | 65 (60.2) | 69 (63.9) | |
| Regular physical exercise at least 3 h/week | 27 (25.0) | 28 (25.9) | |
| Intense physical activity >4 h/week | 0 | 0 | |
aStatus at beginning of cytostatic treatment (i.e. treatment rationale).
bTime since termination of cytostatic treatment calculated in days.
HSCT, Haematopoetic stem cell transplantation.
adherence and safety
Ninety-two percent of patients participated in at least five of six of the in-group counseling sessions. Adherence to the weekly supervised exercise training sessions was 66.6%. Heart rate during supervised exercise sessions was 77 ± 7% of the measured heart rate maximum. Six participants in the PACT group developed lymphedema, but continued to follow the progressive resistance training without exacerbation of symptoms. Adverse events were not monitored in the HE group.
changes in primary outcome measures
self-reported PA
At 12 months, the percentage of patients reporting meeting PA goal behavior (≥3 h/week) was 70.4% (95% CI 58% to 87%; N = 50) in the PACT group compared with 43.4% (95% CI 32% to 55%; N = 33) in the HE group (P = 0.001). Compared with HE, PACT had a superior improvement in self-reported PA from 0 to 6 months (P = 0.002) and from 0 to 12 months (P = 0.026).
cardiorespiratory fitness
Changes in VO2peak are presented in Table 2. Repeated measures analyses showed a statistically significant increase from 0 to 12 months favouring PACT (TER = 1.04 [1.00–1.07], P = 0.032) compared with HE. The mean difference in change from 0 to 12 months between PACT and HE was 0.081 l min−1 (95% CI 0.011–0.151; P = 0.024), and 0.143 l min−1 (95% CI 0.072–0.214, P = <.001) from 0 to 6 months.
Table 2.
Differences in physiological outcomes between PACT and HE at baseline and the 6- and 12-month follow-ups
| Outcome | Baseline |
6 months |
12 months |
Baseline to 6 months (TER) |
6–12 months (TER) |
Baseline to 12 months (TER) |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Geometric mean | 95% CI | Geometric mean | 95% CI | Geometric mean | 95% CI | Ratio of PACT/HE | 95% CI | Between-groups, P-values | Ratio of PACT/HE | 95% CI | Between-groups, P-values | Ratio of PACT/HE | 95% CI | Between-groups, P-values | |
| Primary outcome | |||||||||||||||
| VO₂ peak absolute (l min−1) | |||||||||||||||
| Intervention | 1.97 | 1.89–2.05 | 2.31*** | 2.22–2.42 | 2.34*** | 2.24–2.44 | 1.06 | 1.03–1.10 | <0.001 | 0.98 | 0.94–1.01 | 0.215 | 1.04 | 1.00–1.07 | 0.032 |
| Control | 1.99 | 1.91–2.08 | 2.20*** | 2.11–2.30 | 2.28***†† | 2.18–2.38 | |||||||||
| VO₂ peak relative (ml kg−1min−1) | |||||||||||||||
| Intervention | 28.17 | 26.95–29.44 | 32.80*** | 31.32–34.36 | 33.03** | 31.54–34.60 | 1.06 | 1.02–1.10 | 0.003 | 0.97 | 0.93–1.00 | 0.084 | 1.02 | 0.99–1.06 | 0.209 |
| Control | 27.74 | 26.54–29.02 | 30.55*** | 29.16–32.01 | 31.80***†† | 30.37–33.31 | |||||||||
| Peak power output (Watt) | |||||||||||||||
| Intervention | 169.23 | 162.29–76.45 | 199.93*** | 191.30–208.95 | 201.99*** | 193.26–211.12 | 1.07 | 1.03–1.11 | <0.001 | 0.97 | 0.94–1.08 | 0.175 | 1.04 | 1.00–1.08 | 0.035 |
| Control | 169.99 | 162.96–177.33 | 187.77*** | 179.62–196.30 | 194.92*** | 186.54–203.68 | |||||||||
| HRpeak (bpm) | |||||||||||||||
| Intervention | 171.36 | 168.58–174.18 | 172.73 | 169.75–175.76 | 173.83* | 170.83–176.88 | 1.00 | 0.97–1.02 | 0.821 | 0.99 | 0.98–1.01 | 0.410 | 0.995 | 0.98–1.01 | 0.521 |
| Control | 170.70 | 167.92–173.53 | 171.75 | 168.79–174.77 | 174.06***† | 171.08–177.09 | |||||||||
| HR67watt (bpm) | |||||||||||||||
| Intervention | 129.91 | 127.00–132.88 | 120.41*** | 117.42–123.48 | 120.67*** | 117.66–123.76 | 0.97 | 0.94–1.00 | 0.058 | 1.01 | 0.98–1.05 | 0.381 | 0.99 | 0.96–1.02 | 0.324 |
| Control | 129.21 | 126.26–132.19 | 123.30*** | 120.23–126.44 | 121.84***†† | 118.86–124.89 | |||||||||
| Time to exhaustion (min) | |||||||||||||||
| Intervention | 5.29 | 4.96–5.69 | 6.78*** | 6.33–7.26 | 6.95*** | 6.49–7.44 | 1.09 | 1.02–1.15 | 0.006 | 0.98 | 0.92–1.04 | 0.471 | 1.06 | 1.00–1.13 | 0.042 |
| Control | 533 | 5.00–5.69 | 6.28*** | 5.86–6.72 | 6.58***† | 6.15–7.05 | |||||||||
| Leg press (kg) | |||||||||||||||
| Intervention | 81.76 | 76.34–87.57 | 103.13*** | 95.90–110.90 | 109.68*** | 101.98–117.97 | 1.17 | 1.10–1.24 | <0.001 | 1.04 | 1.01–1.11 | 0.189 | 1.22 | 1.15–1.30 | <0.001 |
| Control | 84.54 | 78.89–90.60 | 91.11*** | 84.74–97.97 | 92.84*** | 86.38–99.77 | |||||||||
| Chest press (kg) | |||||||||||||||
| Intervention | 33.00 | 30.87–35.28 | 37.53*** | 35.01–40.23 | 38.65***†† | 36.05–41.44 | 1.08 | 1.03–1.13 | 0,0012 | 1.02 | 0.97–1.08 | 0.336 | 1.109 | 1.06–1.16 | <0.001 |
| Control | 34.49 | 32.23–36.91 | 36.25** | 33.80–38.89 | 36.43** | 33.98–39.06 | |||||||||
*P < 0.05 **P < 0.01 ***P < 0.001: within group difference compared with baseline.
†P < 0.05; ††P < 0.01; †††P < 0.001: within group difference compared with 6 months.
changes in secondary outcome measures
Compared with HE, PACT had a superior increase in upper and lower body 1RM muscle strength from baseline to 12-month follow-up (TER = 1.22 [1.15–1.30], P < 0.001) (Table 2). The results on QOL are presented in (supplementary Table S3, available at Annals of Oncology online), and show a statistically significant between-group improvements in cognitive function (TER = 1.07 [0.99–1.14], P = 0.046) from 0 to 6 months in favour of PACT. As depicted in (supplementary Table S4, available at Annals of Oncology online), results also show a statistically significant between-group difference in favour of PACT in depression of −0.53 points (TER = 0.57 [0.31–0.83], P = 0.022) and mental health (SF-36) of 4.68 points (TER = 1.06 [1.00–1.12], P = 0.042) from baseline to 12-month follow-up. (supplementary Figure S2, available at Annals of Oncology online) shows effect estimates and confidence intervals for all variables. Clinically significant within-group changes (i.e. ≥10 points) were seen in QOL, role functioning, and fatigue (EORTC QLQ-C30), and in role physical, vitality, social functioning, and role emotional (SF-36).
discussion
In support of our primary hypothesis, we found that PACT was superior to HE in increasing PA behavior and cardiorespiratory fitness. Furthermore, we found an effect of PACT in improving muscular strength and psychological well-being. While we found that VO2peak improved in both groups, this improvement was superior in the PACT group (mean difference 0.08 l min−1 or Cohen's effect size (ES) = 0.33), which is consistent with the weighted mean ES of 0.32 in a meta-analysis of ‘high-quality’ studies [3]. The ability of the intervention to improve fitness via a group-based intervention delivered at fixed time points once weekly is encouraging, and suggests a great transition potential to ‘real-world’ clinical rehabilitation programs with requirements to cater to a heterogeneous group of cancer patients.
Most comparable to our study, Rogers et al. [6] evaluated the effect of a 12-week multidisciplinary PA behavior change intervention including counseling in combination with supervised and home-based exercise (three to five times weekly) in breast cancer survivors (n = 41). The authors reported significantly improved accelerometer PA counts, and a nonsignificant but moderate-to-large effects size increase in fitness in the intervention group versus the control group (4.9 versus 2.0 ml kg−1min−1). In comparison, we documented a statistically significant between-group increase in fitness from 0 to 6 months of 1.82 ml kg−1min−1 (4.6 versus 2.81 ml kg−1min−1) with only one weekly supervised exercise session. A potential explanation for these findings is differences in exercise training intensity. Thus, while several prior exercise oncology trials have incorporated high-intensity aerobic training, PACT is the first trial to exclusively test the efficacy of high-intensity interval training (i.e. cycling intensity at bouts of 90%–95% of measured HRmax).
However, the documented improvement in VO2peak was only borderline significant in comparison to the significant positive change in self-reported PA, which probably may be explained by the lack of continuous individually tailored progression in exercise frequency and intensity. Nevertheless, the clinical importance of the observed magnitude of improvements in PA and VO2peak in PACT is promising. While the strong, independent prognostic value of cardiorespiratory fitness has been demonstrated in numerous clinical populations [17], only two studies to date have examined this question in the oncology setting [18,19].
It is interesting to note also that whereas the improvements in PA behavior and VO2peak at 6 months were maintained to 12 months, the superiority of PACT to HE in psychological well-being (HADS-D and Mental Health) emerged only after completion of the entire intervention period of 12 months. This suggests that the first 6 months of the intervention may have served as a period of physical restoration and habituation, which, only after a period of 6 months, was accompanied by psychological tolerance potentially including a sense of mastery and social reinforcement [20]. However, we did not observe significant between group improvements in QoL and/or functional outcomes. A close examination of the data reveals that the baseline mean of the functional scales was very high indicating a potential ceiling effect.
An important but unexpected finding was the significant improvements in PA and VO2peak observed in the HE (attention control) group, which may be explained by contamination and/or increased program availability and awareness. However, because of the year-long intervention period and the existence of guidelines to support exercise in cancer survivors, we decided that it would be unethical to use a ‘no treatment group’ as control group. Thus, our study is one of the first to show that a minimally intensive intervention, i.e. an in-person health evaluation carried out three times over 12 months, produced significant improvements in PA and VO2peak. If confirmed in larger trials, this finding may have important implications for future exercise promotion efforts in the oncology setting and support the widely held notion that a cancer diagnosis may prompt a teachable moment to initiate healthy lifestyle behavior changes [21]. Thus, based on our data, it appears that not only can health education improve important behavioral outcomes in cancer survivors, but incorporating a once weekly supervised high-intensity session in conjunction with counseling as part of rehabilitation can lead to potentially clinically important improvements in pertinent outcomes.
One important limitation of the Copenhagen PACT Study is its rate of attrition, which is considerably higher than that reported in prior trials. However, the characteristics of participants lost to follow-up did not differ between the randomized groups and was due primarily (60%) to disease recurrence. Another important limitation was the transparent purpose of our trial potentially leading to patient selection bias;, i.e. highly motivated patients interested in healthy lifestyle behaviors and experiencing less treatment-related complications might have been more likely to participate. Other limitations include the lack of monitoring of adverse events in the HE group and the relative limited inclusion of male patients and patients with low socioeconomic status. Thus, our findings are not generalizable to all cancer patients, and we do not yet know whether this type of intervention will induce long-term effects.
In conclusion, a 1-year exercise-based rehabilitation program encompassing both counseling and supervised high-intensity exercise, seem to be an effective strategy to promote PA behavior and increase cardiorespiratory fitness in post-treatment cancer survivors. Additional studies are warranted to determine the optimal approach in assisting survivors becoming and staying physically active in order to prevent and/or mitigate impairments in cardiorespiratory fitness.
funding
This work was supported by a grant from the Velux Foundation. The project has also received founding from the Danish Cancer Society, The Lundbeck Foundation, and The Novo Nordisk Foundation. LWJ is supported by research grants from the National Cancer Institute (CA143254, CA142566, CA138634, CA133895).
disclosure
The authors have declared no conflicts of interest.
Supplementary Material
references
- 1.American Cancer Society. Cancer Facts & Figures 2012 [Online publication] Atlanta: American Cancer Society; 2012. http://www.cancer.org/acs/groups/content/@epidemiologysurveilance/documents/document/acspc-031941.pdf (14 May 2013, date last accessed). [Google Scholar]
- 2.Lakoski SG, Eves ND, Douglas PS, et al. Exercise rehabilitation in patients with cancer. Nat Rev Clin Oncol. 2012;9:288–296. doi: 10.1038/nrclinonc.2012.27. doi:10.1038/nrclinonc.2012.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Speck RM, Courneya KS, Masse LC, et al. An update of controlled physical activity trials in cancer survivors: a systematic review and meta-analysis. J Cancer Surviv. 2010;4:87–100. doi: 10.1007/s11764-009-0110-5. doi:10.1007/s11764-009-0110-5. [DOI] [PubMed] [Google Scholar]
- 4.Fong DY, Ho JW, Hui BP, et al. Physical activity for cancer survivors: meta-analysis of randomised controlled trials. BMJ. 2012;344:e70. doi: 10.1136/bmj.e70. doi:10.1136/bmj.e70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wisloff U, Ellingsen O, Kemi OJ. High-intensity interval training to maximize cardiac benefits of exercise training? Exerc Sport Sci Rev. 2009;37:139–146. doi: 10.1097/JES.0b013e3181aa65fc. doi:10.1097/JES.0b013e3181aa65fc. [DOI] [PubMed] [Google Scholar]
- 6.Rogers LQ, Hopkins-Price P, Vicari S, et al. A randomized trial to increase physical activity in breast cancer survivors. Med Sci Sports Exerc. 2009;41:935–946. doi: 10.1249/MSS.0b013e31818e0e1b. doi:10.1249/MSS.0b013e31818e0e1b. [DOI] [PubMed] [Google Scholar]
- 7.Pinto BM, Papandonatos GD, Goldstein MG, et al. Home-based physical activity intervention for colorectal cancer survivors. Psychooncology. 2013;22:54–64. doi: 10.1002/pon.2047. [DOI] [PubMed] [Google Scholar]
- 8.Rogers LQ, Fogleman A, Trammell R, et al. Effects of a physical activity behavior change intervention on inflammation and related health outcomes in breast cancer survivors: Pilot Randomized Trial. Integr Cancer Ther. 2012 doi: 10.1177/1534735412449687. Jul 24 [epub ahead of print], doi: 10.1177/1534735412449687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vallance JK, Courneya KS, Plotnikoff RC, et al. Randomized controlled trial of the effects of print materials and step pedometers on physical activity and quality of life in breast cancer survivors. J Clin Oncol. 2007;25:2352–2359. doi: 10.1200/JCO.2006.07.9988. doi:10.1200/JCO.2006.07.9988. [DOI] [PubMed] [Google Scholar]
- 10.Midtgaard J. Theoretical and Practical Outline of the Copenhagen PACT narrative-based exercise counselling manual to promote physical activity in post therapy cancer survivors. Acta Oncol. 2013;52:303–309. doi: 10.3109/0284186X.2012.742206. [DOI] [PubMed] [Google Scholar]
- 11.Saltin B, Grimby G. Physiological analysis of middle-aged and old former athletes. Comparison with still active athletes of the same ages. Circulation. 1968;38:1104–1115. doi: 10.1161/01.cir.38.6.1104. doi:10.1161/01.CIR.38.6.1104. [DOI] [PubMed] [Google Scholar]
- 12.Andersen LB. A maximal cycle exercise protocol to predict maximal oxygen uptake. Scand J Med Sci Sports. 1995;5:143–146. doi: 10.1111/j.1600-0838.1995.tb00027.x. doi:10.1111/j.1600-0838.1995.tb00027.x. [DOI] [PubMed] [Google Scholar]
- 13.Levinger I, Goodman C, Hare DL, et al. The reliability of the 1RM strength test for untrained middle-aged individuals. J Sci Med Sport. 2009;12:310–316. doi: 10.1016/j.jsams.2007.10.007. doi:10.1016/j.jsams.2007.10.007. [DOI] [PubMed] [Google Scholar]
- 14.Aaronson NK, Ahmedzai S, Bergman B, et al. The European Organization for Research and Treatment of Cancer QLQ-C30: a quality-of-life instrument for use in international clinical trials in oncology. J Natl Cancer Inst. 1993;85:365–376. doi: 10.1093/jnci/85.5.365. doi:10.1093/jnci/85.5.365. [DOI] [PubMed] [Google Scholar]
- 15.Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatr Scand. 1983;67:361–370. doi: 10.1111/j.1600-0447.1983.tb09716.x. doi:10.1111/j.1600-0447.1983.tb09716.x. [DOI] [PubMed] [Google Scholar]
- 16.Ware JE, Jr, Sherbourne CD. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med Care. 1992;30:473–483. [PubMed] [Google Scholar]
- 17.Warburton DE, Nicol CW, Bredin SS. Health benefits of physical activity: the evidence. CMAJ. 2006;174:801–809. doi: 10.1503/cmaj.051351. doi:10.1503/cmaj.051351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jones LW, Eves ND, Kraus WE, et al. The lung cancer exercise training study: a randomized trial of aerobic training, resistance training, or both in postsurgical lung cancer patients: rationale and design. BMC Cancer. 2010;10:155. doi: 10.1186/1471-2407-10-155. doi:10.1186/1471-2407-10-155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jones LW, Courneya KS, Mackey JR, et al. Cardiopulmonary function and age-related decline across the Breast Cancer Survivorship Continuum. J Clin Oncol. 2012;30:2530–2537. doi: 10.1200/JCO.2011.39.9014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pinto BM, Ciccolo JT. Physical activity motivation and cancer survivorship. Recent Results Cancer Res. 2011;186:367–387. doi: 10.1007/978-3-642-04231-7_16. doi:10.1007/978-3-642-04231-7_16. [DOI] [PubMed] [Google Scholar]
- 21.Demark-Wahnefried W, Clipp EC, Lipkus IM, et al. Main outcomes of the FRESH START trial: a sequentially tailored, diet and exercise mailed print intervention among breast and prostate cancer survivors. J Clin Oncol. 2007;25:2709–2718. doi: 10.1200/JCO.2007.10.7094. doi:10.1200/JCO.2007.10.7094. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.