Abstract
Oxidants are thought to damage cells, and stem cells are viewed as particularly vulnerable to oxidative stress. Now, a new study suggests that the self-renewal of certain stem cells may actually require reactive oxygen species.
It wouldn’t be surprising if people interested in the physiology of reactive oxygen species (ROS) felt a certain kinship for Miles Monroe. As some may recall, Miles Monroe is the name of Woody Allen’s character in the classic comedy Sleeper. In the movie, Allen plays the neurotic owner of the ‘Happy Carrot’ health food store who is cryogenically frozen without his consent. Awaken two hundred years later, he finds that his old breakfast of wheat germ, organic honey and tiger’s milk has been replaced with what science has now established as the far healthier alternatives of deep fat, steak, and cream pies. As the futuristic scientists in the movie explain, all those things people use to think were bad for you are ‘precisely the opposite of what we now know to be true’.
A ‘precisely the opposite’ result can occasionally happen in science, and the latest example can be found in a fascinating new manuscript by Shinohara and colleagues, soon to be published in Cell Stem Cell. In this manuscript, the authors demonstrate that rather than being harmful, ROS are in fact required for spermatogonial stem cell (SSC) self-renewal (Morimoto et al., 2013). This study must be viewed in the context of the relatively recent and rather tumultuous evolution of what is understood about how ROS function in cells. Long viewed as merely toxic byproducts of aerobic respiration, the perception of ROS began to change when oxidants were demonstrated to be purposely produced by cells in response to growth factor stimulation and seemingly necessary for downstream signaling (Sundaresan et al., 1995). In many cases, the enzymatic source of ROS production was not the mitochondria but instead the NOX family of widely expressed NADPH oxidases (Lambeth, 2004). This family of oxidases was first described in phagocytic cells where they generate ROS for host defense and where there activity is regulated by the Ras superfamily of small GTPases. A similar regulatory role for the Ras family of small GTPase is also evident for ROS production in non-phagocytic cells (Sundaresan et al., 1996).
While these studies seemed to partially rehabilitate the reputation of ROS, other work seemed to solidify their toxic nature. This was particularly evident when it came to the biology of stem cells. Analyzing predominantly the hematopoietic stem cell (HSC) system, various genetic models including deletion of the ATM protein kinase, knockout of transcription factors such as Prdm16 or members of the FoxO family or removal of epigenetic factors such as Bmi1, all demonstrated that an increase in stem cell ROS levels resulted in a subsequent impairment in various stem cell properties, including a profound reduction in self-renewal capacity (Wang et al., 2013). Thus, it seemed clear that the unique capacity of stem cells to divide and give rise to new stem cells (e.g. self-renew) appeared exquisitely sensitive to a rise in ROS levels.
In this context, the new result of Shinohara and colleagues further upends what we thought we knew about ROS and stem cell self-renewal. The system they analyzed involved SSCs, cells that undergo continuous self renewal in the male testis in order to produce a continuous supply of spermatozoa. Previous studies had demonstrated that male germline cells could be cultured in vitro with the aid of factors such as glial cell line-derived neurotrophic factor (GDNF) and fibroblast growth factor 2 (FGF2), proteins normally secreted by supporting Seroli cells (Kanatsu-Shinohara et al., 2003). Furthermore, these investigators had shown that germline cells could be maintained in a cytokine free environment when they were engineered to express a constitutively activated form of the Ras GTPase (Lee et al., 2009). These Ras-transduced cells appeared to bypass the need for trophic factors and demonstrated long term self-renewal capacity.
The current study sought to address what might be downstream of Ras that mediates SSC self-renewal. One clue that Shinohara and colleagues pursued in this recent study was that stimulation of germline cells with FGF2 or GDNF appeared to result in increased ROS levels. Moreover, pharmacological treatment with antioxidants or using various chemical inhibitors of the NOX enzymes appeared to reduce the in vitro and in vivo self-renewal capacity of SSCs. In contrast, supplementing germline cultures with low, continuous levels of exogenous hydrogen peroxide appeared to substantially increase the number of SSCs. Taken together, these results suggest that NOX-generated hydrogen peroxide was a necessary requirement for effective self-renewal in SSCs. This point was further emphasized when the authors demonstrated that Nox1 deficient SSCs had a self-renewal deficit that was particularly evident after serial transplantation into recipient testis.
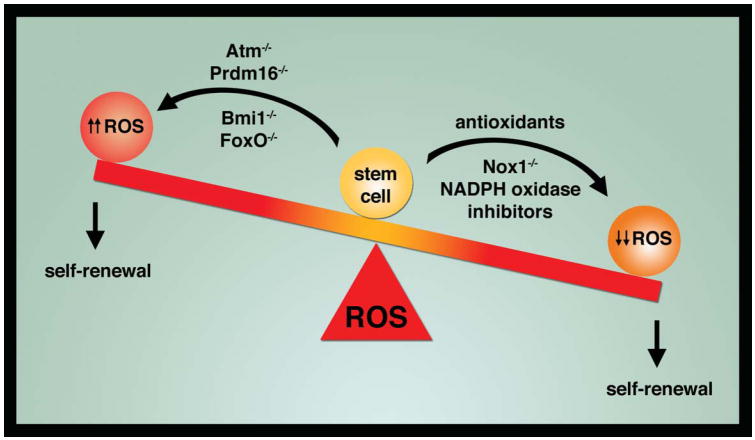
How can we make sense of these latest results that seem to argue that ROS are surprising required for SSC self-renewal? In particular, how does this new result fit in with multiple previous sets of observations suggesting that a rise in ROS is clearly detrimental to stem cell self-renewal capacity? One simple explanation may be that the current study employing SSCs and the past studies employing predominantly HSCs represent observations of different stem cells with different sets of biological requirements. Perhaps more appealing is the issues of degree. Clearly, high, continuous and unregulated levels of ROS appear bad for stem cells. That point was evident in the current study since when high, rather than low, amounts of hydrogen peroxide levels were used, SSCs self-renewal was indeed impaired. In contrast, the current manuscript would argue that reducing ROS too much can also be harmful. Thus, redox homeostasis in stem cells follows what might be called the Goldilocks rule that requires ROS levels to be not too high, not too low but instead, ‘just right’, to maintain functional integrity (Figure).
Figure.
ROS and stem cell self-renewal. Genetic and pharmacological interventions can raise or lower ROS levels in stem cells. Previous studies have established that increasing ROS levels within stem cells impairs self-renewal capacity. A new report suggests that lowering ROS levels can also be detrimental.
Perhaps these results should not come as a complete surprise. For instance, while most observations are consistent with a detrimental effect for ROS, at least one previous study showed that reducing ROS levels too much inhibited the self-renewal capacity of neural stem cells (Le Belle et al., 2011). Similarly, studies in Drosophila suggest that in certain hematopoietic progenitors, increased ROS levels act as a brake that blocks differentiation of these immature progenitor cells into more mature cell types (Owusu-Ansah and Banerjee, 2009). Thus, whether ROS are good are bad, and whether they act as an inhibitor or promoter for ‘stemness’ and self-renewal seems still open for debate. Now, if we could only re-open the debate between wheat germ and cream pies.
Acknowledgments
We are grateful to I. Rovira for help with the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Kanatsu-Shinohara M, Ogonuki N, Inoue K, Miki H, Ogura A, Toyokuni S, Shinohara T. Long-term proliferation in culture and germline transmission of mouse male germline stem cells. Biol Reprod. 2003;69:612–616. doi: 10.1095/biolreprod.103.017012. [DOI] [PubMed] [Google Scholar]
- Lambeth JD. NOX enzymes and the biology of reactive oxygen. Nat Rev Immunol. 2004;4:181–189. doi: 10.1038/nri1312. [DOI] [PubMed] [Google Scholar]
- Le Belle JE, Orozco NM, Paucar AA, Saxe JP, Mottahedeh J, Pyle AD, Wu H, Kornblum HI. Proliferative neural stem cells have high endogenous ROS levels that regulate self-renewal and neurogenesis in a PI3K/Akt-dependant manner. Cell Stem Cell. 2011;8:59–71. doi: 10.1016/j.stem.2010.11.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J, Kanatsu-Shinohara M, Morimoto H, Kazuki Y, Takashima S, Oshimura M, Toyokuni S, Shinohara T. Genetic reconstruction of mouse spermatogonial stem cell self-renewal in vitro by Ras-cyclin D2 activation. Cell Stem Cell. 2009;5:76–86. doi: 10.1016/j.stem.2009.04.020. [DOI] [PubMed] [Google Scholar]
- Morimoto, et al. …Cell Stem Cell… [Google Scholar]
- Owusu-Ansah E, Banerjee U. Reactive oxygen species prime Drosophila haematopoietic progenitors for differentiation. Nature. 2009;461:537–541. doi: 10.1038/nature08313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundaresan M, Yu ZX, Ferrans VJ, Irani K, Finkel T. Requirement for generation of H2O2 for platelet-derived growth factor signal transduction. Science. 1995;270:296–299. doi: 10.1126/science.270.5234.296. [DOI] [PubMed] [Google Scholar]
- Sundaresan M, Yu ZX, Ferrans VJ, Sulciner DJ, Gutkind JS, Irani K, Goldschmidt-Clermont PJ, Finkel T. Regulation of reactive-oxygen-species generation in fibroblasts by Rac1. Biochem J. 1996;318:379–382. doi: 10.1042/bj3180379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang K, Zhang T, Dong Q, Nice EC, Huang C, Wei Y. Redox homeostasis: the linchpin in stem cell self-renewal and differentiation. Cell Death Dis. 2013;4:e537. doi: 10.1038/cddis.2013.50. [DOI] [PMC free article] [PubMed] [Google Scholar]