Abstract
Brain structures related to reproduction are thought to depend on the action of gonadal steroids acting either during early life (organizing irreversible effects) or adulthood (activating transient effects). More recently puberty has become a focus of attention and it was demonstrated that action of sex steroid hormones at this time plays a critical role in the final organization of brain and behavior. We studied by BrdU immunohistochemistry the ontogeny from hatching to sexual maturity of a previously identified cell population in the preoptic area labeled by a BrdU injection at the end of embryonic period (E12) of sexual differentiation in male and female Japanese quail. After an initial increase between E12 and hatching, the density of BrdU-immunoreactive cells decreased until the beginning of puberty but then increased again during sexual maturation in the caudal preoptic area specifically. Divisions of these cells took place in the brain parenchyma as indicated by the large numbers of pairs of labeled cells. No sex difference affecting these processes could be detected at any stage of development. Large numbers of new cells thus arise around puberty in the caudal preoptic area and presumably contribute to the reorganization of this structure that precedes the emergence of adult reproductive behaviors.
Keywords: Embryogenesis, Puberty, Progenitor cell, Preoptic area, Sexual behavior, Brain plasticity
1. Introduction
During ontogeny, exposure to a different endocrine environment leads to the development of sex differences in brain structures that are later implicated in the control of behavioral sex differences (Phoenix et al., 1959). The Japanese quail (Coturnix japonica) is particularly well suited for studying sex differences in sexual behavior (Adkins, 1978; Adkins-Regan, 1983; Ball and Balthazart, 2011; Balthazart and Ball, 1998). In quail, the expression of male-typical sexual behavior is androgen-dependent and the medial preoptic nucleus (POM), a sexually dimorphic structure (larger in males than in females), is required for the activation of male sexual behavior (Aste et al., 1994; Foidart et al., 1995; Panzica et al., 1996). The volume of the POM is significantly larger in males than in females (Panzica et al., 1996) but due to its large spatial heterogeneity, no study has to this date attempted to quantify the total number of cells present in this structure. Multiple neurochemical sex differences have however been identified in this nucleus. They concern for example the number of aromatase-immunoreactive cells, the density of vasotocin-immunoreactive fibers or the turnover of dopamine (see for review: Balthazart et al., 1996).
Sex differences affecting the POM result from both activational and organizational effects of gonadal hormones. For example, the POM volume and the number of aromatase-expressing cells in POM are low and similar in males and females for the first 4–5 weeks after hatching, only increasing in males as the birds reach sexual maturity. The sex differences in these features are thus the result of a differential activation by sex steroids. In contrast, steroids acting during an early critical period of life organize in an irreversible manner the adult responsiveness to sex steroids (see Balthazart et al., 2009 for review). These organizational effects of steroids clearly control sex differences affecting maletypical copulatory behavior in quail. This behavior is readily expressed by castrated males treated with exogenous testosterone but never by ovariectomized females treated with the same or even higher doses of the same steroid (Balthazart et al., 1996). This differential response to testosterone of males and females is the result of a demasculinization of females by their ovarian estrogens before day 12 of embryonic life (E12; Adkins, 1979; Balthazart et al., 1992). Prior to E12, the behavioral phenotype of male and female quail can be completely reversed by treating male embryos with estrogens or female embryos with an inhibitor of aromatase, the enzyme converting T into estradiol (Balthazart et al., 1992). The behavioral sex of quail can thus be controlled by modifying the embryonic hormonal environment, independently of the genetic sex of the birds. However, the cellular mechanisms by which the early estrogen exposure determines the adult behavioral sex are not understood.
We recently demonstrated that the massive wave of neurogenesis that organizes the overall structure of the brain ends in the quail POM before E6 but that cellular proliferations continue at a progressively decreasing rate until E14 (Bardet et al., 2012). New cells that were identified, based on a variety of neurochemical and anatomical features, as slow cycling progenitors are thus produced in the embryonic POM until the end of the critical period of sexual differentiation ending on E12. It was, however, impossible to formally demonstrate that these cell populations do not include a few glial elements because classical glial markers identified in mammals do not work reliably in the quail preoptic area (Bardet et al., 2012). There was also some suggestion that in the adult POM, these cells labeled by the thymidine analog 5-bromo-2′-deoxyuridine (BrdU) on E12 were more numerous in adult females than in males (Bardet et al., 2012). In an attempt to better understand the mechanisms that underlie the sexual differentiation of this brain region, we studied here in males and females the ontogeny from hatching until adulthood of this preoptic cell population labeled at E12 by BrdU. We demonstrate that there is an active proliferation of these cells around the time of puberty suggesting a role of these new cells in the structural organization and plasticity of the adult preoptic area.
2. Results
2.1. Experiment 1: sex difference observed in cells labeled by BrdU injection on E12
To determine whether cells labeled by a BrdU injection on E12 at the end of the period of sexual differentiation are present in statistically different numbers in adults (PN56) as suggested by a previous experiment (Bardet et al., 2012), BrdU-ir cells were counted in a group of 7 males and 7 females who had been injected with BrdU on E12 and killed on PN56. Counts were obtained separately in the rostral (CA-4), intermediate (CA-2), and caudal (CA) levels of the POA.
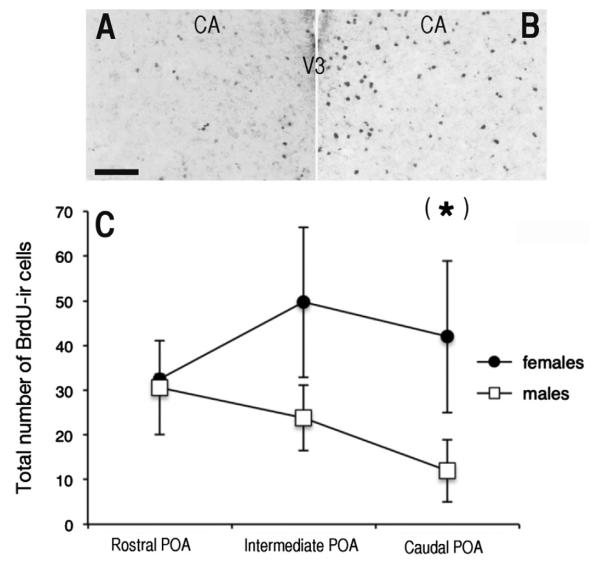
On average, more labeled cells were observed in females than in males at the CA and CA-2 levels (Fig. 1). A two-way ANOVA indicated the presence of a barely significant sex difference (F1,40=8.93, p=0.049) in the number of BrdU-ir cells in the POA, but no effect of position within the area (F2,40=0.75, p=0.843) and no interaction (F2,40=4.23, p=0.386) Although the absence of significant interaction suggested that the sex difference was present throughout the sampling area, visual inspection of data in Fig. 1 strongly suggested that the effect of sex was driven by the most posterior levels of the POA. This was confirmed by descriptive t-tests indicating a significant difference at the level of the anterior commissure (CA: t12=2.23 p=0.046) but not at the more rostral levels (CA-2: t13=1.48, p=0.163: CA-4: t14=1.11, p=0.913).
Fig. 1.
A, B. Photomicrograph of a section through a male (A) and a female (B) quail brain injected with BrdU at E12 and killed at PN56. Scale bar=100 μm. C. Numbers of BrdU-ir cells at three rostro-caudal levels of the preoptic area (POA) of male and female quail injected with BrdU at E12 and killed at PN56. Data are means±standard errors of the mean (SEM); (*)=p<0.05 by a descriptive t-test compared to the other sex at the same rostro-caudal level. V3=third ventricle, CA=anterior commissure.
2.2. Experiment 2: cell proliferation between E12 and E13
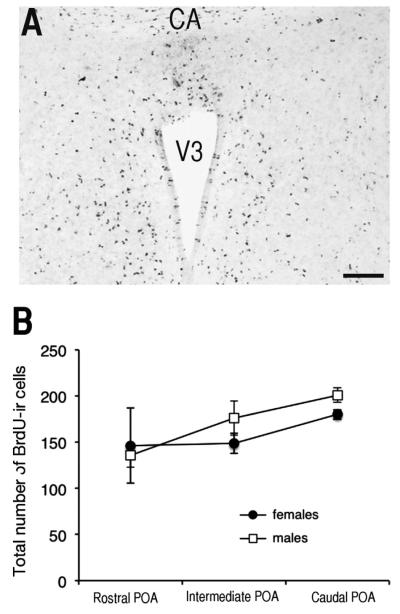
The sex difference observed in adult birds in the number of preoptic cells labeled by a BrdU injection on E12 could be due to differential cell proliferation, migration, or survival. To help differentiate between these alternatives, an additional set of eggs was injected with BrdU on E12 and brains were collected 24 h later on E13. Large numbers of BrdU-ir cells were observed throughout the parenchyma of the preoptic area and also to a lesser extent along the walls of the third ventricle in both males and females (Fig. 2A).
Fig. 2.
Cell proliferation in the male and female quail preoptic area (POA) as demonstrated by a BrdU injection on E12 and brain collection for histological visualization on E13. A. Photomicrograph of BrdU-ir cells observed along the ventricle and in the parenchyma of the POA of female at low magnification: Scale bar =100 μm. CA=anterior commissure, V3=third ventricle B. Number of BrdU-ir cells counted in the POA of males and female quail at three different rostrocaudal levels. No sex difference in proliferation was detected but more cells were present in the caudal than in the rostral POA. Data are means±standard errors of the mean (SEM).
The number of BrdU-ir cells in the POA was much higher in brains collected at E13 (150–200 cells per camera field) as compared to numbers collected at PN56 (30–40 cells). This difference is presumably explained by a combination of factors including brain growth and apoptosis between E13 and PN56 and dilution of BrdU in labeled cells as a result of multiple divisions (label is no longer detectable after more than 4 divisions; Hayes and Nowakowski, 2002).
A two-way ANOVA identified no sex difference (F1,16=0.19, p=0.193) in the number of BrdU-ir cells in the POA; there was an effect of position within the nucleus (F2,16=5.43, p=0.015) but no interaction (F2,16=0.63, p=0.544). Post-hoc tests indicated that largest numbers of BrdU-ir cells were present in the caudal POA as compared to the intermediate and rostral POA (p=0.046 and p=0.014 respectively; Fig. 2B).
2.3. Experiment 3: proliferation and survival of cells labeled by a BrdU injection at E12
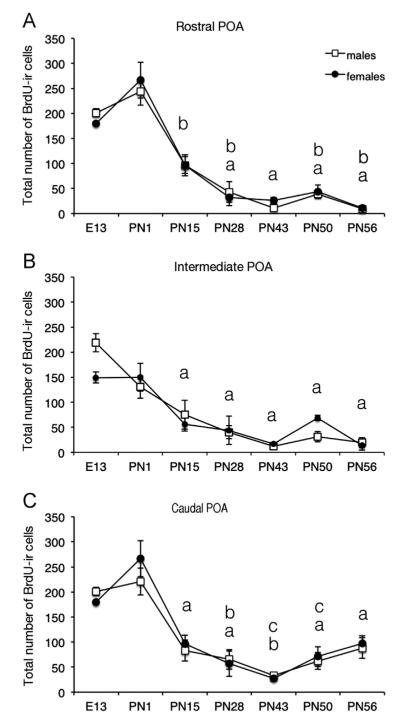
To follow the proliferation and survival of the cells labeled by a BrdU injection on E12 from E13 until the adult age, we counted the cells in the POA at the level of the anterior commissure (CA) or 240 and 480 μm rostral to the CA (CA-2 and -4) in brains collected at key points from hatching (taking place on E17) until early adulthood: PN1 (when a large peak in plasma T is observed), PN15, PN28, PN43 (just after sexual maturation), PN50 or PN56 (Fig. 3).
Fig. 3.
Number of BrdU-ir cells counted at three rostrocaudal levels of the preoptic area (POA) in male and female quail injected with BrdU on embryonic day 12 and killed at key points during development until adulthood between embryonic day 13 (E13) and postnatal day 56 (PN56). Data for each brain area were analyzed by a two-way ANOVA (sex and age of birds as factors) that were followed by Newman–Keuls post-hoc tests analyzing the significant age effect whose results are indicated by letters in the figure. Time points labeled by a same letter are not different, time points that do not share a same letter are significantly different for p<0.05.
2.3.1. Total number of BrdU-ir cells
A two-way ANOVA of the total number of BrdU-ir cells was run independently for each rostro-caudal level of the POA. Significant main effects of age were identified for the rostral, intermediate and caudal POA (respectively F6,52=33.92; p<0.001; F6,52=10.67; p<0.001; F6,52=24.99; p<0.001). No sex difference was detected (rostral F1,52=0.14; p=0.714; intermediate F1,52=0.55; p=0.462; and caudal F1,52=0.48; p=0.493) and there was no interaction between the age and the sex of the birds in the three POA levels (F6,52=0.43, p=0.856; F6,52=0.76, p=0.604; F6,52=0.58, p=0.746). Therefore, the main effect of age was further analyzed using the Newman–Keuls post-hoc test for each level of POA for both sexes simultaneously.
The total number of the BrdU-ir cells increased slightly but significantly between E13 and PN1 in the caudal and rostral POA. In contrast, in the intermediate POA, the average number of BrdU-ir cells did not change significantly between PN1 and E13 (Fig. 3). The BrdU-ir cell number then decreased dramatically from PN1 to PN15 in all rostrocaudal levels of the POA (rostral, intermediate and caudal, PN1 vs. PN15). After PN15, the number of BrdU-ir cells remained stable until PN56 in the intermediate POA. In contrast, some additional decrease in the number of BrdU-ir cells took place between PN15 and PN43 in the rostral and caudal POA (see Fig. 3). Quite interestingly, in the caudal POA, the total number of BrdU-ir cells then increased significantly between PN43 and PN56 (Fig. 3C) in contrast to the rostral and intermediate POA where this number remained unchanged.
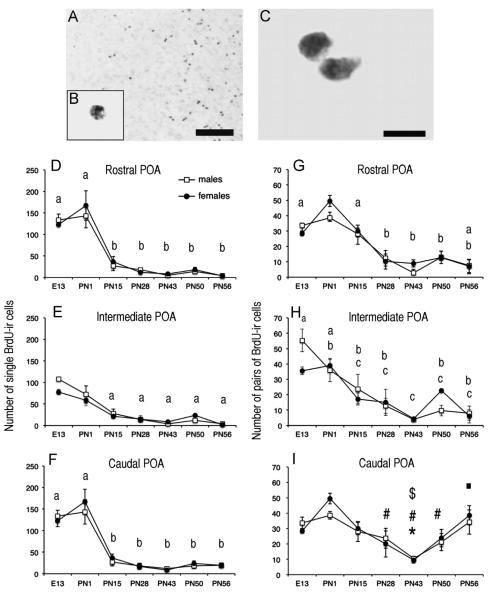
Qualitative observation of these BrdU-ir cells indicated that they were often present in pairs. It was therefore decided to separately quantify the number of single cells (Fig. 4 D–F) and the number of BrdU-ir cells observed in pairs (“doublets” i.e. cells in close proximity separated by a distance equal or smaller to 10 μm that were presumably derived from the division of a single cell; Fig. 4G–I).
Fig. 4.
Changes with age in the numbers of BrdU-ir cells at three rostro-caudal levels of the preoptic area (POA). A. Photomicrographs of the POA of a female quail injected with BrdU on E12 and killed at PN56. The insert (B) contains a photomicrograph at higher magnification of a single labeled cell and a pair of labeled cells (doublet) is illustrated in panel C. Scale bar=100 μm for A, 20 lm for B (same bar as in A) and 10 μm for C. D–I. Number of single BrdU-ir cells (D–F) and of BrdU-ir doublets (G–I) present at three rostro-caudal levels of the POA in male and female quail injected with BrdU on embryonic day 12 and killed at key points during development until adulthood from the embryonic day 13 (E13) to the postnatal day 56 (PN56). Data for each brain area were analyzed by a two-way ANOVA (sex and age of birds as factors) that were followed by Newman–Keuls post-hoc tests analyzing the significant age effect whose results are indicated by letters in the figure. In D through H, time points labeled by a same letter are not different, time points that do not share a same letter are significantly different for p<0.05. In I, *=p<0.05 vs. E13; #=p<0.05 vs. PN1; $=p<0.05 vs. PN15 and =p<0.05 vs. PN43.
2.3.2. Number of single BrdU-ir cell
Like the total numbers of BrdU-ir cells, the numbers of single BrdU-ir cells were analyzed independently by a two-way ANOVA for each rostro-caudal level of the POA. Significant main effects of age were again identified for the rostral, intermediate and caudal POA (respectively F6,52=41.76; p<0.001; F6,52=33.74; p<0.001; F6,52=37.71; p<0.001) while no sex difference (rostral F1,52=0.13; p=0.724; intermediate F1,52=0.89; p=0.350; and caudal F1,52=0.29; p=0.594) and no interaction between age and sex (F6,52=0.36, p=0.902; F6,52=1.32, p=0.265; F6,52=0.34, p=0.908) were detected. The main effects of age were then further analyzed with Newman–Keuls post-hoc tests for each level of POA.
There was no significant change in single cells numbers between E13 and PN1 in the rostral and caudal POA levels although clear trends toward an increase were present (p=0.060 and p=0.068) while there was a significant although moderate decrease at this stage in the intermediate level (p<0.001). After birth, the profile of the number of the single cells was similar for the three rostro-caudal levels: a major decrease was observed between PN1 and PN15 (p<0.001) and then the number of single cells remained stable and very low until adulthood for the three levels of the POA ((see Fig. 4 D–F) for the list of significant differences).
2.3.3. Number of BrdU-ir cells present in pairs of cells (doublets)
The three two-way ANOVA analyzing the numbers of BrdU-ir cells present in pairs identified significant main effects of age at the rostral, intermediate and caudal POA levels (respectively F6,52=10.14; p<0.001; F6,52=3.79; p<0.001; F6,52=7.22; p<0.001). No sex difference was detected (rostral F1,52=0.06; p=0.810; intermediate F1,52=0.31; p=0.578; and caudal F1,52= 0.39; p=0.535) and there was no interaction between age and sex in the three levels POA (F6,52=0.27, p=0.950; F6,52=0.42, p=0.861; F6,52=0.57, p=0.752). Analysis of the main effects of age with Newman–Keuls post-hoc tests showed that the number of doublets increased during early development (between E13 and PN1) in the rostral and caudal but not in the intermediate POA. The number of the doublets then decreased progressively to reach a nadir at PN43 in the three brain regions. These numbers then stayed at these low values in the rostral and intermediate POA but quite interestingly increased significantly between PN43 and PN56 in the caudal POA (see fig. 4G–I).
A qualitative observation of the BrdU-ir cells seemed to indicate that over time the number of doublets compared to the number of single cells increased substantially. To better visualize this trend, we calculated the percentage of BrdU-ir cells that were present in doublets among the total number of BrdU-ir cells (Fig. 5).
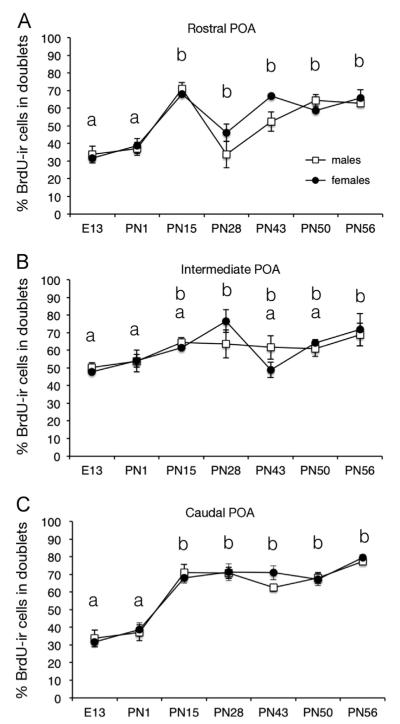
Fig. 5.
Percentage of cells present in doublets among the total number of BrdU-ir cells counted at three rostro-caudal levels of the preoptic area (POA) at key points during development between embryonic day 13 (E13) and postnatal day 56 (PN56). One doublet contributing for two cells in this calculation. Data for each brain area were analyzed by a two-way ANOVA (sex and age of birds as factors) that were followed by Newman–Keuls post-hoc tests analyzing the significant age effect whose results are indicated by letters in the figure. Time points labeled by a same letter are not different, time points that do not share a same letter are significantly different for p<0.05.
2.3.4. Percentage of BrdU-ir cells present in pairs (doublets)
We also analyzed the percentage of the total number of cells that were actually included in doublets (one doublet contributing for two cells in this total). Analyses by two-way ANOVA of the percentage of BrdU-ir cells present in pairs identified significant main effects of age in the rostral, intermediate and caudal POA (respectively F6,52=19.48; p<0.001; F6,52=3.61; p<0.001; F6,52=38.03; p<0.001). Again, no sex difference (rostral F1,52=0.16; p=0.695; intermediate F1,52=0.01; p=0.916; and caudal F1,52= 0.01; p=0.907) and no interaction between age and sex were detected in the three POA levels (F6,52=1.85, p=0.107; F6,52=1.74, p=0.131 F6,52=0.93, p=0.480).
Newman–Keuls post-hoc tests analyzing the effects of age identified stable percentages of doublets between E13 and PN1 at the three levels of the POA followed by an increase between PN1 and PN15 in the rostral and caudal levels where levels then remained stable until adulthood (PN56). In the intermediate POA, this increase was less prominent and a significant difference with the early ages was only detected at PN56 (and transiently at PN28).
2.4. Cells labeled by a BrdU injection on embryonic day 12 divide during puberty
Since we observed in the caudal POM between PN43 and PN56 an increase in the total number of cells labeled by a BrdU injection at E12 and in the number of doublets, we tested whether these BrdU-ir cells actually divide at PN43 (early sexual maturation), PN50 (sexual maturation) and PN56 (young adults). To this aim, we double labeled sections for BrdU and the proliferating cell nuclear antigen (PCNA, an endogenous marker of proliferation) in males and females that had been injected with BrdU at E12 and killed at PN43, PN50 and PN56. PCNA expression increases during the G1 phase of the cell cycle, peaks at the transition from the G1 to the S phase, and then decreases through the G2 phase. PCNA-positive cells are thus in the process of preparing for mitosis.
This experiment identified in the caudal POA the presence of double-labeled cells (BrdU and PCNA) at the three ages that were investigated (PN43, PN50 and PN56; Fig. 6) thus confirming that some of the cells labeled by a BrdU injection at E12 are still cycling at these peripubertal ages.
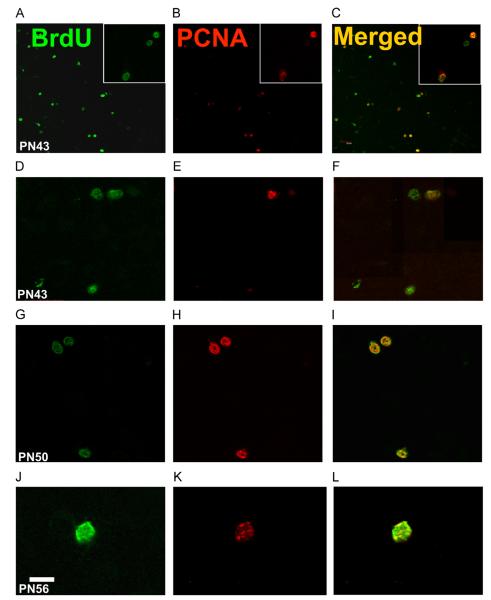
Fig. 6.
Photomicrographs of sections through the caudal POA stained by immunofluorescence from birds injected with BrdU at E12 and killed at PN43 (A–F), PN50 (G–I) or PN56 (J–L). The sections were double-labeled for BrdU (green; panels A, D, G and J) and the proliferation marker, PCNA (red; panels B, E, H and K). Labels at the top of each column and beginning of each line refer to the entire column or line. Panels C, F, I and L present the overlap of both signals. Panels A–C and G–I are from females, panels D–F and J–L are from males. In A–C, several BrdU-ir nuclei are clearly co-localized with PCNA-ir nuclei, but some BrdU-ir cells do not express PCNA. D–F illustrates one PCNA-ir cell labeled with BrdU within a doublet. In G-I all BrdU-ir cells are co-localized with PCNA. J–L illustrates at higher magnification a single cell labeled by both BrdU and PCNA. Scale bar=60 μm in A–B but 30 lm in inserts, 20 μm in D–I and 10 μm in J–L.
The presence of PCNA-immunoreactive cells in the POA was somewhat surprising since the POA is not classically considered as “a neurogenic zone” that continues to produce new neurons into adulthood. Substantial numbers of cells labeled by a BrdU injection on E12 nevertheless expressed PCNA in the caudal POA at PN43 (Fig. 6 panel A–F), PN50 (Fig. 6 panel G–I) and PN56 (Fig. 6 panel J–L). These dividing cells were observed in both sexes (Panels A–C and G–I are in females and panel D–F and J–L in males). PCNA-positive cells at PN43, PN50 and PN56 were found as single cells as well as in doublets. More surprisingly we also found BrdU-ir doublets in which one cell of the pair only expressed PCNA suggesting that the cell cycle is not synchronized between the two cells of a same BrdU-ir doublet that are presumably derived from a same precursor. These cells double-labeled for PCNA and BrdU were observed at all rostro-caudal levels of the POM.
Since increases in the number of BrdU-ir cells were observed specifically in the caudal POA between PN43 and PN56, counts of PCNA and BrdU positive cells were performed in the caudal POA for two males and two females at each peripubertal age (E12+PN43, E12+PN50 and E12+PN50) and results are shown in Table 1.
Table 1.
Mean and standard deviation (SD) of the numbers of PCNA-immunoreactive (+) and BrdU-immunoreactive (+) cells and of double-labeled cells counted in males and females at PN43, PN50 and PN56 after an injection of BrdU at E12. The table also presents the ratios of PCNA+ and BrdU+ cell numbers as well as the percentages of PCNA+ and of BrdU+ cells that were double-labeled. Two birds were available for each sex and age except in two cases where one sample was accidentally lost.
| PCNA+ | BrdU+ | PCNA/BrdU | PCNA+BrdU+ | PCNA+BRdU− | % PCNA+BrdU+/PCNA+ | % PCNA+BrdU+/BrdU+ | |
|---|---|---|---|---|---|---|---|
| FEMALES | |||||||
| PN43 | 19 | 42 | 0.45 | 17 | 2 | 89.47 | 40.48 |
| PN43 | 13 | 49 | 0.27 | 10 | 3 | 76.92 | 20.41 |
| PN50 | 21 | 54 | 0.39 | 20 | 1 | 95.24 | 37.04 |
| PN50 | 25 | 60 | 0.42 | 13 | 12 | 52.00 | 21.67 |
| PN56 | 24 | 74 | 0.32 | 14 | 10 | 58.33 | 18.92 |
| Mean | 20.40 | 55.80 | 0.37 | 14.80 | 5.60 | 74.39 | 27.70 |
| SD | 4.77 | 12.13 | 0.07 | 3.83 | 5.03 | 18.89 | 10.21 |
| MALES | |||||||
| PN43 | 5 | 17 | 0.29 | 4 | 1 | 80.00 | 23.53 |
| PN50 | 14 | 71 | 0.20 | 13 | 1 | 92.86 | 18.31 |
| PN50 | 15 | 60 | 0.25 | 11 | 4 | 73.33 | 18.33 |
| PN56 | 16 | 30 | 0.53 | 9 | 7 | 56.25 | 30.00 |
| PN56 | 10 | 22 | 0.45 | 8 | 2 | 80.00 | 36.36 |
| Mean | 12.00 | 40.00 | 0.35 | 9.00 | 3.00 | 76.49 | 25.31 |
| SD | 4.53 | 24.05 | 0.14 | 3.39 | 2.55 | 13.34 | 7.83 |
There were in general two to three times more BrdU-ir than PCNA-ir cells in the caudal POA (see Table 1). A very large fraction (over 70%) of the PCNA-ir cells were also labeled with BrdU indicating that this population of cells is especially mitotically active around puberty. It could indeed be estimated that approximately 25% of the BrdU-ir cells were cycling and expressing PCNA at the time points considered. However, we also observed PCNA-ir cells (around 30% of the total) that were not double labeled with BrdU indicating that either other cells populations that were not labeled by a BrdU injection on E12 also cycle at this time or that these cells had been labeled on E12 but they continued cycling during the early post-natal life when free BrdU was not longer available for incorporation.
No obvious difference between the sexes and across peripubertal ages could be detected but this conclusion should clearly be confirmed by a more extensive quantitative analysis. These results are consistent with the lack of sex difference in the survival of BrdU-ir cells during puberty. Nevertheless, further investigations should be performed to determine potential significant differences in cell division rates at different peripubertal ages.
3. Discussion
The sexually dimorphic POM of the preoptic area plays a critical role in the control of male sexual behavior in quail but the mechanisms controlling its development and in particular the sex differences affecting different features of this nucleus have not been clearly identified. In the present experiments, we characterized the development of this nucleus by analyzing the survival of a cell population labeled by a BrdU injection on E12, the day marking the end of the critical period controlling the sexual differentiation of behavior. This cell population was suggested earlier to be sexually differentiated in adulthood but this was not confirmed here. We report that this cell population is apparently not associated with a sex difference in proliferation rate nor in cell death. The previously detected sex difference is thus not observed in all groups of birds and resulted from unidentified factors. We demonstrate here that the density of these cells labeled by a BrdU injection on E12 per unit surface progressively decreases during the first 6–7 postnatal weeks but then increases during the following weeks that correspond to the period of sexual maturation of the birds. There is thus an increase in brain plasticity taking place around puberty in Japanese quail that possibly contributes to explain the development of sexually differentiated features of the preoptic area. The specific role of cells labeled by a BrdU injection on E12 in this plasticity should now be investigated. Below we discuss several key questions raised by these original findings.
3.1. Sex differences in cell number in adults are not due to a difference in initial rate of BrdU incorporation
Our previous study indicated that cells labeled by BrdU in quail embryos between E8 and E14 are present in similar numbers in adult male and female quail in most brain regions, with the notable exception of three steroid-sensitive regions (the POM, bed nucleus of the stria terminalis and ventromedial nucleus of the hypothalamus) where BrdU injected at E12, but not at other developmental times, resulted in larger number of cells in the adult females as compared to males (Bardet et al., 2012). The presence of these sex differences specifically in steroid-sensitive areas but not in control regions (e.g., nidopallium, nucleus rotundus and cerebellum) suggested a direct or indirect control by steroids. It should be noted, however, that these differences were statistically significant only in post-hoc analyses that were secondarily focused on embryonic day 12 based on the visual evaluation of the data. Differences were not significant in the general ANOVA globally assessing effects of sex and age of BrdU injection.
In a first experiment, we confirmed here the previously reported sex difference (females>males) in the number of BrdU-ir cells labeled by a BrdU injection at E12 in the caudal POA (Bardet et al., 2012). The difference was, however, present only in the caudal part of the POA (it extended more rostrally in our previous study) and it was barely reaching statistical significance (p=0.046).
In an attempt to understand the origins of this sex difference observed in the caudal POA, we analyzed in a second experiment whether it relates to a sex difference in short term BrdU labeling. In birds injected with BrdU on E12 but killed 24 h later (on E13), no sex difference in the number of BrdU-ir cells could be detected at three rostro-caudal levels of the POA covering the entire area. This lack of sex difference in labeling was again confirmed in experiment 3 (no sex difference at E13). The sex difference observed in adult birds after BrdU injection on E12 thus cannot be the result of an initial difference in proliferation. It should result from a differential cell death later in life with more cells of the labeled cell population disappearing in males than in females.
However, during experiment 3 we completely failed to identify any sex difference in the numbers of cells labeled by BrdU on E12 at any time point between hatching and adulthood. Furthermore, in this experiment, the number of these cells was nearly identical in males and in females at PN56 (adulthood), the age when previous experiments had suggested the presence of a sex difference favoring females.
The origins of this discrepancy remain unclear at present. The sex difference may concern exclusively a cell population that incorporated BrdU within a very narrow window of development (only a part of E12) that was missed during experiment 3. We had originally checked the correspondence between incubation ages in quail and the Hamburger & Hamilton stages (see Bardet et al., 2012), but this confirmation was not obtained here and minor differences in the rate of maturation of the embryos could have taken place between experiments so that injections of BrdU on E12 would have concerned here embryos that were slightly more (or less) mature. This would be consistent with the observation that the number of BrdU-ir cells labeled on E12 and counted at PN56 in the caudal POA was lower in experiment 1 (about 40 per counting field in females and less than 10 in males) than in experiment 3 (around 90 in both sexes). Alternatively, the rate of postnatal development might have been different between experiments so that brains collected at PN56 did not correspond to the same developmental stage. This seems, however, improbable since birds in all experiments were sexually mature as attested by the fully developed size of their gonads.
3.2. Proliferation and survival of cells labeled by a BrdU injection at E12
The main goal of the present studies was to follow the development of the population of preoptic cells labeled by a BrdU injection at E12 in order to better understand their survival and potential significance. These BrdU-ir cells were thus quantified at several key periods of development between hatching and sexual maturity. We found a general increase of the total number of BrdU-ir cells labeled at E12 in the caudal and rostral POA between E13 and PN1. After PN1, a progressive but very marked decline of these cell populations was detected, and finally we observed an increase of the total number of BrdU-ir cells and in particular the doublets during sexual maturation (after PN43) specifically in the caudal POA. These observations suggest complex controls of the proliferation and survival in these cell populations during three distinct periods of the bird’s life and interesting functional consequences.
3.2.1. Increase in number of BrdU-ir cells labeled by a BrdU injection on E12 from E13 to PN1
A significant increase in the number of preoptic cells labeled by a BrdU injection on E12 was observed during the latter half of embryonic development, from E13 to PN1 in the caudal and rostral POA but surprisingly not in the intermediate POA (Fig. 3). This indicates that populations of cells in different regions of the POA are differentially modulated during the same time period. This was already suggested by the observation that populations of aromatase-immunoreactive cells do not follow the same developmental pattern at these different rostro-caudal levels of the POA (Balthazart et al., 2000).
The additional cells counted at PN1 as compared to E13 could have two origins. They could first result from the incorporation of “free” BrdU in cells that would have replicated their DNA during this period. Indeed if BrdU injected in an animal has a relatively short half life in the order of a few hours (Packard et al., 1973), it has been reported that in an avian egg, BrdU can remain available for several days if injected in large quantity as done here (LaVail and Cowan, 1971; Tsai et al., 1981a, 1981b; Yurkewicz et al., 1981). Alternatively, it is also conceivable that little or no free BrdU was available between E13 and PN1 but that some of the cells that had incorpored BrdU on E12 went through one or two additional rounds of divisions which dilutes the incorporated BrdU but without rendering it undetectable. This second interpretation is supported by the fact that during the same period there was also an increase in the absolute number of doublets, suggesting the existence of divisions of previously labeled cells. It is however impossible to fully discriminate between these interpretations with the available data (See (Bardet et al., 2012) for further discussion).
3.2.2. Decline of BrdU-ir cell numbers before puberty
From hatching (PN1) to PN43, we observed a continuous decrease in the total number of BrdU-ir cells per unit surface in all rostro-caudal locations of the POA. The increase in brain size that is observed at that time when neuronal proliferation is markedly reduced or absent probably contributes in a significant manner to this diminished cell density. This could potentially be tested by counting total numbers of cells in POM rather than cell numbers per unit surface. However, besides the pragmatic problems that would be associated with the quantification of huge numbers of cells in many subjects, this approach would also raise the question of the definition of the boundaries of the POM and whether these boundaries themselves changes during ontogeny.
Despite the limitations associated with the quantification of cells per unit surface, we interestingly observed some differences between the rate of decline at different locations with the major decrease in numbers occurring earlier (between PN1 and PN15) in the rostral and caudal POA than at intermediate levels (see detail of statistical comparisons in Fig. 3). A parallel decrease in the absolute number of doublets was also observed during this period. It is likely that at least part of the decrease in cell density is the result of cell death presumably by apoptosis. This interpretation could, however, not be confirmed in this study due to the poor labeling observed with activated caspase-3, a specific marker of apoptosis. A less likely explanation is that multiple cell divisions also take place during this period and potentially dilute the incorporated BrdU so that it becomes invisible in some cells. It is indeed the case that the number of doublets decreases less rapidly than the number of single cells (Fig. 4) so that there is actually an increase in the proportion of cells present in doublets during this early postnatal life. This is particularly the case in the caudal POM where there is an abrupt increase in the percentage of doublets between PN1 and PN15. It is noticeable that whereas the period between PN1 and PN15 corresponds to the sharpest decrease in total cell numbers in the caudal POA (numbers will remain nearly constant afterward (see Fig. 3C)), this is also the time when the percentage of doublets increases from embryonic values observed at E13 and PN1 to adult-typical values observed from PN15 till PN56 (Fig. 5C). One could imagine that during this period, cell death is preferentially affecting single cells (presumably older) as compared to doublets (that divided more recently) as confirmed by the fact that numbers of single cells decrease sharply (Fig. 4C) while numbers of doublets remain nearly constant (Fig. 4F). Alternatively, it is also possible and probably more likely that the differential rate of decrease of single cells and of doublets is due to the fact that some single cells actually divide during this period to form doublets. This phenomenon would thus partly compensate the disappearance of doublets and create an apparent rate of decline that would be slower than for single cells.
We suggest, however, that cell death rather than extensive cell proliferation (and the associated dilution of the BrdU label) explains the overall decrease in BrdU-ir cells. Our previous work (Bardet et al., 2012) has indeed shown that cell proliferation decreases sharply during the end of the incubation period and becomes apparently very low after E14. One would thus have to assume the existence of a massive period of post-natal proliferation to explain the disappearance of BrdU-ir cells by dilution of the label and there is no reason (e.g., no prominent peak on plasma sex steroids) to suspect the existence of such a peak that has also not been detected in other avian or mammalian species. The cellular mechanism mediating this decrease in BrdU-ir cell numbers and the differential decrease affecting single cells and doublets should however receive additional attention.
3.2.3. Increase in the number of BrdU-ir cells and doublets during puberty in the caudal POA
Quite interestingly, we observed an increase in the number of BrdU-ir cells after PN43 in the caudal POA (Fig. 3). This increase was not associated with an increase in single cells (their number remains stable; Fig. 4F) but rather with an increase in the absolute number of doublets (Fig. 4I) so that in adulthood, about 80% of the BrdU-ir cells were present in doublets versus only about 30% in early development (Fig. 5). This strongly suggests that the increase in total number of cells is due to divisions of previously labeled cells taking place in the parenchyma of the brain during puberty. The detection of BrdU-ir cells double-labeled with the marker of cell divisions PCNA clearly confirms this interpretation. We show here that about 75% of the cells expressing PCNA in the caudal POA during puberty contain BrdU indicating that they divided on E12 or soon thereafter (Table 1). Based on the small sample size investigated, we found no evidence for sex difference in the percentage of BrdU-ir cells that express PCNA; males and females also had roughly equal ratios of PCNA-ir and BrdU-ir cells. These data, although they need to be confirmed by more extensive studies, are nevertheless consistent with the finding that both sexes show similar numbers of BrdU-ir doublets during puberty. Further studies with larger number of subjects would, however, be needed to test these tentative observations.
We found an increase in the number of doublets during puberty (suggesting that single cells divide and produce a doublet), but no decrease in the total number of single cells. This suggests that the expected loss of some single cells (that formed doublets and were thus no longer counted as « single cells ») was compensated by the separation of cells that were previously in doublets and moved apart sufficiently (≥10 μm) to be counted as single cells.
It is important to note that these doublets were present in adult birds as well as in juveniles in the entire parenchyma of the POA suggesting the presence of cell divisions at this location and not only at the ventricle wall as observed during the description of adult neurogenesis in the telencephalon of adult songbirds (Alvarez-Buylla and Nottebohm, 1988; Alvarez-Buylla and Kirn, 1997). This was actually already the case in embryos on E13: one day after the BrdU injection labeled single cells and doublets were found in the entire preoptic area. The POA thus contains a cell population throughout its parenchyma that is able to divide. However, these divisions only occur at specific time points and locations, for example in the caudal POA at the time of puberty (between PN43 and PN56). These cells remained dormant until sexual maturation. If divisions were taking place continuously, the BrdU label would be lost before or soon after hatching: the BrdU signals is indeed reported to disappear after 3 or 4 divisions (Hayes and Nowakowski, 2002).
3.3. Nature and potential function of additional BrdU-ir cells dividing at puberty
Brain function or structures related to reproduction are often thought to depend on the action of gonadal steroids acting either during early life (organizing, irreversible effects) or in adulthood (activating, reversible effects). More recently, however, the period of sexual maturation, commonly called puberty in mammals, has become a focus of attention and it has been demonstrated in some cases and suggested in others that the action of sex steroid hormones at this time plays a critical role in the final organization of brain and behavior (Ahmed et al., 2008; Patlak, 2012; Schulz et al., 2009; Sisk and Zehr, 2005). Puberty has thus been suggested as a second critical period of brain and behavior organization by sex steroids (Schulz et al., 2009).
Especially relevant for the present work is the study of Ahmed and collaborators demonstrating that, in rats, injections of BrdU just before puberty (on days PN 20-22, 30-32 and 40-42, i.e. pre-, early- and mid-puberty) label 20 days later cell populations in the sexually dimorphic nucleus of the preoptic area (SDN), the medial amygdala and the anteroventral periventricular nucleus of the hypothalamus (AVPV; Ahmed et al., 2008). This last nucleus is larger in females than in males whereas the SDN and medial amygdala are larger in males. Correlatively, more BrdU-ir cells were detected in the AVPV of females as compared to males but the reverse was true in the two other nuclei. It was therefore speculated that the differential cell proliferation in males and females around puberty correlates and may be a (partial) cause of the volumetric differences observed in adult rats (Ahmed et al., 2008).
Double-label experiments indicated that a substantial part of these new cells in the amygdala and AVPV (about 25 and 50% respectively) turn into neurons and express the neuronal marker NeuN (Ahmed et al., 2008). Additionally, in the medial amygdala, more BrdU-ir cells co-expressed the glial marker GFAP than the neuronal marker NeuN. In contrast, however, in the SDN of the POA, BrdU-ir cells expressed neither NeuN nor GFAP and it was proposed that these cells were either going to die by apoptosis or represent a pool of cells that could differentiate on demand at a later time. This study (Ahmed et al., 2008) thus suggested that the fate of cells born around puberty varies from one brain region to another. It was also notable that the BrdU-ir cells observed in the SDN were present in doublets in the brain parenchyma suggesting that they divided at these locations (Kokoeva et al., 2007).
These cells of the rat SDN labeled by BrdU injected peripubertally thus display similar characteristics as the cell population studied here in quail. In quail, cells labeled by a BrdU injection on E12 and analyzed on PN56 do not express classical neuronal (Hu, NeuN, aromatase) nor glial (GFAP, S100,vimentin) markers and they cannot be stained by classical mammalian markers of undifferentiated progenitors such as BLBP, nestin or Sox2 although a few of them express PCNA (the proliferating cell nuclear antigen) a marker of cell cycling and proliferation (see Bardet et al., 2012) and they have divided in the brain parenchyma far away from the ventricles (Bardet et al., 2012; this study). We further demonstrate here that these cells dividing around puberty were labeled by a BrdU injection much earlier during embryonic life at the end of the critical period for sexual differentiation but remained quiescent or cycled very slowly until this peripubertal reactivation. Several converging arguments confirm this increased rate of cell divisions around puberty in the caudal POA: (a) the increase in total number of BrdU-ir cells, (b) the increase in the number of doublets, (c) the presence of PCNA labeled cells and their colocalization with BrdU indicating that the mitoses concern at least in part cells that were labeled by BrdU on E12 or soon thereafter and were quiescent in between since otherwise their BrdU would be too diluted to be detectable. The fact that this dormancy ends for a substantial number of cells around puberty suggests that the renewed proliferation is linked to the gonadal steroid hormones produced at this time as previously reported in rodents where gonadectomy significantly reduced the number of BrdU-ir cells (Ahmed et al., 2008).
Interestingly, this peripubertal addition of cells in the POA temporally coincides with the emergence of sexual behavior and with multiple neuroanatomical and neurochemical changes in the POA of quail (e.g., increase in both sexes of the POM volume (Thompson and Adkins-Regan, 1992), of preoptic aromatase activity and of the number of aromatase-immunoreactive cells (Balthazart et al., 2000)). The divisions of cells labeled by a BrdU injection on E12 observed here at the beginning of sexual maturation may directly or indirectly participate to this peripubertal plasticity of the caudal POA. Their specific role remains, however, to be determined.
In conclusion, we identified here a population of presumably non-neuronal cells (i.e., not expressing Hu nor NeuN; see Bardet et al., 2012) labeled by BrdU at E12 or soon after that remain quiescent during the first post-hatching month and then multiply during puberty in the caudal POA, a critical site for the activation of adult sexual behavior in the Japanese quail. These newly generated cells potentially play a significant role in the neural plasticity that takes place concurrently in the POA but further investigations will be necessary to elucidate the specific mechanisms controlling this cellular proliferation and its functional significance
4. Experimental procedures
4.1. Experimental animals
All experiments were carried out on Japanese quail (Coturnix japonica) hatched from eggs incubated in our laboratory that were obtained either from a local breeder in Belgium or from our laboratory breeding colony. All experimental procedures were in accordance with the relevant Belgian laws regarding the Protection and Welfare of Animals and the Protection of Experimental Animals, and all protocols were approved by the Ethics Committee for the Use of Animals at the University of Liège.
Eggs were incubated, narrow end down, at 39 °C, 50–60% relative humidity in a forced-air incubator on trays that were rotated 90° twice daily. The day when eggs were set in the incubator was counted as embryonic day zero (E0). On E15, eggs were transferred into separate wire mesh boxes for hatching and placed at the bottom of the incubator with no further rotation until they hatched on E17.
Throughout their life in the laboratory, birds were maintained on a photoperiod simulating long summer days (16L:8D) with access to food and water ad libitum. During the first three weeks after hatching, chicks were kept in mixed sex groups in brooder cages at 30 °C. Temperature was then progressively lowered to room temperature until around 5 weeks of age, when all birds were transferred to individual cages.
4.2. Experiment 1: sex difference in the number of cells labeled by a BrdU injection at E12 surviving to adulthood in the POM
To test the reliability of the sex difference in the number of adult POM cells labeled on E12 by the thymidine analog 5-bromo-2′-deoxyuridine (BrdU) that had been previously suggested (Bardet et al., 2012), eggs were injected with BrdU on E12 (Fluka 16880) to label cells during the S phase of their cycle. Eggs were cleaned with alcohol, the shell at the narrow end of the egg (opposite to the air chamber) was pierced with a flame-sterilized needle, and 200 μg of BrdU dissolved in 20 μl sterile 0.9% NaCl was injected with an automatic pipette fitted with autoclaved tips inserted approximately 5 mm into the albumen. The hole in the shell was sealed with melted paraffin and the egg immediately returned to the incubator until hatching. At post-natal day 56 (PN56), birds were killed by decapitation and their brains rapidly (within 3 min) removed from the skull. Brains were placed in the aldehyde fixative acrolein (5% in Phosphate-buffer 0.01 M-saline 0.9% [PBS], pH 7.2) for 2.5 h, washed twice in PBS (30 min) and cryoprotected in 30% sucrose for 24 h at 4 °C. Brains were then frozen on dry ice and stored at −80 °C until processed for immunohistochemistry. Gonadal sex of each bird was determined by dissection under a stereomicroscope. A total of 14 brains (7 males and 7 females) were collected for this part of the experiment.
4.3. Experiment 2: cellular proliferation at E12
To investigate whether the sex difference in the number of BrdU-positive cells observed in adult birds injected on E12 was due to a differential proliferation at the time of injection, BrdU was administered as described in experiment 1 to Japanese quail eggs at embryonic day 12 (E12). Ten embryos (5 males and 5 females) were killed 24 h later (on E13) by decapitation. Brains were quickly removed from the skull and processed as described for experiment 1. The sex of the embryos was determined by visualizing the gonads under a stereomicroscope.
4.4. Experiment 3: proliferation and survival from hatching into adulthood of cells labeled by a BrdU injection at E12
To follow the proliferation and survival of labeled by a BrdU injection on E12, another batch of eggs (n=250) was injected with BrdU at E12 as described in experiment 1. Male and female brains were collected at key points during development until adulthood: postnatal day 1 (PN1, just after hatching that takes place on E17), PN15, PN28 (just before puberty), PN43 and PN50 (during puberty) and PN56 (young adults). Five birds of each sex were used at each of the earliest stages (PN1, PN15, PN28), and six birds of each sex were used at each of the later stages (PN43, PN50, PN56). Tissue was processed as described for experiment 1.
4.5. BrdU immunocytochemistry
The forebrain of each subject from the level of the tractus septopallio-mesencephalicus (TSM) to the caudal end of the telencephalon was cut in the coronal plane with a cryostat (Leica) at −18 °C in three (for embryos at E13 only) or four (from PN1 up to adults) series of 30 μm-thick sections that were collected in phosphate-buffered saline (PBS; 0.1 M, pH 7.4). One series of free-floating sections from each brain was stained by immunohistochemistry for BrdU. Aldehydes were first deactivated by a 15 min incubation in 0.1% sodium borohydride in phosphate-buffered saline (PBS; 0.1 M, pH 7.4) and rinsed 3 times with PBS. Endogenous peroxidases were then inactivated by a 20 min incubation in 0.6% H2O2 in PBS. Sections were treated with 2N HCL for 20 min at 37 °C to denature DNA, neutralized in 0.1 M sodium borate buffer at pH 8.5 for 10 min, and rinsed 5 min in PBS with 0.1% Triton X-100 (PBST). Non-specific binding was blocked during 30 min in 10% horse serum in PBST and sections were incubated overnight at 4 °C with a monoclonal rat anti-BrdU antibody (AbD Serotec, OBT-0030, 1/2000 in PBST). After 3 washes in PBST, sections were incubated in a biotinylated donkey anti-rat antibody (Jackson Inc, 712-065-150, 1/2000 in PBST) for 2 h and then washed 3 times in PBST. The sections were placed in an avidin-biotin-peroxidase solution (Vector ABC Elite kit in PBST) for 90 min, followed by two consecutive rinses in PBST and one in PBS. Finally, peroxidase activity was revealed with the chromogen 3,3′–diaminobenzidine tetrahydrochloride (DAB, 0.04% in PBS) and 0.012% H2O2 for 10 min. After repeated washes in PBS, sections were mounted on microscope slides and covered with a gelatin-phenol aqueous mounting medium.
4.6. Double-labeling BrdU and PCNA
To determine whether BrdU-ir cells labeled on E12 were cycling and dividing during puberty, we labeled sections throughout the brains of 2 males and 2 females killed on PN43, PN50, and PN56 after an injection of BrdU at E12 by fluorescent double immunohistochemistry for BrdU and for the Proliferating Cell Nuclear Antigen (PCNA), a marker of cell proliferation (Charvet and Striedter, 2008, 2009). BrdU labeling was performed as described above, with an overnight incubation in the primary antibody at 1/200 (concentration increased to optimize fluorescent labeling). After 3 washes in PBST, sections were incubated with a goat anti-rat antibody tagged with the fluorescent Alexa 546 dye (Jackson Immunoresearch, 1/200 in PBST) for 2 h, washed 3 times in PBST, and blocked for 30 min in 5% normal goat serum in PBST. The sections were then incubated overnight at 4 °C with the monoclonal mouse NCL-L-PCNA antibody (Novocastra Leica, Clone PC10, 1/500). After 3 washes in PBST, sections were incubated with a goat anti-mouse antibody tagged with the fluorescent Alexa 488 dye. After washing in PBS, sections were mounted on microscope slides and cover-slipped with Vectashield Hard Set Mounting Medium (Vector Laboratories). Fluorescent sections were photographed with a confocal microscope (Olympus Fluoview FV1000) equipped with an inverted microscope (Olympus IX81). Images were processed to homogenize contrast and brightness within a single plate with Adobe Photoshop 7.0.1 or Adobe Photoshop Elements 6.
4.7. Data analysis
During experiments 1, 2 and 3, the number of BrdU-ir cells was quantified by an experimenter blind to the sex of the birds using computer-assisted image analysis in a standardized microscope field (536 μm × 360 μm) centered on the POM in the medial preoptic area (POA). BrdU incorporation was quantified at 3 rostro-caudal levels defined in a standardized manner based on anatomical landmarks (e.g., the third ventricle, commissura anterior and tractus septopallio-mesencephalicus): the caudal POA at the level of the largest extension of the commissura anterior (CA), the intermediate POA located two stained sections (i.e. 160 or 240 μm in chicks or adults respectively) more rostrally (CA-2) and the rostral POA located 4 sections more rostrally (CA-4). In each case, the microscope field was centered on the POM but because the size and boundaries of the nucleus vary during development, it is likely that at some developmental stages, parts of the area where BrdU cells were quantified were not within the architectonic limits of POM. Therefore, we prefer to conservatively use the term medial POA to define the area under investigation.
Images were acquired with a video camera attached to a microscope (Olympus BH2, 20X objective) connected to a Macintosh computer running the image analysis software Image J 1.36b (NIH, USA). Digital images were made binary and the threshold set manually to discriminate labeled material from background staining. Unless otherwise specified, the computer then automatically counted the number of labeled objects in the entire digitized camera field (536 μm × 360 μm; field always in landscape orientation). Exclusion thresholds were set at 20 (low threshold) and 200 (high threshold) pixels to disregard dark objects that did not correspond to the size of a cell nucleus.
Fluorescent sections (BrdU and PCNA staining) were photographed with an Olympus Fluoview FV1000 confocal system equipped with an Olympus IX81 inverted microscope (Olympus). Sections from two males and two females stained by double-label immunohistochemical procedures for BrdU and PCNA were photographed with a video camera attached to a Leica microsope (20 × objective) connected to a computer running the image analysis software Leica. Cells were counted in these photomicrographs in a 449 × 335 μm or 0.150 mm2 field of view at the caudal level POA (Table 1). The mean numbers of cells counted in two animals per group are presented in the results.
4.8. Statistical analyses
All data were analyzed by two way Analyses of Variance (ANOVA) with the sex of the birds and location in the POA as independent and repeated factors respectively (Experiments 1 and 2) or with the sex of the birds and the age at brain collection (independent factor) as factors (Experiment 3). Data were further analyzed as appropriate using the Newman–Keuls post-hoc test. All results are expressed as mean 7standard error of the mean (S.E.M) of the total number of BrdU-ir cells in the counting area. Differences between groups were considered significant when p≤0.05.
Acknowledgments
This work was supported by a postdoctoral incoming fellowship of the University of Liège (KM) and Grants from the National Institutes of Mental Health (NIMH RO1 MH50388) and the University of Liège (Fonds spéciaux 2009) to JB. We would like to thank Dr. J.M Barker for our discussions and her English proofreading. We also thank the GIGA imaging platform and in particular N. Wolkoff for her help with the confocal microscope.
REFERENCES
- Adkins EK. Sex steroids and the differentiation of avian reproductive behavior. Am. Zool. 1978;18:501–509. [Google Scholar]
- Adkins EK. Effect of embryonic treatment with estradiol or testosterone on sexual differentiation of the quail brain. Neuroendocrinol. 1979;29:178–185. doi: 10.1159/000122920. [DOI] [PubMed] [Google Scholar]
- Adkins-Regan E. Sex steroids and the differentiation and activation of avian reproductive behaviour. In: Balthazart J, Gilles R, editors. Hormones and Behaviour in Higher Vertebrates. Springer-Verlag; Berlin: 1983. pp. 219–228. [Google Scholar]
- Ahmed EI, et al. Pubertal hormones modulate the addition of new cells to sexually dimorphic brain regions. Nat. Neurosci. 2008;11:995–997. doi: 10.1038/nn.2178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvarez-Buylla A, Kirn JR. Birth, migration, incorporation, and death of vocal control neurons in adult songbirds. J. Neurobiol. 1997;33:585–601. [PubMed] [Google Scholar]
- Alvarez-Buylla A, Nottebohm F. Migration of young neurons in adult avian brain. Nature. 1988;335:353–354. doi: 10.1038/335353a0. [DOI] [PubMed] [Google Scholar]
- Aste N, et al. Morphometric studies demonstrate that aromatase-immunoreactive cells are the main target of androgens and estrogens in the quail medial preoptic nucleus. Exp. Brain Res. 1994;101:241–252. doi: 10.1007/BF00228744. [DOI] [PubMed] [Google Scholar]
- Ball GF, Balthazart J. Japanese quail as a model system for studying the neuroendocrine control of reproductive and social behaviors. ILAR J. 2011;51:310–325. doi: 10.1093/ilar.51.4.310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balthazart J, Ball GF. The Japanese quail as a model system for the investigation of steroid-catecholamine interactions mediating appetitive and consummatory aspects of male sexual behavior. Ann. Rev. Sex Res. 1998;9:96–176. [PubMed] [Google Scholar]
- Balthazart J, De Clerck A, Foidart A. Behavioral demasculinization of female quail is induced by estrogens: Studies with the new aromatase inhibitor, R76713. Horm. Behav. 1992;26:179–203. doi: 10.1016/0018-506x(92)90041-s. [DOI] [PubMed] [Google Scholar]
- Balthazart J, Tlemçani O, Ball GF. Do sex differences in the brain explain sex differences in the hormonal induction of reproductive behavior? What 25 years of research on the Japanese quail tells us. Horm. Behav. 1996;30:627–661. doi: 10.1006/hbeh.1996.0066. [DOI] [PubMed] [Google Scholar]
- Balthazart J, et al. Ontogeny of aromatase and tyrosine hydroxylase activity and of aromatase-immunoreactive cells in the preoptic area of male and female Japanese quail. J. Neuroendocrinol. 2000;12:853–866. doi: 10.1046/j.1365-2826.2000.00532.x. [DOI] [PubMed] [Google Scholar]
- Balthazart J, Arnold AP, Adkins-Regan E. Sexual differentiation of brain and behavior in birds. In: Pfaff DW, Arnold AP, Etgen AM, Fahrbach SE, Rubin RT, editors. Hormones, Brain and Behavior. Academic Press; San Diego, CA: 2009. pp. 1745–1787. [Google Scholar]
- Bardet SM, Mouriec K, Balthazart J. Birth of neural progenitors during the embryonic period of sexual differentiation in the Japanese quail brain. J. Comp. Neurol. 2012;520:4226–4253. doi: 10.1002/cne.23153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charvet CJ, Striedter GF. Developmental species differences in brain cell cycle rates between northern bobwhite quail (Colinus virginianus) and parakeets (Melopsittacus undulatus): implications for mosaic brain evolution. Brain Behav. Evol. 2008;72:295–306. doi: 10.1159/000184744. [DOI] [PubMed] [Google Scholar]
- Charvet CJ, Striedter GF. Developmental origins of mosaic brain evolution: morphometric analysis of the developing zebra finch brain. J. Comp. Neurol. 2009;514:203–213. doi: 10.1002/cne.22005. [DOI] [PubMed] [Google Scholar]
- Foidart A, et al. Critical re-examination of the distribution of aromatase-immunoreactive cells in the quail forebrain using antibodies raised against human placental aromatase and against the recombinant quail, mouse or human enzyme. J. Chem. Neuroanat. 1995;8:267–282. doi: 10.1016/0891-0618(95)00054-b. [DOI] [PubMed] [Google Scholar]
- Hayes NL, Nowakowski RS. Dynamics of cell proliferation in the adult dentate gyrus of two inbred strains of mice. Brain Res. Dev. Brain Res. 2002;134:77–85. doi: 10.1016/s0165-3806(01)00324-8. [DOI] [PubMed] [Google Scholar]
- Kokoeva MV, Yin H, Flier JS. Evidence for constitutive neural cell proliferation in the adult murine hypothalamus. J. Comp. Neurol. 2007;505:209–220. doi: 10.1002/cne.21492. [DOI] [PubMed] [Google Scholar]
- LaVail JH, Cowan WM. The development of the chick optic tectum. II Autoradiographic studies. Brain Res. 1971;28:421–441. [PubMed] [Google Scholar]
- Packard DS, Jr., Menzies RA, Skalko RG. Incorporation of thymidine and its analogue, bromodeoxyuridine, into embryos and maternal tissues of the mouse. Differentiation. 1973;1:397–404. doi: 10.1111/j.1432-0436.1973.tb00137.x. [DOI] [PubMed] [Google Scholar]
- Panzica GC, Viglietti-Panzica C, Balthazart J. The sexually dimorphic medial preoptic nucleus of quail: a key brain area mediating steroid action on male sexual behavior. Front. Neuroendocrinol. 1996;17:51–125. doi: 10.1006/frne.1996.0002. [DOI] [PubMed] [Google Scholar]
- Patlak M. Hormones and the teen brain: they do more than foster adolescent angst. Endocr. News. 2012;37:29–31. [Google Scholar]
- Phoenix CH, et al. Organizational action of prenatally administered testosterone propionate on the tissues mediating behavior in the female guinea pig. Endocrinology. 1959;65:369–382. doi: 10.1210/endo-65-3-369. [DOI] [PubMed] [Google Scholar]
- Schulz KM, Molenda-Figueira HA, Sisk CL. Back to the future: the organizational-activational hypothesis adapted to puberty and adolescence. Horm Behav. 2009;55:597–604. doi: 10.1016/j.yhbeh.2009.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sisk CL, Zehr JL. Pubertal hormones organize the adolescent brain and behavior. Front. Neuroendocrinol. 2005;26:163–174. doi: 10.1016/j.yfrne.2005.10.003. [DOI] [PubMed] [Google Scholar]
- Thompson RR, Adkins-Regan E. Ontogeny of a sexually dimorphic nucleus in the preoptic area of the Japanese quail (Coturnix japonica) Dev. Brain Res. 1992;70:231–237. doi: 10.1016/0165-3806(92)90202-8. [DOI] [PubMed] [Google Scholar]
- Tsai HM, Garber BB, Larramendi LM. 3H-thymidine autoradiographic analysis of telencephalic histogenesis in the chick embryo: I. Neuronal birthdates of telencephalic compartments in situ. J. Comp. Neurol. 1981a;198:275–292. doi: 10.1002/cne.901980207. [DOI] [PubMed] [Google Scholar]
- Tsai HM, Garber BB, Larramendi LM. 3H-thymidine autoradiographic analysis of telencephalic histogenesis in the chick embryo: II. Dynamics of neuronal migration, displacement, and aggregation. J. Comp. Neurol. 1981b;198:293–306. doi: 10.1002/cne.901980208. [DOI] [PubMed] [Google Scholar]
- Yurkewicz L, et al. 3H-thymidine long survival autoradiography as a method for dating the time of neuronal origin in the chick embryo: the locus coeruleus and cerebellar Purkinje cells. J. Comp. Neurol. 1981;203:257–267. doi: 10.1002/cne.902030207. [DOI] [PubMed] [Google Scholar]