Abstract
We compared egg survivorship and egg development time at different soil moistures for two closely related grasshopper species from divergent habitats: marsh-inhabiting Romalea microptera (Beauvois) versus desert-inhabiting Taeniopoda eques (Burmeister). These two species can interbreed and produce viable offspring. In nature, both species have a similar 8–9 mo subterranean egg stage, but their soil environments differ dramatically in water content. We predicted that the eggs of the two species would exhibit differential survivorship and development times under different moisture levels. Our laboratory results show that the eggs of both species survived a wide range of soil moistures (≈ 0.5 to 90%), maintained for 3 mo. However, the eggs of the marsh grasshopper, R. microptera, better tolerated the highest soil moistures (95 and 100%), whereas the eggs of the desert species, T. eques, better tolerated the lowest soil moistures (0.0 and 0.1%). Sixty-five percent of marsh-inhabiting R. microptera eggs, but no desert T. eques eggs, survived 3 mo submersion under water. In contrast, 49% of desert T. eques eggs, but only 3.5% of R. microptera eggs, survived after being laid into oven-dried sand and then maintained with no additional water until hatch. In the laboratory at 26°C, the two species differed significantly in the mean length of the oviposition-to-hatch interval: 176 d for R. microptera versus 237 d for T. eques. These divergent traits presumably benefit these insects in their divergent habitats. Our results suggest the evolution of physiological divergence that is consistent with adaptations to local environments.
Keywords: egg, egg pod, oviposition, hatching, soil moisture
Comparisons among closely related populations and species living in divergent habitats provide some of the best evidence in support of adaptive evolution and incipient speciation (Ghalambor et al. 2003, Willmer et al. 2005, Grace et al. 2010). Because closely related taxa only recently diverged from a common ancestor, we would normally expect them to possess similar characteristics (i.e., phylogenetic conservatism; Prinzing et al. 2001, Willis et al. 2008). However, related species that come to live in radically different environments experience divergent selective pressures, which can lead to the evolution of divergent adaptive characteristics (divergent evolution) (Thornhill and Alcock 1983, Kawecki and Ebert 2004). Hence, a trait-by-trait comparison of related taxa surviving in different habitats can help us identify those specific traits that may have recently undergone rapid evolution. Such comparisons are also the first step in identifying the specific adaptations that allow populations and species to survive in divergent and challenging habitats (Willmer et al. 2005).
The lubber grasshoppers Taeniopoda eques (Burmeister) and Romalea microptera (Beauvois) (Romaleidae fam.) are ideal species for examining trait divergence because they are closely related but survive in dramatically different habitats. Although the two species are currently assigned to different genera, this is probably a misrepresentation of their true systematic relationship (Mutun and Borst 2004, D. W. Whitman, unpublished data). The two species are very similar, but dissimilar to all other American grasshoppers. The nymphs are nearly indistinguishable, with similar patterns of aposematic yellow, orange, or red stripes on a black background (Fig. 1) (Hebard 1925). Both species aggregate as early nymphs, roost at night, are flightless, sluggish, and polyphagous, but favor liliaceous food plants (Whitman and Orsak 1985, Whitman 1988a, 1990, Hatle and Whitman 2001). Both species are chemically defended with both internal toxins and a unique metathoracic tracheal defense gland, and are the only grasshoppers known to possess this odd and complex structure (Whitman et al. 1985, 1991). The two species are the largest grasshoppers in North America, north of Mexico (Rehn and Grant 1961, Whitman and Vincent 2008). The strongest evidence for their close relatedness is that the two species can mate and produce viable hybrids in the laboratory (D. W. Whitman, unpublished data). These shared phenotypic characteristics, mitochondrial DNA evidence (Mutun and Borst 2004), and an associated biogeographical hypothesis (Rehn and Grant 1959, 1961) suggest that these two allopatric species only recently split from a common ancestor.
Fig. 1.

Nymphal (A) R. microptera and (B) T. eques closely resemble one another with a similar aposematic pattern of yellow, orange, or red lines on a black background. These closely related, allopatric species can interbreed to produce fertile hybrids in the laboratory.
In contrast to these biological similarities, these two species live in very dissimilar environments. T. eques inhabits the dry Chihuahuan Desert of northern Mexico and the southwestern United States (Rehn and Grant 1959, 1961), whereas R. microptera ranges across the humid southeastern United States (Hebard 1925, Rehn and Grant 1961). In south Florida, R. microptera inhabits the Everglades, a wetlands environment that is the antithesis of the Chihuahuan Desert.
The Chihuahuan Desert of northern Mexico and southwestern USA is characterized by low and highly variable annual precipitation (≈29 cm/yr), and low soil moisture (Brown 1982, NOAA 1997–2006). The sparse rains come in late summer and fall, after a spring-to-midsummer drought, and are localized and uneven (Fig. 2) (Tinkham 1948, Maddox et al. 1995). Desert shrubs dominate the Chihuahuan Desert, and their small leaves, open architecture, and spaced distribution expose the desert soils to intense solar heating that fosters soil desiccation (Brown 1982). Indeed, summer soil surfaces can dry to 0.02% moisture content and reach 69°C (Whitman 1987, Thoma et al. 2006, NOAA 1997–2006), which is far above the upper-thermal, long-duration survival-limit of ≈31°C for lubber eggs (Chladny and Whitman 1998).
Fig. 2.
Mean environmental conditions for Douglas, AZ, and Everglades City, FL, from 1997 to 2006. Columns indicate mean precipitation (centimeter) for that month averaged over 10 yr for each particular location. The plotted line indicates the mean air temperature (30.5 cm above the ground) for that month averaged over 10 yr for each location. The horizontal black bars indicate the typical length of egg stage for each species within its corresponding habitat. Note that each species spends 8–9 mo in the egg stage.
In contrast, the South Florida Everglades experiences high precipitation (≈140 cm/yr), as well as high and fluctuating water tables and soil surface moisture levels (Chen and Gerber 1990, Lodge 2010). Much of the Everglades area floods during the summer rainy season, and in some locations, R. microptera egg pods reside under water for several months. South Florida vegetation varies, but tends to be lush (Myers and Ewel 1990, Lodge 2010), and the soil often remains wet and shaded from solar radiation, and, hence, cool (van Wingerden et al. 1991, Getz 2005). The annual temperature range is relatively narrow (Fig. 2) (Chen and Gerber 1990, NOAA 1997–2006).
In southern Arizona and New Mexico, T. eques oviposits in the fall (late September to late October). Rarely, a few older T. eques continue to oviposit into November, but only if the early winter weather remains uncharacteristically warm and moist, and there are no hard freezes. The eggs diapause through the winter and aestivate through the hot and dry spring-summer drought. They hatch in July, in synchrony with the beginning of the late-summer rainy season (Fig. 2) (Whitman and Orsak 1985). Newly hatched T. eques do not feed on desert perennial shrubs, but instead feed on the short-lived, late-summer desert annuals that sprout only if and after there are suitable rains in July or late June (Whitman and Orsak 1985). Hatching before the summer rains would be lethal for T. eques, because of the high temperatures, low humidity, and lack of food (Fig. 2).
In contrast, most of the eggs of R. microptera from the Everglades region of south Florida, are laid in the summer (June through August). However, because of an extended hatching period and the long sub-tropical growing season in south Florida, which allows both population asynchrony and long adult lifespan, adults can be present in south Florida from late-April through January, and it is conceivable that at least a few eggs can be laid throughout the 8.5-mo early May through mid-January period. Nearly all R. microptera eggs pass through the mild Florida winter and hatch in February and March, in synchrony with rising spring temperatures (Fig. 2); however, a few pods hatch as early as late December or as late as mid-May (range = 4.5 mo). In the field T. eques eggs are subjected to extreme drought, whereas south Florida R. microptera eggs can experience prolonged flooding. R. microptera eggs hatch when soil temperatures in the field are moderate and slowly rising, whereas T. eques eggs fail to hatch under dramatically faster and higher temperatures increases (Fig. 2). When cultured at 26°C in the laboratory, eggs of both species undergo diapause, which, based on the timing, would synchronize egg diapauses with winter in the field (T.W.S., unpublished data).
Previous studies have already documented trait divergence in these sister species. For example, the two species differ in phenology (Whitman and Orsak 1985, Stauffer and Whitman 2007), adult body color (Rehn and Grant 1959, 1961), oviposition behaviors (Stauffer and Whitman 1997, 2007), and egg pod characteristics (Stauffer and Whitman 2007), and all of these trait differences are in a logical direction, in that they presumably increase fitness given their respective habitats (Whitman 1987, Whitman 1988b, Stauffer and Whitman 1997, 2007).
In this paper, we continue to explore trait divergence in lubber grasshoppers by examining egg survival and hatching time under different soil moistures. Grasshopper egg physiology is an appropriate subject for evolutionary studies, because many grasshopper species spend the majority of their life cycle as eggs buried in soil (Stauffer and Whitman 1997). Many temperate species exist as nymphs and adults for only 3 to 4 mo, but remain underground in the egg stage 9 mo (Uvarov 1966, Capinera and Sechrist 1982). Hence, soil could be considered their primary habitat, and the short, above-ground period, their secondary habitat. As such, we would expect lubbers to have evolved adaptations that favor survival in the specific soil conditions of their respective habitats. In this paper, we test the hypothesis that grasshopper eggs are adapted to their specific habitat, by culturing egg pods under different soil moistures and monitoring survival. We predicted that survival in desert T. eques would be high in dry soil and low in wet soil, and that marsh-inhabiting R. microptera would show an opposite response.
Materials and Methods
Insects
Grasshoppers were collected as late-instar nymphs from near Fakahatchee Strand State Preserve, FL (25°57′13″ N and 81°21′21″ W), at the western end of the Everglades region (Romalea microptera) and from the Chihuahuan Desert, near Rodeo, NM (31°50′07″ N and 109°01′52″ W) (Taeniopoda eques), transferred to Illinois State University in Normal, IL, and maintained as per Matuszak and Whitman (2001) under a 14:10 L:D photoperiod. Hence, the late-instar and adult females of both species experienced similar laboratory thermal, photoperiod, and diet conditions, before ovipositing.
Egg Cups and Sand Moisture
Experiments were conducted in 1-liter plastic cups with tight-fitting lids, filled three-fourths full with 1,100 g of clean, fine grade, white, oven-dried sand, moistened to a specific level with deionized (DI) water (Chladny and Whitman 1998, Matuszek and Whitman 2001). A mated female grasshopper was allowed to lay a single egg pod of ≈66 eggs (T. eques) or ≈44 eggs (R. microptera) in each cup, and each cup represented a replicate. The difference in cup mass before versus after oviposition represented the mass of the deposited egg pod. Both the mass of the egg pod and the total mass of the sand-filled cup + pod were recorded. Subsequent weighing of egg cups allowed us to monitor any water loss, which was corrected by adding DI water. The cups were sealed with a tight-fitting lid and generally lost ≈0.05 g/H2O per week.
Different treatments contained different moisture levels calculated as a percentage of totally flooded sand. Totally flooded sand is sand containing no air bubbles, with standing water exactly at the top surface of the sand, and in our system represents 1,100 g oven dried sand containing 220 g of water. Hence, treatments of 100, 50, and 1.0% moisture content represent 220, 110, and 2.2 g water per egg cup (or per 1,100 g oven-dried sand), respectively. To change the moisture level, we removed the lid and either added DI water or used a fan at room temperature to evaporate water from the open cup.
Experiment 1: Thirteen Different Moisture Treatments
Our goal was to test water physiology in eggs that had tanned, as opposed to water physiology in soft, untanned, just-laid eggs. Therefore, we allowed mated T. eques and R. microptera females to lay egg pods into sand cups of 7% moisture. This moisture level allowed the eggs to tan and develop their water-resistance under “optimal” soil-moisture levels before the start of the experiment. Ten days after oviposition, when eggs had tanned and developed their water-resistant capabilities, cups were randomly assigned to, and adjusted to, one of 13 different moisture treatments (0.125, 0.25, 0.5, 1.0, 2, 4, 8, 15, 35, 75, 90, 95, and 100%).
Each treatment was replicated ten times. Treatments were maintained for 90 d at their precise moisture levels by weighing weekly and adding any lost water. At 100 d from oviposition, the moisture levels of all 13 treatments were brought back to 7% by either adding or evaporating away water. This was done to assure that hatching for all treatments occurred at the same moisture level. For the 100% moisture treatment, evaporation to 7% required 6 d. The entire experiment was conducted at room temperature (mean = 26°C; range = 24–28°C). We recorded the number and date of all hatchlings that emerged from each cup. We compared the effects of soil moisture on egg survival and hatching time. For egg survival, we excavated the pods 60 d after the last hatch (day 415), counted the number of eggs laid and the number of eggs hatched, and calculated the percent hatch for each cup. Lubber eggs are ≈9 mm long, with a tough chorion (egg shell) that remains in the soil after hatching. In unhatched eggs, the chorion is unbroken and the interior of the egg contains living or dead material. Unhatched eggs are easily distinguished from hatched eggs, which consist of an empty egg shell, split open in a characteristic way. For time to hatch, we used date of first hatch per pod for our analysis to eliminate the problem of non-independence, where a single hatching egg might influence other eggs in the same pod to hatch simultaneously. In this laboratory experiment, hatching times within a single pod varied: in some pods, most of the eggs hatched over a 1–2 d period, whereas in others hatching extended to over a month.
Experiment 2: Pods Laid Into and Maintained at Low Moisture Levels
After completing experiment 1, we were surprised at the ability of T. eques eggs to survive low soil moistures. Therefore, we conducted experiment 2 to test both lower moisture levels and longer exposure to dry conditions. In experiment 2, female R. microptera and T. eques laid into standard egg cups that were comprised of four soil moisture treatments (0.0, 0.1, 1.0, and 4.0% water) (N = 15 pods per species per treatment). Containers were monitored every 4 d and maintained at these moisture levels at room temperature throughout the entire egg development period of 4–10 mo, depending on species and treatment. Time to first hatch and any subsequent hatching occurrences were recorded. For our analyses, we used time to first hatch, as described in experiment 1. Sixty days after the last egg hatch was recorded, pods were excavated and the total number of eggs laid and percent hatch was determined.
Length of Egg Stage: T. eques versus R. microptera
In a separate analysis, we used data from experiment 1 to compare the mean length of the egg stage for the two species, under stable laboratory-room temperatures of 26 ± 2°C. We used nonstressed pods, defined as those displaying >40% survival (n = 74 pods for T. eques and 67 pods for R. microptera), because moisture stress might alter the egg incubation period. Thus, pods subjected to extremely low or high moisture levels, and which were more likely to exhibit both poor hatching and altered developmental rates, were not used. For this analysis, we used all hatchlings for each pod, as opposed to using only the first hatch per pod, to calculate a separate mean time of hatching for each pod (each pod produced an independent mean). We then averaged all of these pod means to derive a grand mean for each species.
Statistical Analyses
The effects of moisture and species on percent hatch and time to first hatch were tested using analysis of variance (ANOVA) and multiple ANOVA (MANOVA) (SAS 9.1; SAS Institute 2004). Some pods had individuals that hatched, but failed to reach the sand surface. For these pods, we had no data on hatching date, but we did have data on survival to hatch. Our analysis with MANOVA omits these pods. Post hoc comparisons with two-way ANOVA or Tukey’s honestly significant difference (HSD) were conducted to determine significance between species and between moisture levels for both experiment 1 and 2. Length of egg stage was tested using a t-test.
Results
Experiment 1: Egg Survival After 90 d at 13 Specific Moisture Levels
The eggs of both species survived a surprisingly wide range of soil moistures; only moisture levels below 0.5% or above 90% produced substantial egg mortality (Fig. 3).
Fig. 3.
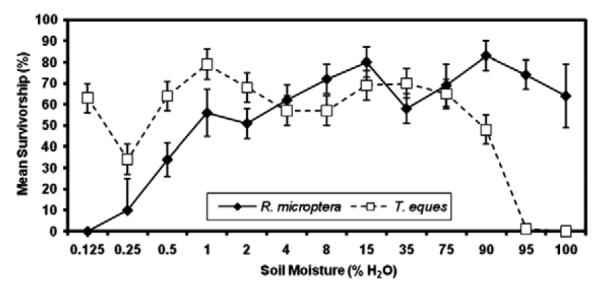
Mean percent hatch (±SE) for R. microptera and T. eques egg pods subjected to various moisture treatments for 90 d beginning on day 10 from oviposition.
Moisture significantly affected egg survivorship (F1,12 = 3.06; P = 0.0007) whereas species did not significantly affect overall survivorship (F1,12 = 0.96; P = 0.3285). However, the interaction of moisture level and species significantly affected egg survivorship (F1,9 = 3.28; P = 0.0011). Post hoc two-way ANOVA showed that marsh R. microptera survived better than desert T. eques at high soil moisture levels of 90% (P = 0.0260) and 95% (P < 0.0001). Indeed, no T. eques hatched from the 100% moisture treatments, and only 1.3% from the 95% moisture treatment (Fig. 3). In contrast, desert T. eques survived better than R. microptera at 0.25% moisture (P = 0.0109) and 0.125% moisture (P < 0.0001). At 0.25% moisture, 34% of T. eques eggs versus 10% of R. microptera eggs survived, and at 0.125% moisture, 63% of T. eques and 0% of R. microptera survived.
Time to first hatch (the interval between oviposition and the time to the first hatch for each pod) was not significantly affected by the interaction of moisture and species (F1,9 = 1.65, P = 0.1052). Across all moisture levels combined, the two species displayed significantly different times to first hatch per pod: R. microptera (mean ± SE = 164 ± 6.1 d) versus T. eques (217.4 ± 4.7 d) (F1,12 = 550.57; P < 0.0001). Moisture level alone (independent of species differences) significantly affected time at first hatch (F1,12 = 2.63; P = 0.0031), with hatching slightly accelerated in dry soils (Fig. 4). The range in hatching times for all hatched eggs across all treatments was 133–224 d for R. microptera, and 195–355 d for T. eques.
Fig. 4.

Mean (±SE) time to first hatch per pod for R. microptera and T. eques egg pods subjected to various moisture treatments for 90 d beginning on day 10 from oviposition.
Experiment 2: Constant Low Moisture Throughout Egg Development
In this experiment, egg survival was significantly affected by the interaction of moisture level and species (F1,3 = 5.21; P = 0.0029), with T. eques eggs surviving better than R. microptera at the lower soil moistures (Fig. 5). Surprisingly, 48.6% of T. eques eggs survived being laid into absolutely dry sand and then maintained for 8 mo with no additional water. In contrast, only 3.5% of R. microptera eggs survived in completely dry sand (Fig. 5). Egg survival for all pods combined was significantly affected by moisture level on its own (F1,3 = 24.52; P < 0.0001) and species on its own (F1,3 = 4.74; P = 0.0333). In this experiment, which tested very low soil moistures, eggs survived better at the higher moisture levels used (i.e., 1 and 4% moisture levels), and eggs from desert-inhabiting T. eques survived better overall.
Fig. 5.

Mean percent hatch (±SE) for R. microptera and T. eques egg pods laid into soil at specific low soil moisture levels and maintained at those levels for the duration of the egg stage.
In experiment 2, the time to first hatch/pod was significantly affected by the interaction of moisture level and species (F1,3 = 6.59; P = 0.0006). The mean time for the first hatch per pod was significantly longer for R. microptera at 0% than at both 1% (ANOVA: Tukey-Kramer; P = 0.0047) and 4% (P = 0.0019) (Fig. 6). Overall, there was a highly significant species effect (F1,3 = 893.83; P < 0.0001), with R. microptera hatching sooner than T. eques. Finally, moisture level independently and significantly affected hatching time (F1,3 = 4.64; P = 0.0055). Hatching time for R. microptera was later at the lowest moisture levels used in the experiment (Fig. 6).
Fig. 6.

Mean time (±SE) to first hatch per pod for R. microptera and T. eques egg pods laid into soil at specific low soil moisture levels and maintained at those levels for the duration of the egg stage.
Length of Egg Stage: T. eques versus R. microptera
Under relatively constant laboratory-room temperatures of ≈26°C, the two species differed significantly in average hatching time per pod: R. microptera (mean ± SE = 175.5 ± 1.4 d); T. eques (236.6 ± 1.6 d) (t-test: P < 0.0001) (Fig. 7).
Fig. 7.

Distribution of mean hatch times per pod for egg pods from experiment 1 for R. microptera (n = 67 egg pods; ranging from 2 to 95% H2O treatments) and T. eques (n = 74 egg pods; ranging from 1 to 90% H2O treatments).
Discussion
In this study, two closely related grasshopper species have evolved divergent egg physiologies. The eggs of a marsh-inhabiting grasshopper survived better at high soil moistures than at low soil moistures, whereas the eggs of a desert grasshopper survived better at low than high soil moisture levels. Remarkably, 64% of eggs of R. microptera, from the Everglades wetlands, survived and hatched after 90 d of total submergence under water. Likewise, nearly 50% of eggs of T. eques, from the Chihuahuan Desert, survived when laid into oven-dried sand containing no water, and then maintained with no additional moisture throughout the 8-mo egg stage. These species differences appear to be beneficial given their divergent habitats. Other authors have noted that the eggs of xerophilic grasshopper species are much more resistant to drying, but more susceptible to drowning than are the eggs of hydrophilic species (Shulov 1952, Ingrisch 1983, Hewitt 1985, Colvin 1996, Gehrken and Doumbia 1996).
Our two species also differed in egg incubation times. Under similar laboratory conditions of 26 ± 2°C, the mean oviposition-to-hatch period for T. eques eggs was 2 mo longer than for R. microptera (237 vs. 176 d) (Fig. 7). Ongoing studies in our laboratory show that this difference is primarily because of a much longer diapause period in T. eques (T. W. Stauffer, unpublished data). These differences in length of the egg stage are presumably beneficial in that they allow each species to hatch at the appropriate time of year, in their respective habitats. Similar to many temperate-zone grasshoppers (Uvarov 1966, 1977, Capinera and Sechrist 1982, Pfadt 1994), Florida R. microptera hatch at the beginning of spring and thus must be ready to hatch as soon as soil temperatures begin to rise (Fig. 2). Egg eclosion in spring-hatching grasshoppers is often triggered by rising soil temperatures that exceed a threshold temperature (Shotwell 1929, 1941, Pickford 1976, Hewitt 1979, Koehler et al. 1999). For R. microptera, there should be little fitness loss for rapidly maturing embryos, as long as actual hatching is delayed by low winter soil temperatures, as is the case for other spring-hatching grasshopper species (Shotwell 1929, Uvarov 1966, 1977, Wardhaugh et al. 1969, Kemp and Sánchez 1987). Even exceptionally early hatching (in early January) would not be immediately lethal for R. microptera, because food plants and sunshine to heat the black nymphs are available year-round in sub-tropical south Florida.
In comparison, desert T. eques do not hatch in spring, but, like many other desert grasshoppers, have evolved to hatch at the beginning of the rainy season (Whitman and Orsak 1985, Whitman 1988b, Colvin 1996, Moizuddin 1999, 2005, Maiga et al. 2008). For T. eques, spring or midsummer hatching would be lethal, because the searing desert heat, low humidity, and lack of edible annuals during midsummer would quickly kill the tiny hatchlings. Hence, the longer egg development time for desert T. eques probably prevents early hatching, even during rising or high soil temperatures.
In many grasshoppers, low soil moisture can slow or stop development, and hence greatly extend the egg stage (Farrow 1975, Uvarov 1977, Mukerji and Gage 1978, Hunter et al. 2001), in some cases up to several years (Shulov and Pener 1961, Colvin 1996, Maiga et al. 2008). In others, high soil moisture prolongs the egg stage (Parker 1930, Shulov and Pener 1961). However, in our experiment, soil moisture did not dramatically alter egg development time for either species. There were some slight, but significant differences in length of egg incubation at the lowest moisture levels (Figs. 4 and 6). This may (or may not) be an artifact because of low survival (low N) for R. microptera at the lowest soil moistures (Figs. 3 and 5).
Our study shows that these related species have evolved different egg physiologies. These trait changes appear to be beneficial for each species given their respective habitats. These results are not surprising, as grasshoppers and other insects readily adapt their physiologies and life histories to local conditions. Numerous studies document apparently adaptive geographic physiological variability in closely related grasshopper species or populations along altitude (Chappell 1983, Hadley and Massion 1985, Dingle et al. 1990, Dingle and Mousseau 1994, Berner et al. 2004), latitude (Dingle and Mousseau 1994, Telfer and Hassall 1999, Fielding 2006, 2008, Fielding and DeFoliart 2007, 2008, Tanaka and Zhu 2008, Zhao et al. 2009), or habitat/microhabitat gradients (Schädler and Witsack 1999, van Wingerden et al. 1991, Fisher 1997, Grace et al. 2009). Such ecotypic differences often have a genetic basis (Groeters and Shaw 1992, Dingle and Mousseau 1994, Berner et al. 2004, Grace et al. 2009, Tan et al. 2008).
In this current study, the egg physiologies of two grasshopper species differ, and these differences are presumably beneficial given their disparate climates. Previous studies of these two related insects show divergence in other traits, and those differences also appear to be adaptive. For example, although both species are univoltine and exhibit an obligatory egg diapause under constant 26°C, T. eques eggs diapause immediately after laying, whereas R. microptera eggs enter diapause ≈2 mo after being laid (T. W. Stauffer, unpublished data). This difference allows each species to synchronize egg diapause with winter (Fig. 2). Furthermore, in the field, desert T. eques lay deeper egg pods than R. microptera (9.3 vs. 3.9 cm to the bottom of the pod) (Stauffer and Whitman 2007), which presumably keeps the eggs away from the hot and dry desert soil surface (Wardhaugh et al. 1969). In contrast there may be advantages for marsh-inhabiting R. microptera to lay pods near the soil surface, because deep pods experience greater flooding, and flooding can kill grasshopper eggs (Popov 1959, Shulov and Pener 1961, Uvarov 1966, Farrow 1975, Shah et al. 1998). Almost all grasshopper species oviposit underground, and it is well known that desert grasshoppers tend to lay deeper egg pods than do mesic or hydric species (Stauffer and Whitman 1997). A few marsh-inhabiting grasshopper species actually lay pods above the ground, on or in vegetation, and this is thought to counter the disadvantages of laying in flooded soils (Braker 1989, Stauffer and Whitman 1997). Hence, as with differences in egg water physiology (this paper), lubber differences in egg pod depth also appear to be beneficial (Stauffer and Whitman 2007).
R. microptera and T. eques also differ in ovipositionsite selection. R. microptera tend to lay in sunny, open spots, whereas desert T. eques tend to lay under bushes, where they and the resulting pods are shaded from direct desert sunlight (Stauffer and Whitman 2007). Shaded desert soils experience less heating and drying than sun-exposed desert soils (Farrow 1975, Fisher 1993). Finally, although the topography of the Everglades is relatively flat (Myers and Ewel 1990, Lodge 2010), R. microptera tend to oviposit at elevated sites (Stauffer and Whitman 2007). This presumably lessens exposure of the pods to the seasonal flooding that occurs in the Everglades area, and reduces the chance that the eggs would have to hatch underwater, which is almost always lethal to the vermiform larvae.
Our experiments were conducted in the laboratory under relatively warm and stable temperatures and unchanging photoperiod. In nature, these variables fluctuate, and we would expect different egg development rates, hatching times, and survival under different thermal regimes, including cold-shocks, which are known to induce or end diapause in the eggs of some grasshopper species (Uvarov 1966, 1977, Pickford 1975, Oma et al. 1990, Fisher et al. 1996, Fisher 1997), and warm temperatures, which alter diapauses or elicit hatching in mature embryos (Shotwell 1929, 1941, Parker 1930, Wardhaugh et al. 1969, Guo et al. 2009, Zhu et al. 2009). Photoperiod experienced by adult females can also influence diapause in subsequent eggs of some grasshopper species (Wardhaugh 1977, Dean 1982, Tanaka 1994). However, to date there is no evidence that photoperiod influences the eggs of lubber grasshoppers.
In conclusion, our data support the hypotheses that the eggs of each species are adapted to their specific edaphic conditions. Furthermore, this and previous studies suggest rapid divergence of some traits (e.g., egg development time, timing of egg diapause, egg survival at extreme moisture levels, overall phenology, egg pod depth, egg and clutch size, adult body color, etc.) and little divergence of others (e.g., hatching success at moderate moisture levels, nymphal color pattern, sluggishness, flightlessness, aggregative, acoustic, roosting, thermoregulatory, and threat-display behaviors, a preference for feeding on lilies, internal toxins, and a shared tracheal secretory defense gland) in these sister species (Hebard 1925, Rehn and Grant 1961, Whitman and Orsak 1985, Whitman et al. 1985, 1991, 1992, 1994, Whitman 1987, 1988a, 1988b, 1990, Hatle and Whitman 2001, Stauffer and Whitman 2007, Whitman and Vincent 2008). Given that these two insects can interbreed and produce viable hybrids in the laboratory, it is not surprising that they share most traits. Yet, they differ in a host of other traits, and these differences appear to be specifically related to, and beneficial in, their respective desert versus marsh environments (e.g., Stauffer and Whitman 2007). This suggests that these differences evolved in direct response to their divergent environments.
On a broader scale, this study adds to the extensive catalog of cases demonstrating ecotypic differentiation in related organisms (Hoeksema and Forde 2008, Leimu and Fischer 2008, Tanaka and Zhu 2008, Hereford 2009, Zhao et al. 2009). Under the right conditions, populations and species quickly adjust to local conditions via genetic change (Loxdale 2010) or phenotypic plasticity (Whitman and Ananthakrishnan 2009), or both (Grace et al. 2010). Such evolution can be surprisingly rapid (Carroll et al., 2003, Carroll 2008, Bradshaw and Holzapfel 2010, Huang et al. 2010, Loxdale 2010). And, it can occur over stunningly small spatial scales, perhaps as close as insects living on different branches on the same tree (Alstad and Corbin 1990), and even in the face of interpopulational genetic exchange (Mopper et al. 2000).
Acknowledgments
This project was supported by National Science Foundation grants DBI-9978810 and DBI-0442412.
References Cited
- Alstad DN, Corbin KW. Scale insect allozyme differentiation within and between host trees. Evol. Ecol. 1990;4:43–56. [Google Scholar]
- Berner D, Körner C, Blanckenhorn WU. Grasshopper populations across 2000m of altitude: is there life history adaptation? Ecography. 2004;27:733–740. [Google Scholar]
- Bradshaw WE, Holzapfel CM. Light, time and the physiology of biotic response to rapid climate change in animals. Annu. Rev. Physiol. 2010;72:147–166. doi: 10.1146/annurev-physiol-021909-135837. [DOI] [PubMed] [Google Scholar]
- Braker HE. Oviposition on host plants by a tropical forest grasshopper (Microtylopteryx hebardi: Acrididae) Ecol. Entomol. 1989;14:141–148. [Google Scholar]
- Brown DE. Biotic communities of the American southwest-United States and Mexico. Desert Plants. 1982;4:1–342. [Google Scholar]
- Capinera JL, Sechrist TS. Grasshoppers (Acrididae) of Colorado. Colorado Agric. Exp. Sta. Bull. 1982;584S [Google Scholar]
- Carroll SP. Facing change: forms and foundations of contemporary adaptation to biotic invasions. Mol. Ecol. 2008;17:361–372. doi: 10.1111/j.1365-294X.2007.03484.x. [DOI] [PubMed] [Google Scholar]
- Carroll SP, Dingle H, Famula TR. Rapid appearance of epistasis during adaptive divergence following colonization. Proc. R. Soc. Lond. B (Suppl.) 2003;270:S80–S83. doi: 10.1098/rsbl.2003.0019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chappell M. Metabolism and thermoregulation in desert and montane grasshoppers. Oecologia. 1983;56:126–131. doi: 10.1007/BF00378228. [DOI] [PubMed] [Google Scholar]
- Chen E, Gerber JF. Climate. In: Myers RL, Ewel RL, editors. Ecosystems of Florida. University of Central Florida Press; Orlando, FL: 1990. pp. 11–34. [Google Scholar]
- Chladny TA, Whitman DW. The effects of temperature, soil moisture, and ventilation on the eggs of the grasshopper Romalea guttata. Trans. Ill. Acad. Sci. 1998;91:155–159. [Google Scholar]
- Colvin J. Diapause duration, survival in relation to desiccation and egg-pod morphology of the Senegalese grasshopper, Oedaleus senegalensis. Physiol. Entomol. 1996;21:173–178. [Google Scholar]
- Dean JM. Control of diapauses induction by a change in photoperiod in Melanoplus sanguinipes. J. Insect Physiol. 1982;28:1035–1040. [Google Scholar]
- Dingle H, Mousseau TA. Geographic variation in embryonic development time and state of diapause in a grasshopper. Oecologia. 1994;97:179–185. doi: 10.1007/BF00323147. [DOI] [PubMed] [Google Scholar]
- Dingle H, Mousseau TA, Scott SM. Altitudinal variation in life cycle syndromes of California populations of the grasshopper, Melanoplus sanguinipes (F.) Oecologia. 1990;84:199–206. doi: 10.1007/BF00318272. [DOI] [PubMed] [Google Scholar]
- Farrow RA. The African migratory locust in its main outbreak area of the Middle Niger: quantitative studies of solitary populations in relation to environmental factors. Locusta. 1975;11 [Google Scholar]
- Fielding DJ. Optimal diapauses strategies of a grasshopper, Melanoplus sanguinipes. J. Insect Sci. 2006;6:1–16. doi: 10.1673/1536-2442(2006)6[1:ODSOAG]2.0.CO;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fielding DJ. Diapause traits of Melanoplus sanguinipes and Melanoplus borealis (Orthoptera: Acrididae) Ann. Entomol. Soc. Am. 2008;101:439–448. [Google Scholar]
- Fielding DJ, DeFoliart LS. Growth, development, and nutritional physiology of grasshoppers from subarctic and temperate regions. Physiol. Biochem. Zool. 2007;80:607–618. doi: 10.1086/521801. [DOI] [PubMed] [Google Scholar]
- Fielding DJ, DeFoliart LS. Discriminating tastes: self-selection of macronutrients in two populations of grasshoppers. Physiol. Entomol. 2008;33:264–273. [Google Scholar]
- Fisher JR. Location of Aulocara elliotti eggs pods in a crested wheatgrass field in Montana. J. Kansas Entomol. Soc. 1993;65:416–420. [Google Scholar]
- Fisher JR. Embryonic diapause in Aulocara elliotti and Ageneotettix deorum Orthoptera: Acrididae): low-temperature relationships. Environ. Entomol. 1997;26:906–911. [Google Scholar]
- Fisher JR, Kemp WP, Pierson FB. Aulocara elliotti (Orthoptera: Acrididae): diapauses termination, postdiapause development, and prediction of hatch. Environ. Entomol. 1996;25:1158–1166. [Google Scholar]
- Gehrken U, Doumbia YO. Diapause and quiescence in eggs of a tropical grasshopper Oedaleus senegalensis (Krauss) J. Insect Physiol. 1996;42:483–491. [Google Scholar]
- Getz LL. Use of national weather station temperature records in field studies. Trans. Ill. Acad. Sci. 2005;98:49–54. [Google Scholar]
- Ghalambor CK, Walker JA, Reznick DN. Multi-trait selection, adaptation, and constraints on the evolution of burst swimming performance. Integr. Comp. Biol. 2003;43:431–438. doi: 10.1093/icb/43.3.431. [DOI] [PubMed] [Google Scholar]
- Grace T, Joern A, Apple JL, Brown SJ, Wisely SM. Highly polymorphic microsatellites in the north American snakeweed grasshopper, Hesperotettix viridis. J. Orthop. Res. 2009;18:19–21. [Google Scholar]
- Grace T, Wisely SM, Brown SJ, Dowell FE, Joern A. Divergent host plant adaptation drives the evolution of sexual isolation in the grasshopper Hesperotettix viridis (Orthoptera: Acrididae) in the absence of reinforcement. Biol. J. Linn. Soc. 2010;100:866–878. [Google Scholar]
- Groeters FR, Shaw DD. Association between latitudinal variation for embryonic development time and chromosome structure in the grasshopper Caledia captiva (Orthoptera: Acrididae) Evolution. 1992;46:245–257. doi: 10.1111/j.1558-5646.1992.tb01999.x. [DOI] [PubMed] [Google Scholar]
- Guo K, Hao SG, Sun OJ, Kang L. Differential responses to warming and increased precipitation among three contrasting grasshopper species. Global Change Biol. 2009;15:2539–2548. [Google Scholar]
- Hadley NF, Massion DD. Oxygen consumption, water loss and cuticular lipids of high and low elevation populations of the grasshopper Aeropedellus clavatus (Orthoptera: Acrididae) Comp. Biochem. Phys. 1985;80A:307–311. [Google Scholar]
- Hatle JD, Whitman DW. Sluggish movement of conspicuous insects as a defense mechanism against motion-oriented predators. In: Ananthakrishnan TN, editor. Insect and Plant Defense Dynamics. Science Publishers; Enfield, NH: 2001. pp. 209–228. [Google Scholar]
- Hebard M. The group Taeniopodae as found in the United States (Orthoptera) Trans. Am. Entomol. Soc. Phila. 1925;52:1–12. [Google Scholar]
- Hereford J. A quantitative survey of local adaptation and fitness trade-offs. Am. Nat. 2009;173:579–588. doi: 10.1086/597611. [DOI] [PubMed] [Google Scholar]
- Hewitt GB. Hatching and development of rangeland grasshoppers in relation to forage growth, temperature, and precipitation. Environ. Entomol. 1979;8:24–29. [Google Scholar]
- Hewitt GB. Review of factors affecting fecundity, oviposition, and egg survival of grasshoppers in North America. U.S. Dep. Agric. Agric. Res. Serv. 1985;36:1–35. [Google Scholar]
- Hoeksema JD, Forde SE. A meta-analysis of factors affecting local adaptation between interacting species. Am. Nat. 2008;171:275–290. doi: 10.1086/527496. [DOI] [PubMed] [Google Scholar]
- Huang X, Schmitt J, Dorn L, Griffiths C, Effgen S, Takao S, Koornneef M, Donohue K. The earliest stages of adaptation in an experimental plant population: strong selection on QTLS for seed dormancy. Molec. Ecol. 2010;19:1335–1351. doi: 10.1111/j.1365-294X.2010.04557.x. [DOI] [PubMed] [Google Scholar]
- Hunter DM, Walker PW, Elder RJ. Adaptations of locusts and grasshoppers to the low and variable rainfall of Australia. J. Orthop. Res. 2001;10:347–351. [Google Scholar]
- Ingrisch S. Zum einfluß der feuchte auf die schlupfrate und entwicklungsdauer der eier mitteleuropäischer feldheuschrecken (Orthoptera: Acrididae) Dtsche. Entomol. Z. 1983;30:1–15. [Google Scholar]
- Kawecki TJ, Ebert D. Conceptual issues in local adaptation. Ecol. Lett. 2004;7:1225–1241. [Google Scholar]
- Kemp WP, Sanchez NE. Differences in postdiapause thermal requirements of eggs of two rangeland grasshoppers. Can. Entomol. 1987;119:653–661. [Google Scholar]
- Koehler G, Klaus R, Roman A. Biology of the alpine grasshopper Miramella formosanta (Fruhstorfer, 1921) Mitt. Schweiz. Entomol. Ges. 1999;72:315–328. [Google Scholar]
- Leimu R, Fischer M. A meta-analysis of local adaptation in plants. PLOS. 2008;3 e. 4010:1–8. doi: 10.1371/journal.pone.0004010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lodge TE. The everglades handbook. CRC; Boca Raton, FL: 2010. [Google Scholar]
- Loxdale HD. Rapid genetic changes in natural insect populations. Ecol. Entomol. 2010;35:155–164. [Google Scholar]
- Maddox RA, McCollum DM, Howard KW. Large-scale patterns associated with severe summertime thunderstorms over central Arizona. Weather Forecast. 1995;10:763–778. [Google Scholar]
- Maiga IH, Lecoq M, Kooyman C. Ecology and management of the Senegalese grasshopper Oedaleus senegalensis (Krauss 1877) (Orthoptera: Acrididae) in West Africa: review and prospects. Ann. Soc Entomol. Fr. 2008;44:271–288. [Google Scholar]
- Matuszek JV, Whitman DW. Captive rearing of eastern lubber grasshopper Romalea microptera. Proceedings: Invertebrates in Captivity Conference; 2001; Ron Rico, AZ: Sonoran Arthropod Studies Institute; 2001. pp. 56–65. [Google Scholar]
- Moizuddin M. Studies on habitats and life cycle of African migratory locust Locusta migratoria migratoroides (Riche and Fairmaire) (Orthoptera: Acridoidea) in the desert area of Lasbela, Balochistan. Pakistan J. Zool. 1999;31:111–116. [Google Scholar]
- Moizuddin M. Habitat and life cycle of grasshopper Acrotylus humbertianus Saussure (Orthoptera: Acridoidae) in the desert area of Lasbela, Balochistan. Proc. Pakistan Congr. Zool. 2005;25:65–71. [Google Scholar]
- Mopper S, Stiling P, Landau K, Simberloff D, van Zandt P. Spatiotemporal variation in leafminer population structure and adaptation to individual oak trees. Ecology. 2000;81:1577–1587. [Google Scholar]
- Mukerji MK, Gage SH. A model for estimating hatch and mortality of grasshopper egg populations based on soil moisture and heat. Ann. Entomol. Soc. Am. 1978;71:183–190. [Google Scholar]
- Mutun S, Borst DW. Intraspecific micochondrial DNA variation and historical biogeography of the Eastern Lubber grasshopper, Romalea microptera. Ann. Entomol. Soc. Am. 2004;97:681–696. [Google Scholar]
- Myers RL, Ewel JJ. Ecosystems of Florida. University of Central Florida Press; Orlando, FL: 1990. [Google Scholar]
- NOAA Archived data. 1997–2006 ( www.noaa.gov)
- Oma EA, Streett DA, Henry JE. Establishment of a nondiapause Melanoplus differentialis (Thomas) (Orthoptera: Acrididae) colony. Can. Entomol. 1990;122:583–584. [Google Scholar]
- Parker JR. Some effects of temperature and moisture upon Melanoplus mexicanus mexicanus Saussure and Camnula pellucida Scudder (Orthoptera) B. Montana Agric. Exp. Stn. 1930;(No. 223):1–132. [Google Scholar]
- Pfadt RE. Field guide to common western grasshoppers. Wyo. Agric. Exp. Stn. Bull. 1994;912 [Google Scholar]
- Pickford R. Water uptake in eggs of Camnula pellucida (Orthoptera: Acrididae) and its relationship to embryogenesis. Can. Entomol. 1975;107:533–542. [Google Scholar]
- Pickford R. Embryonic growth and hatchability of eggs of the two-striped grasshopper, Melanoplus bivittatus (Orthoptera: Acrididae), in relation to date of oviposition and weather. Can. Entomol. 1976;108:621–626. [Google Scholar]
- Popov GB. Ecological studies on oviposition by Locusta migratoria migratorioides (R. & R.) in its outbreak area in the French Sudan. Locusta. 1959;6:3–64. [Google Scholar]
- Prinzing A, Durka W, Klotz S, Brandl R. The niche of higher plants: evidence for phylogenetic conservatism. Proc. R. Soc. Lond. B. 2001;268:2383–2389. doi: 10.1098/rspb.2001.1801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rehn JAG, Grant HJ. A review of the Romaleinae (Orthoptera; Acrididae) found in America north of Mexico. Proc. Acad. Nat. Sci. Phila. 1959;111:190–271. [Google Scholar]
- Rehn JAG, Grant HJ. A monograph of the Orthoptera of North America. Volume I. Mono. Acad. Nat. Sci. Phila. 1961;(No. 12):1–257. [Google Scholar]
- SAS Institute . PROC user’s manual. version 9.1 ed SAS Institute; Cary, NC: 2004. [Google Scholar]
- Shah PA, Godonou I, Gbongboui C, Hossou A, Lomer CJ. Survival and mortality of grasshopper egg pods in semi-arid cereal cropping areas of northern Benin. Bull. Entomol. Res. 1998;88:451–459. [Google Scholar]
- Schädler M, Witsack W. Variation of postembryonic development time and number of nymphal instars on a small spatial scale in Central European grasshoppers (Caelifera: Acridiae) Entomol. Gener. 1999;24:125–135. [Google Scholar]
- Shotwell RL. Some notes on the grasshopper situation in north central Montana. J. Econ. Entomol. 1929;22:581–588. [Google Scholar]
- Shotwell RL. Life Histories and habits of some grasshoppers of economic importance on the Great Plains. Tech. Bull. U.S. Dep. Agric. 1941;(No. 774):1–47. [Google Scholar]
- Shulov A. The development of eggs of Schistocerca gregaria (Forskål) in relation to water. Bull. Entomol. Res. 1952;43:469–476. [Google Scholar]
- Shulov A, Pener MP. Environmental factors in interruption of development of Acrididae eggs. In: Grossowicz N, editor. Cryptobiotic Stages in Biological Systems. Elsevier; Amsterdam, London: 1961. pp. 144–153. [Google Scholar]
- Stauffer TW, Whitman DW. Grasshopper oviposition. In: Gangwere SK, Muralirangan MC, Muralirangan M, editors. The Bionomics of Grasshoppers, Katydids, and Their Kin. CAB International; Wallingford, United Kingdom: 1997. pp. 232–280. [Google Scholar]
- Stauffer TW, Whitman DW. Divergent oviposition behaviors in a desert vs a marsh grasshopper. J. Orthop. Res. 2007;16:103–114. [Google Scholar]
- Tan R-H, Zhu D-H, Yang Y-P. The diapauses rate of hybrid offsprings among three geographic populations in a grasshopper, Oxya chinensis. Chinese Bull. Entomol. 2008;45:394–397. [Google Scholar]
- Tanaka H. Embryonic diapause and life cycle in the Migratory Locust, Locusta migratoria L. (Orthoptera: Acrididae) in Kyoto. Appl. Entomol. Zool. 1994;29:179–191. [Google Scholar]
- Tanaka S, Zhu D-H. Geographic variation in embryonic diapause, cold-hardiness and life cycles in the migratory locust Locusta migratoria (Orthoptera: Acrididae) in China. Entomol. Sci. 2008;11:327–339. [Google Scholar]
- Telfer MG, Hassall M. Ecotypic differentiation in the grasshopper Chorthippus brunneus: life history varies in relation to climate. Oecologia. 1999;121:245–254. doi: 10.1007/s004420050926. [DOI] [PubMed] [Google Scholar]
- Thoma D, Moran MS, Bryant R, Rahman MM, Collins C. D. Holifield, Skirvin SM, Sano EE, Slocum K. Comparison of four models for determining surface soil moisture from c-band radar imagery. Water Resour. Res. 2006;42:1–12. [Google Scholar]
- Thornhill R, Alcock J. The evolution of insect mating systems. Harvard University Press; Cambridge, MA: 1983. [Google Scholar]
- Tinkham ER. Faunistic and ecological studies on the Orthoptera of the Big Bend region of Tans-Pecos Texas, with especial reference to the orthopteran zones and faunae of Midwestern North America. Am. Midl. Nat. 1948;40:521–663. [Google Scholar]
- Uvarov B. Grasshoppers and locustsk. vol. 1. Cambridge University Press; London: 1966. [Google Scholar]
- Uvarov B. Grasshoppers and locusts. vol. 2. Centre Overseas Pest Research; London: 1977. [Google Scholar]
- van Wingerden WKRE, Musters JCM, Maaskamp FIM. The influence of temperature on the duration of egg development in West European grasshoppers (Orthoptera: Acrididae) Oecologia. 1991;87:417–423. doi: 10.1007/BF00634600. [DOI] [PubMed] [Google Scholar]
- Wardhaugh KG. The effects of temperature and photoperiod on the morphology of the egg-pod of the Australian plague locust (Chortoicetes terminifera Walker, Orthoptera: Acrididae) Aust. Ecol. 1977;2:81–88. [Google Scholar]
- Wardhaugh K, Ashour Y, Ibrahim AO, Khan AM, Bassonbol M. Experiments on the incubation and hopper development periods of the Desert Locust (Schistocerca gregaria Forskål) in Saudi Arabia. Anti-Locust Bull. 1969;45:38. [Google Scholar]
- Whitman DW. Thermoregulation and daily activity patterns in a black desert grasshopper, Taeniopoda eques. Anim. Behav. 1987;35:1814–1826. [Google Scholar]
- Whitman DW. Allelochemical interactions among plants, herbivores, and their predators. In: Barbosa P, Letourneau D, editors. Novel Aspects of Insect-Plant interactions. Wiley; New York: 1988a. pp. 11–64. [Google Scholar]
- Whitman DW. The function and evolution of thermoregulation in the grasshopper Taeniopoda eques. J. Anim. Ecol. 1988b;57:369–383. [Google Scholar]
- Whitman DW. Grasshopper chemical communication. In: Chapman RF, Joern A, editors. Biology of Grasshoppers. Wiley; New York: 1990. pp. 357–391. [Google Scholar]
- Whitman DW, Orsak L. The biology of Taeniopoda eques (Orthoptera: Acrididae) in southeastern Arizona. Ann. Entomol. Soc. Am. 1985;78:811–825. [Google Scholar]
- Whitman DW, Vincent S. Large size as an antipredator defense in an insect. J. Orthop. Res. 2008;17:353–371. [Google Scholar]
- Whitman DW, Ananthakrishnan TN, editors. Phenotypic plasticity in insects: mechanisms and consequences. Science Publishers; Enfield, NH: 2009. [Google Scholar]
- Whitman DW, Blum MS, Jones CG. Chemical defense in Taeniopoda eques (Orthoptera: Acrididae): role of the metathoracic secretion. Ann. Entomol. Soc. Am. 1985;78:451–455. [Google Scholar]
- Whitman DW, Billen JPJ, Alsop D, Blum MS. Anatomy, ultrastructure, and functional morphology of the metathoracic tracheal defensive glands of the grasshopper Romalea guttata. Can. J. Zool. 1991;69:2100–2108. [Google Scholar]
- Whitman DW, Jones CG, Blum MS. Defensive secretion production in lubber grasshoppers (Orthoptera:Romaleidae): influence of age, sex, diet, and discharge frequency. Ann. Entomol. Soc. Am. 1992;85:96–102. [Google Scholar]
- Whitman DW, Blum MS, Slansky F., Jr . Carnivory in phytophagous insects. In: Ananthakrishnan TN, editor. Functional Dynamics of Phytophagous Insects. Oxford & IBH; New Delhi, India: 1994. pp. 161–205. [Google Scholar]
- Willis CG, Ruhfel B, Primack RB, Miller-Rushing AJ, Davis CC. Phylogenetic patterns of species loss in Thoreau’s woods are driven by climate change. Proc. Nat. Acad. Sci. 2008;105:17029–17033. doi: 10.1073/pnas.0806446105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willmer P, Stone G, Johnston I. Environmental physiology of animals. Blackwell; Malden, MA: 2005. [Google Scholar]
- Zhao Q, Zhu D-H, Yang Y-P, Tan R-H. Variation of embryonic diapause intensity and life-cycle pattern in five geographic populations of the Chinese rice grasshopper, Oxya chinensis (Orthoptera: Acridoidea: Catantopidae) from China. Acta Entomol. Sinica. 2009;52:183–190. [Google Scholar]
- Zhu D-H, Yang Y-P, Zhiwei L. Reversible change in embryonic diapause intensity by mild temperature in the Chinese rice grasshopper, Oxya chinensis. Entomol. Exp. et Appl. 2009;133:1–8. [Google Scholar]



