Abstract
Objective
To compare the accuracy of common clinical tests for left neglect with that of a computerized reaction time Posner test in a stroke population.
Design
Neglect measures were collected longitudinally in stroke patients at the acute (≈2wk) and chronic (≈9mo) stage. Identical measures were collected in a healthy control group.
Setting
Inpatient and outpatient rehabilitation.
Participants
Acute stroke patients (n=59) with left neglect, 30 of whom were tested longitudinally; healthy age-matched controls (n=30).
Interventions
Not applicable.
Main Outcome Measures
A receiver operating characteristic analysis, ranking the measures' sensitivity and specificity using a single summary statistic.
Results
Most clinical tests were adequately accurate at the acute stage, but many were near chance at the chronic stage. The Posner test was the most sensitive test at both stages, the most sensitive variable being the reaction time difference for detecting targets appearing on the left compared to the right side.
Conclusions
Computerized reaction time tests can be used to screen for subtle but potentially clinically relevant left neglect, which may not be detectable by conventional clinical tests, especially at the chronic stage. Such tests may be useful to assess the severity of the patients' deficits and provide more accurate measures of the degree of recovery in clinical trials than established clinical measures.
Keywords: Rehabilitation, Stroke
Hemispatial neglect is a tendency to miss or ignore stimuli on the side opposite the brain lesion, frequently associated with nonspatially selective deficits in attention, such as slower processing detection speed in both hemifields1,2. Neglect occurs in roughly 20 to 30% of patients with acute stroke1,3,4 and is more common for right-sided lesions1,3,4,5. Symptoms often attenuate with time but can persist into the chronic stage6, with a prevalence of about 10% to 15% a year of more after stroke according to some studies.7-10
Neglect contributes significantly to disability following stroke and has been identified as a predictor of poor rehabilitation outcome.11,12 Sensitive screening tests for neglect are important, as this deficit may be less obvious to patients, families, and the medical team than physical symptoms such as hemiparesis. One recent study estimated that 61% of cases of acute neglect go undetected.13
The most common standardized screening tests in the clinical setting are paper-and-pencil tests and other tabletop tests involving simulation of everyday activities.14 These tests may be more sensitive than a nonstandardized bedside screening,13 but there is insufficient evidence of the reliability, validity, and sensitivity of such tests. Agrell,15 Lindell,16 Bailey,17 and colleagues reported detection rates of 46%, 41%, and 75% for the BIT Star Cancellation Subtest, respectively, in right hemisphere stroke patients with neglect about 9 to 11 weeks poststroke. In general authors have recommended using a battery of tests to increase diagnostic accuracy, at the expense of increased diagnostic burden.
Paper-and-pencil tests may be especially poor at detecting neglect in the chronic stage. After learning compensatory strategies or partially recovering, patients may be able to pass a test in which they have unlimited time to identify static targets while they may still be impaired in reacting promptly to briefly presented targets.
One longitudinal study found that only 6 out of 27 patients with left neglect at the acute stage (<7d poststroke) continued to show neglect at 3 months according to the BIT8, while another found that 8 of 14 patients with left neglect at the subacute stage (<3mo) continued to show neglect at 6 months according to the BIT.18 However, it is unknown how many of the patients with “recovered” neglect may have still had neglect symptoms undetectable by the BIT.
Computerized RT tests offer a promising alternative approach for neglect screening.19-21 RT tests can capture deficits in speed of response, identifying subtle but significant neglect that a static paper and pencil test might miss. Deouell et al,21 for instance, found that a computerized RT test was more sensitive to neglect than paper-and-pencil tests in subacute (≈7wk poststroke) right hemisphere stroke patients in a rehabilitation hospital. Fifty percent of patients who passed the BIT nevertheless showed signs of neglect on the RT test. One noteworthy case report within this study profiled a patient followed after discharge from the hospital. The patient returned to driving and “was involved in 9 car accidents, all concerning the left side of his car.” This patient tested positive for neglect on the RT test but passed the BIT. To date, however, no study has assessed the performance of an RT neglect test longitudinally in a group of patients with stroke.
In the present study, we compare longitudinally the accuracy of an RT test with a battery of clinical tests, using ROC analysis. The clinical tests are representative of those commonly used in investigational settings22 and in clinical practice.23 The battery included tests of both personal and peripersonal neglect as well as scores for both lateralized performance (difference in performance between the left and right hemispaces) and nonlateralized (overall performance across both left and right hemispaces) performance. For the RT test we selected the Posner cueing paradigm,24 as it was designed to measure both delays in detecting targets in the contralesional space and difficulty disengaging from stimuli on the ipsilesional side, 2 processes hypothesized to be impaired in neglect.24,25
We hypothesized that the reaction time test would be more accurate in detecting neglect than the clinical tests, at both the acute and chronic stages.
Methods
Patients
Patients with stroke met the following inclusion criteria: (1) 18 years of age or older; (2) single acute right hemisphere cerebrovascular lesion; (3) awake, alert, and able to consent to enrollment and complete the study tests; and (4) clinically significant left neglect as reported by treating physician or therapist. We defined clinically significant left neglect as impaired self-care or difficulty with higher-level activities such as reading or navigating the environment due to decreased attention to the left, including asomatognosia, visual, sensory, or motor neglect. Clinician diagnosis of left neglect at the acute stage was then the criterion standard used in defining the presence of neglect in our analysis.
Exclusion criteria were as follows: (1) CT or MRI evidence of other lesions, although we allowed up to 2 clinically silent lacunae with a diameter less than 15mm and evidence of periventricular white matter disease up to grade 5 on Longstreth's classification 28 (as a higher level of disease has been found to be significantly associated with cognitive decline); (2) pre-existing neurologic or psychiatric conditions, such as dementia, schizophrenia, or previous stroke or brain injury; (3) visual problems such as macular degeneration or homonymous hemianopsia (although patients with quadrantanopsia were included, because we were always able to position the stimuli outside their field cut), evaluated clinically by the study physician; and (4) score of 9 or greater on the Short Blessed Test,29 a brief dementia screen.
We recruited 59 patients with left neglect after stroke from Barnes-Jewish Hospital and the Rehabilitation Institute of St. Louis. Patients provided informed consent according to procedures approved by the Washington University Institutional Review Board. Thirty healthy, age-matched subjects served as controls. The latter group comprised elderly participants who volunteered to undergo yearly neurologic and cognitive assessment as part of an ongoing, longitudinal study of age-related cognitive changes.
Mean time from stroke onset to acute testing was 16±11 days (mean ± SD). We invited all patients to return 6 to 9 months later. Thirty patients completed testing in the chronic stage. Patients who did not return for chronic testing, due to a change in medical status, relocation, or other reason, did not differ significantly from those who did return in stroke severity, age, or neglect severity at the acute stage. Mean time from stroke onset to chronic testing was 39±16.7 weeks.
All patients had a first-ever right hemisphere stroke (55 ischemic, 4 hemorrhagic), diagnosed by a neurologist and confirmed with head CT or MRI. Lesions included right hemispheric cortical and subcortical lesions; a map of the group-wise lesion overlap for 30 of the patients is shown in figure 1. (The patients who returned for chronic testing had an anatomical MRI.)
Fig 1.
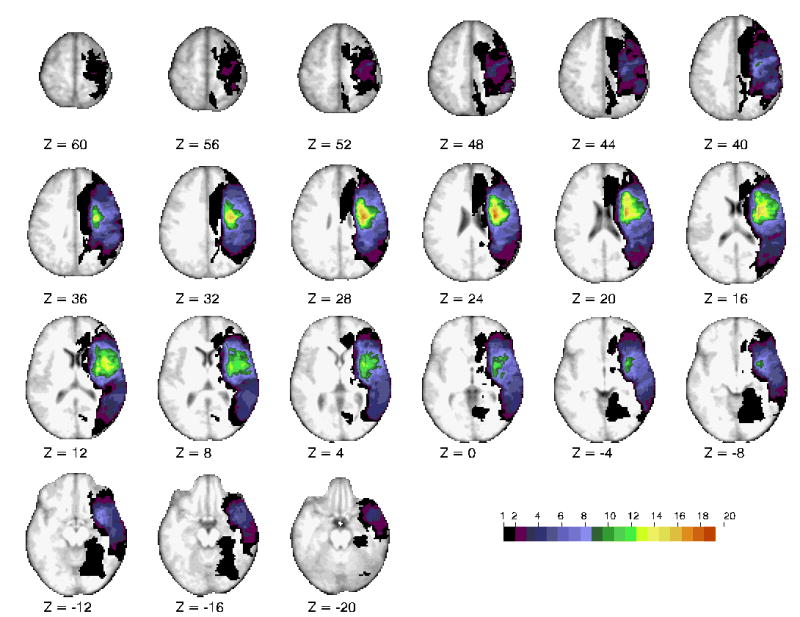
Horizontal slices of anatomical MRI standardized in Talairach atlas showing the lesion distribution for 30 representative patients, obtained at the chronic testing session. The color scale represents the number of patients with damage in a specific voxel.
Mean age was 60.5±15.2 years, and 49% of the patients were women. Most patients were right handed (88%) per the Edinburgh Handedness Inventory.26 All patients underwent acute inpatient or outpatient rehabilitation. Overall stroke severity ranged from mild to severe as measured by National Institutes of Health Stroke Scale score27, assessed within 24 hours of admission. Average National Institutes of Health Stroke Scale was 11.7±5.9 (moderate severity).
Mean age ± SD of the control group was 63.2±15.6 years, and 53% were women.
Procedures
Patients completed a 1-hour testing session in a quiet room at the Rehabilitation Institute of St. Louis.
Clinical tests
Tests had no time limits. The examiner presented stimuli at midline and provided no feedback other than to ask “Are you finished?” after the patient stopped working.
Line cancellation30
A pseudo-random array of 40 diagonal lines, presented in peripersonal space. We measured “nonlateralized spatial attention” as the total number of misses and “lateralized spatial attention” as the difference between the number of left and right misses (L-R difference).
Behavioral Inattention Test: star cancellation subtest31
A pseudo-random array of 54 targets with 52 distracters, presented in peripersonal space. We recorded total misses and L-R difference.
Mesulam Test: random array symbol cancellation subtest32,33
A pseudo-random array of 60 target symbols with several hundred distracters, presented in peripersonal space. We recorded total misses and L-R difference.
Behavioral Inattention Test: Article reading subtest31
A 3-column article, presented in peripersonal space, to be read out loud. We recorded total words missed and L-R difference.
Clock drawing test
Patients were to draw the face of a clock and set the time. We rated drawings using the 15-point scale devised by Freedman.34
Baking tray test35
A functional test of neglect, presented in peripersonal space. Patients were to place 16 plastic “cookies” on a tray as if they were going to bake them. Each cookie placed left or right of midline counted for 1 point, up to 16 points per side. Next, the examiner placed the cookies in a symmetric pattern and asked patients to replicate the pattern. We recorded before and after demonstration total misses and L-R difference.
Shape test36
This test is intended to identify patients whose neglect might be due to difficulty disengaging their attention from the ipsilesional field. Patients were to touch 16 colored wooden shapes arrayed on a felt board, presented in peripersonal space, under 4 randomly-ordered conditions. In the first, the examiner removed each shape as the patient touched it (“visually-guided with removal”). In the second, the shapes were not removed (“visually-guided without removal”). In the third and fourth, patients were blindfolded (“tactilely-guided with removal” and “tactilely-guided without removal”). We recorded total misses and L-R difference.
Fluff test37
A test of personal neglect. The examiner attached 6 targets to the blindfolded patient's left arm, leg, and trunk. Patients were to remove the targets with their right hand. We recorded misses.
Computerized Reaction Time Test: Posner Cueing Paradigm
Stimuli were generated by an Apple Power Macintosh computer and displayed on a 17 inch Apple Monitor. Behavioral responses were acquired through a Carnegie Mellon button box interfaced with the computer. The experimenter visually screened for eye movements and encouraged visual fixation whenever a fixation break occurred. The display contained a central fixation cross and two eccentric, square frames (side 1 degree, center of frame at 3.3° from the fixation cross) positioned along the horizontal meridian to the left and right of fixation. For the 2 patients with a quadrantanopsia, we presented stimuli in the visible part of the field on symmetrically opposite positions across the vertical meridian. The onset of a new trial was signaled by a color change, from red to green, of the fixation cross. Then 800ms later an arrow cue pointing left or right appeared at fixation for 2360ms. Following a delay ranging from 1000 to 2000ms the target (an asterisk) appeared for 300ms within 1 of the 2 frames (left or right). On 75% of the trials, the target appeared at the location indicated by the cue (valid condition), while on 25% of the trials it appeared at the opposite location (invalid condition). Patients had to detect the target as quickly as possible with a right hand keypress. The RTs were recorded. An intertrial interval of 2360ms separated subsequent trials. Blocks contained 40 trials (30 valid, 10 invalid). Each patient completed 2 blocks. The test took a total of 15 minutes to administer, including a practice block.
We measured lateralized spatial attention (Posner L-R difference) as relative delay in RTs for targets presented in the left versus right visual field. Because most patients with stroke missed many trials in the left field, the longest possible RT (2000ms) was substituted for missed trials in order to create a unified index that took into account both accuracy and speed. No patient missed every trial for any condition. We measured nonlateralized spatial attention (average RT) as the average of the RTs across all 4 conditions.
Data Analysis
Previous research has assessed the accuracy of neglect tests by comparing scores of neglect patients to those of healthy control subjects. The cut-off scores for classifying a patient as having neglect are typically set using high confidence intervals,17,28,31,32 which ensures high specificity (the ability to minimize false positives), at the cost of low sensitivity (the ability to minimize false negatives). Rather than comparing sensitivities of different scores given an arbitrary level of specificity, we used an ROC-based analysis which combines sensitivity and specificity into a single variable, to quantify the accuracy of the different instruments in differentiating between test and control groups.
The ROC analysis trades-off sensitivity and specificity. The curve plots the estimated false negative rate, using a given score as a cut-off, against the false positive rate for the same cut-off score. The curve is traced by varying the cut-off score over the range of possible score values. The AUC is a direct measure of the diagnostic power of the test. An AUC of 1.0 indicates a perfect test, that is all patients with neglect are classified as such while none of the subjects without neglect is diagnosed as having neglect, while an AUC of 0.5 indicates a test that does no better than chance at differentiating patients with neglect from participants without neglect.39
Because the AUC is not a normally distributed variable, conventional parametric procedures cannot be used. In order to establish whether tests for neglect differed in their diagnostic accuracy, we used a nonparametric method based on a bootstrap resampling procedure.40 This procedure allows one to test the significance of the difference between the AUCs for 2 different tests. The procedure involved the following steps. First the scores were transformed into ordinal values. Thus the worst score was given a value of 1, the second worst score a value of 2 and so forth. This had no affect on the AUCs, because the AUC only depends on the ordinal relationship between the scores in the patient and control group. Second, on each iteration, paired pseudo-samples were created by sampling with replacement the joined set of scores. Each of the 2 samples thus obtained contained ordinal values obtained from both scores. The significance for the 2-tailed, paired comparison was obtained by computing the proportion of iteration, in which the absolute value of the difference between the AUC computed from bootstrapped samples was greater that the absolute value of the difference between the AUCs computed from the original data. To ensure that we could accurately estimate the p-value to the second decimal position, that is, at a significance level of 0.01, estimates of the p-value were based on 100,000 iterations.
Results
As shown in tables 1 and 2, almost all tests showed worst mean performance for acute patients (most missed targets or slowest RTs), intermediate mean performance for chronic patients, and best mean performance for control subjects. Each neglect patient scored below the cut-off published in the test manual or original paper on at least one clinical test at the acute stage (see table 3 for cut-off scores), and 27 of 30 scored below cut-offs on at least 1 test at the chronic stage.
Table 1. Clinical Tests, Mean Percent Errors.
| Acute | ±SD | Chronic | ±SD | Control | ±SD | |
|---|---|---|---|---|---|---|
| Lines total % misses | 9.8% | ±21.8 | 0.7% | ±2.2 | 0.4% | ±1.6 |
| Lines L-R difference | 7.6% | ±17.2 | -0.2% | ±2.5 | -0.1% | ±0.5 |
| Stars total % misses | 30.8% | ±29.7 | 13.5% | ±22.2 | 0.4% | ±0.9 |
| Stars L-R difference | 27.3% | ±31.5 | 4.8% | ±10.7 | 0.0% | ±0.0 |
| Mesulam total % misses | 41.1% | ±31.0 | 8.3% | ±8.6 | 1.1% | ±2.2 |
| Mesulam L-R difference | 21.8% | ±22.4 | 3.0% | ±10.2 | 0.0% | ±2.6 |
| Reading total % misses | 17.1% | ±27.4 | 3.8% | ±10.5 | 0.3% | ±0.7 |
| Reading L-R difference | 16.9% | ±32.1 | 6.8% | ±26.1 | 0.1% | ±0.9 |
| Clock errors | 22.2% | ±20.3 | 11.7% | ±11.1 | 4.0% | ±7.6 |
| Fluff L % misses | 18.4% | ±26.2 | 1.3% | ±4.6 | 4.4% | ±10.7 |
| Baking before total % misses | 29.9% | ±19.8 | 15.6% | ±16.3 | 11.3% | ±17.3 |
| Baking before L-R difference | 48.4% | 52.5 | 18.8% | ±41.4 | 0.8% | ±20.7 |
| Baking after total % misses | 21.2% | ±20.8 | 1.3% | ±1.6 | 0.0% | ±0.0 |
| Baking after L-R difference | 33.0% | ±46.2 | 12.5% | ±39.1 | 0.0% | ±0.0 |
| Shapes v/nr total % misses | 11.3% | ±18.6 | 2.8% | ±7.0 | 0.0% | ±0.0 |
| Shapes v/nr L-R difference | 8.7% | ±23.3 | -0.4% | ±9.7 | 0.0% | ±0.0 |
| Shapes v/r total % misses | 4.6% | ±14.4 | 3.7% | ±18.6 | 0.0% | ±0.0 |
| Shapes v/r L-R difference | 5.7% | ±20.3 | -0.4% | ±27.3 | 0.0% | ±0.0 |
| Shapes t/nr total % misses | 23.7% | ±21.5 | 7.8% | ±10.3 | 0.0% | ±1.1 |
| Shapes r/nr L-R difference | 17.1% | ±35.1 | 1.3% | ±16.1 | 0.0% | ±2.3 |
| Shapes r/r total % misses | 11.3% | ±16.9 | 15.7% | ±30.7 | 1.7% | ±9.1 |
| Shapes t/r L-R difference | 4.8% | ±21.0 | -0.9% | ±14.7 | 0.0% | ±0.0 |
Note. Displays the raw data for each clinical test for acute patients, chronic patients, and control subjects. For the Shape test, v=visual, t=tactile, r=removal of touched targets, nr= no removal of touched targets. Acute (n=59), chronic (n=30), control (n=30).
Table 2. Computerized RT Test (Posner cueing paradigm), Mean RT (in ms).
| Acute | ±SD | Chronic | ±SD | Control | ±SD | |
|---|---|---|---|---|---|---|
| Posner average RT | 1104.52 ms | ±356.2 | 660.36 ms | ±220.2 | 428.37 ms | ±75.1 |
| Posner L-R difference | 851.20 ms | ±525.6 | 349.47 ms | ±379.6 | 7.33 ms | ±74.4 |
Note. Displays the raw data for the Posner test for acute patients, chronic patients, and control subjects. Acute (n=59), chronic (n=30), control (n=30).
Table 3. Clinical Tests, Published Cut-Off Scores.
| Maximum misses or errors | Cut-off score | |
|---|---|---|
| Lines total misses | 40 | 2 |
| Stars total misses | 54 | 3 |
| Mesulam total misses | 60 | 2 |
| Reading total misses | 153 | 13 |
| Clock errors | 15 | 3 |
| Fluff L misses | 6 | 1 |
| Baking, ratio of contra:ipsi misses | 16:0 | 9:7 |
| Shapes test | 16 | None available |
Figure 2 displays the AUC for the tests at the acute and chronic stages. All tests had higher than chance accuracy at the acute stage (chance=0.5), but only some did at the chronic stage. The Posner L-R RT score had the highest AUC at both stages. Interestingly, the AUC was greater for total scores than L-R scores for all the clinical tests except the Baking tray test, while for the Posner task the L-R RT AUC score was slightly greater than the average RT AUC score.
Fig 2.
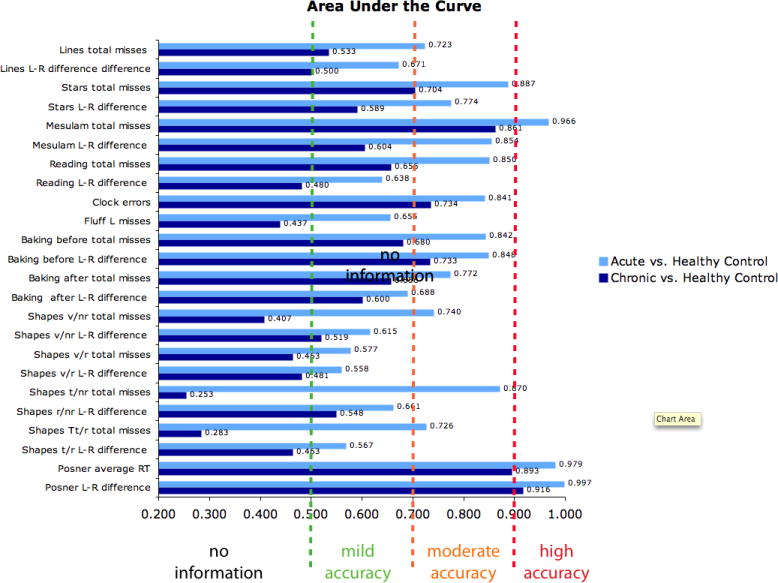
Displays the Area Under the Curve (x-axis) for each test (y-axis) for acute and chronic patients.
Table 4 shows the statistical significance of the differences between the AUC scores based on the Posner L-R RT and Posner average RT, and the AUC scores based on the L-R misses or total misses on the other tests at both acute and chronic stage. The Posner test showed significantly better sensitivity than most traditional tests at the acute stage with the exception of the Mesulam and BIT stars. At the chronic stage, the Posner average score was similar to the Mesulam total score, and not significantly better than several other pencil-and-paper scores emphasizing detection in both visual fields (Stars total, Clock, Baking tray after total, Shapes total misses with and without removal). However, the Posner L-R index was significantly superior to all pencil-and-paper except the Mesulam total score. The 2 Posner indexes (average and L-R) showed similar sensitivity.
Table 4. P Values of the 2-Tailed Comparisons Between the Posner and Clinical Tests.
| Acute | Chronic | |||
|---|---|---|---|---|
| Posner Average RT | Posner L-R Difference | Posner Average RT | Posner L-R Difference | |
| Lines total misses | <.01* | <.01* | <.01* | <.01* |
| Lines L-R difference | <.01* | <.01* | <.01* | <.01* |
| Stars total misses | <.01* | 0.06 | 0.07 | 0.01* |
| Stars L-R difference | <.01* | <.01* | <.01* | <.01* |
| Mesulam total misses | 0.08 | 0.91 | 0.61 | 0.31 |
| Mesulam L-R difference | <.01* | 0.06 | <.01* | <.01* |
| Reading total misses | <.01* | <.01* | 0.01* | <.01* |
| Reading L-R difference | <.01* | <.01* | <.01* | <.01* |
| Clock errors | <.01* | 0.02* | 0.17 | 0.01* |
| Fluff L misses | <.01* | <.01* | 0.01* | <.01* |
| Baking before total misses | <.01* | <.01* | <.01* | <.01* |
| Baking before L-R difference | <.01* | <.01* | 0.01* | <.01* |
| Baking after total misses | <.01* | 0.01* | 0.06 | 0.02* |
| Baking after L-R difference | <.01* | <.01* | <.01* | <.01* |
| Shapes v/nr total misses | <.01* | 0.02* | 0.18 | 0.04* |
| Shapes v/nr L-R difference | <.01* | <.01* | <.01* | <.01* |
| Shapes v/r total misses | <.01* | <.01* | 0.11 | 0.01* |
| Shapes v/r L-R difference | <.01* | <.01* | <.01* | <.01* |
| Shapes t/nr total misses | <.01* | <.01* | <.01* | <.01* |
| Shapes t/nr L-R difference | <.01* | <.01* | <.01* | <.01* |
| Shapes t/r total misses | <.01* | <.01* | <.01* | <.01* |
| Shapes r/r, L-R difference | <.01* | <.01* | <.01* | <.01* |
| Posner average RT | . | 0.06 | . | 0.53 |
| Posner L-R difference | 0.06 | . | 0.53 | . |
Note. Displays the significance of differences between the Posner test and the clinical tests for acute and chronic patients. For the Shape test, v=visual, t=tactile, r=removal of touched targets, nr= no removal of touched targets.
P<.05.
Discussion
Our analysis suggests that using an RT test may provide a more efficient and accurate assessment of neglect than clinical tests, particularly at chronic time points when some recovery has occurred.
The ROC analysis indicated that the clinical tests varied in accuracy, with rankings in a similar order to those found in previous reports.1,16,17,41,42 Interestingly, all clinical tests were mildly to moderately accurate at the acute stage, with BIT stars (total and L-R), Mesulam (L-R), BIT total reading, clock, and Baking tray (total and L-R) being the most accurate. The Mesulam and BIT total scores, with AUC values approximately .90 consistent with high sensitivity, performed comparably to the Posner cueing task. These tests should therefore be first choice for the accurate diagnosis of hemispatial neglect at the acute stage. The highest sensitivity of the total scores emphasize the clinical importance to take in consideration bilateral deficits in hemispatial neglect.
Accuracy dropped from the acute to the chronic stage, with some tests near or below chance at the chronic stage. Because patients were classified based on the presence of significant clinical left neglect at the acute stage, one could argue that the clinical tests picked up fewer cases of chronic neglect simply because the symptoms had recovered. However, our finding that most subjects at the chronic stage continued to score below cut-offs on at least one clinical test suggests that recovery is a graded phenomenon and that only a few had fully recovered.
Importantly, the high accuracy of the Posner test in discriminating controls from chronic patients also indicates that even in the chronic stage many patients had neglect, and further suggests that RT measures are a sensitive indicator of chronic neglect. Conversely, a clinician would fail to pick up neglect in many cases by using just pencil-and-paper tasks. A notable exception is the Mesulam test where the total miss score seems to have the same accuracy at the chronic stage as the Posner cueing RT task. Previous studies20,21 in small groups of patients with chronic stroke have indicated that an RT test may pick up neglect symptoms undetectable by clinical tests. Our longitudinal analysis confirms the persistence of relatively impaired performance on the neglected side in a group of 30 stroke patients. Clinical trials comparing the effectiveness of rehabilitative interventions for neglect and clinics that treat a high volume of patients with stroke and brain injury may consider investing in equipment capable of reaction-time tests.
Study Limitations
A limitation of our study is that the results of our bootstrap resampling procedure did not account for the inflation of the significance value introduced by multiple comparisons. However, while multiple comparison corrections ensure that a few of the many comparisons reported will not be significant by chance, in the present case, many of the reported comparisons between the RT measures and the traditional measures were significant. Therefore, the present conclusion concerning the overall superiority of the Posner RT measure in comparison to traditional tests was well supported by the results.
Our study compared subjects with and without stroke. Differences between these groups may be attributable to generalized effects of brain damage rather than neglect-specific impairment, thus inflating the value of the AUCs. We speculate, however, that lateralized RT measures, which showed slightly higher accuracy than average RT in classifying neglect patients at the acute and chronic stages, may be significantly more accurate than average RT as well as traditional tests in discriminating stroke patients with neglect from stroke patients suffering other types of symptoms than total RT measures. It would be interesting in future work to examine this hypothesis.
This study did not attempt to correlate performance on the RT task with real-life performance of visual attention tasks. It would have been beneficial to compare clinician assessment at the chronic stage with the RT task. We intended to use the Catherine Bergego Scale42, 43 for this purpose as it systematically classifies clinical impression of severity of neglect and has been shown to be more sensitive than paper-and-pencil measures. Unfortunately, at the chronic time point nearly all patients had been discharged from therapies, either because of insurance limitations or because they were deemed recovered, so we had no way to catalogue clinical impression of the treating therapist at this stage. It is possible that some patients had neglect on the RT task that was insignificant in their daily lives, while other patients may have had meangingful symptoms undetectable by clinical tests. Future research to determine the clinical relevance of given L-R RT differences would be helpful in applying this type of test.
Finally, it should be noted that the RT test was limited to the exploration of visual neglect. Because neglect is a complex phenomenon that can involve tactile, auditory, and motor systems as well as visual perception, it is possible that this type of RT test is insensitive to some types of neglect. It would be valuable to study the interactions among different types of neglect in association with a visual RT task.
Conclusions
While most commonly-used clinical tests of left neglect are sensitive in the florid acute stage, they may be insensitive to subtle neglect, especially at the chronic stage as patients acquire compensatory strategies. We found that a single, rapidly-administered reaction time test showed the highest accuracy of all the tests we evaluated in classifying patients with neglect at both acute and chronic stages, and this accuracy was significantly better than that of many clinical tests at the chronic stage. Therapists should consider using a RT test rather than a paper-and-pencil test especially when assessing patients for ability to return to higher-level tasks such as driving.
Acknowledgments
The authors would like to thank the clinical staff at Barnes-Jewish Hospital and the Rehabilitation Institute of St. Louis for their help in identifying study candidates and facilitating study testing.
Supported by the National Institute of Mental Health (grant no. R01 MH71920-06), National Institute of Neurological Disorders and Stroke (grant no. R01 NS48013), and by the James S. McDonnell Foundation.
List of Abbreviations
- AUC
area under the curve
- BIT
Behavioral Inattention Test
- CT
computed tomography
- MRI
magnetic resonance imaging
- RT
reaction time
- ROC
receiver operating characteristic
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Buxbaum LJ, Ferraro MK, Veramonti T, et al. Hemispatial neglect: subtypes, neuroanatomy, and disability. Neurology. 2004;62:749–56. doi: 10.1212/01.wnl.0000113730.73031.f4. [DOI] [PubMed] [Google Scholar]
- 2.Robertson IH. Do we need the “lateral” in unilateral neglect? Spatially nonselective attention deficits in unilateral neglect and their implications for rehabilitation. NeuroImage. 2001;14:S85–90. doi: 10.1006/nimg.2001.0838. [DOI] [PubMed] [Google Scholar]
- 3.Ringman JM, Saver JL, Woolson RF, Clarke WR, Adams HP. Frequency, risk factors, anatomy, and course of unilateral neglect in an acute stroke cohort. Neurology. 2004;63:468–74. doi: 10.1212/01.wnl.0000133011.10689.ce. [DOI] [PubMed] [Google Scholar]
- 4.Pedersen PM, Jorgensen HS, Nakayama H, Rasschou HO, Olsen TS. Hemineglect in acute stroke--incidence and prognostic implications. The Copenhagen Stroke Study. Am J Phys Med Rehabil. 1997;6:122–7. doi: 10.1097/00002060-199703000-00007. [DOI] [PubMed] [Google Scholar]
- 5.Weintraub S, Mesulam M. Right cerebral dominance in spatial attention. Arch Neurol. 1987;44:621–5. doi: 10.1001/archneur.1987.00520180043014. [DOI] [PubMed] [Google Scholar]
- 6.Farne A, Buxbaum LJ, Ferraro M, et al. Patterns of spontaneous recovery of neglect and associated disorders in acute right brain-damaged patients. J Neurol Neurosurg Psychiatry. 2004;75:1401–10. doi: 10.1136/jnnp.2002.003095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Linden T, Samuelsson H, Skoog I, Blomstrand C. Visual neglect and cognitive impairment in elderly patients late after stroke. Acta Neurol Scand. 2005;111:163–8. doi: 10.1111/j.1600-0404.2005.00391.x. [DOI] [PubMed] [Google Scholar]
- 8.Cassidy T, Lewis S, Gray C. Recovery from visuospatial neglect in stroke patients. J Neurol Neurosurg Psychiatry. 1998;64:555–7. doi: 10.1136/jnnp.64.4.555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kotila M, Niemi M, Laaksonen R. Four-year prognosis of stroke patients with visuospatial inattention. Scand J Rehabil Med. 1986;18:177–9. [PubMed] [Google Scholar]
- 10.Patel M, Coshall C, Rudd A, Wolfe C. Natural history of cognitive impairment after stroke and factors associated with its recovery. Clin Rehabil. 2003;17:158–66. doi: 10.1191/0269215503cr596oa. [DOI] [PubMed] [Google Scholar]
- 11.Katz N, Hartman-Maeir A, Ring H, Soroker N. Functional disability and rehabilitation outcome in right hemisphere damaged patients with and without unilateral spatial neglect. Arch Phys Med Rehabil. 1999;80:379–84. doi: 10.1016/s0003-9993(99)90273-3. [DOI] [PubMed] [Google Scholar]
- 12.Gillen R, Tenne H, McKee T. Unilateral spatial neglect: Relation to rehabilitation outcomes in patients with right hemisphere stroke. Arch Phys Med Rehabil. 2005;86:763–7. doi: 10.1016/j.apmr.2004.10.029. [DOI] [PubMed] [Google Scholar]
- 13.Edwards D, Hahn M, Baum C, Perlmutter M, Sheedy C, Dromerick A. Screening patients with stroke for rehabilitation needs: Validation of the post-stroke rehabilitation guidelines. Neurorehabil Neural Repair. 2006;20:42–8. doi: 10.1177/1545968305283038. [DOI] [PubMed] [Google Scholar]
- 14.Menon-Nair A, Korner-Bitensky N, Wood-Dauphine S, Robertson E. Assessment of unilateral spatial neglect post stroke in Canadian acute care hospitals: are we neglecting neglect? Clin Rehabil. 2006;20:623–34. doi: 10.1191/0269215506cr974oa. [DOI] [PubMed] [Google Scholar]
- 15.Agrell B, Dehlin O, Dahlgren C. Neglect in elderly stroke patients: a comparison of five tests. Psychiatry Clin Neurosci. 1997;51:295–300. doi: 10.1111/j.1440-1819.1997.tb03201.x. [DOI] [PubMed] [Google Scholar]
- 16.Lindell A, Jala M, Tenovuo O, Brunila T, Voeten M, Hamalainen H. Clinical assessment of Hemispatial neglect: evaluation of different measures and dimensions. Clin Neuropsychol. 2007;21:479–97. doi: 10.1080/13854040600630061. [DOI] [PubMed] [Google Scholar]
- 17.Bailey MJ, Riddock MJ, Crome P. Evaluation of a test battery for hemineglect in elderly stroke patients for use by therapists in clinical practice. NeuroRehabilitation. 2000;14:39–50. [PubMed] [Google Scholar]
- 18.Cherney L, Halper A. Unilateral visual neglect in right-hemisphere stroke: a longitudinal study. Brain Inj. 2001;15:585–92. doi: 10.1080/02699050010009090. [DOI] [PubMed] [Google Scholar]
- 19.Schendel K, Robertson L. Using reaction time to assess patients with unilateral neglect and extinction. J Clin Exp Neuropsychol. 2002;24:941–50. doi: 10.1076/jcen.24.7.941.8390. [DOI] [PubMed] [Google Scholar]
- 20.Pflugshaupt T, Almoslochner Bopp S, Heinemann D, et al. Residual oculomotor and exploratory deficits in patients with recovered hemineglect. Neuropsychologia. 2004;42:1203–11. doi: 10.1016/j.neuropsychologia.2004.02.002. [DOI] [PubMed] [Google Scholar]
- 21.Deouell L, Sacher Y, Soroker N. Assessment of spatial attention after brain damage with a dynamic reaction time test. J Int Neuropsychol Soc. 2005;11:697–707. doi: 10.1017/S1355617705050824. [DOI] [PubMed] [Google Scholar]
- 22.Jepson R, Despain K, Keller D. Unilateral neglect: assessment in nursing practice. J Neurosci Nurs. 2008;40:142–9. [PubMed] [Google Scholar]
- 23.Menon-Nair A, Korner-Bitensky N, Ogourtsova T. Occupational therapists' identification, assessment, and treatment of unilateral spatial neglect during stroke rehabilitation in Canada. Stroke. 2007;38:2556–62. doi: 10.1161/STROKEAHA.107.484857. [DOI] [PubMed] [Google Scholar]
- 24.Posner MI, Walker JA, Friedrich FJ, Rafal RD. Effects of parietal injury on covert orienting of attention. J Neurosci. 1984;4:1863–74. doi: 10.1523/JNEUROSCI.04-07-01863.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ladavas E, Carletti M, Gori G. Automatic and voluntary orienting of attention in patients with visual neglect: horizontal and vertical dimensions. Neuropsychologia. 1994;32:1195–208. doi: 10.1016/0028-3932(94)90102-3. [DOI] [PubMed] [Google Scholar]
- 26.Oldfield RC. The assessment and analysis of handedness: the Edinburgh Inventory. Neuropsychologia. 1979;9:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- 27.Goldstein L, Samsa G. Reliability of the National Institutes of Health Stroke Scale Extension to non-neurologists in the context of a clinical trial. Stroke. 1997;28:307–10. doi: 10.1161/01.str.28.2.307. [DOI] [PubMed] [Google Scholar]
- 28.Longstreth W, Arnold A, Beauchamp N, et al. Incidence, manifestations, and predictors of worsening white matter on serial cranial magnetic resonance imaging in the elderly: the Cardiovascular Health Study. Stroke. 2005;36:56–61. doi: 10.1161/01.STR.0000149625.99732.69. [DOI] [PubMed] [Google Scholar]
- 29.Katzman R, Brown T, Fuld P, Peck A, Schechter T, Schimmel H. Validation of a short orientation-memory-concentration test of cognitive impairment. Am J Psychiatry. 1983;140:734–9. doi: 10.1176/ajp.140.6.734. [DOI] [PubMed] [Google Scholar]
- 30.Albert MI. A simple test of visual neglect. Neurology. 1973;23:658–64. doi: 10.1212/wnl.23.6.658. [DOI] [PubMed] [Google Scholar]
- 31.Wilson B, Cockburn J, Halligan P. Development of a behavioral test of visuospatial neglect. Arch Phys Med Rehabil. 1987;68:98–102. [PubMed] [Google Scholar]
- 32.Mesulam MM. Principles of behavioral neurology. Philadelphia: F.A. Davis; 1985. pp. 102–3. [Google Scholar]
- 33.Lowery N, Ragland JD, Gur RC, Gur RE, Moberg PJ. Normative data for the symbol cancellation test in young healthy adults. Appl Neuropsychol. 2004;11:218–21. doi: 10.1207/s15324826an1104_8. [DOI] [PubMed] [Google Scholar]
- 34.Freedman M, Leach L, Kaplan E, Winocur G, Shulman KI, Delis DC. Clock Drawing: a neuropsychological analysis. New York: Oxford Univ pr; 1994. [Google Scholar]
- 35.Tham K, Tegner R. The baking tray task: a test of spatial neglect. Neuropsychol Rehabil. 1996;6:9–26. doi: 10.1080/713755496. [DOI] [PubMed] [Google Scholar]
- 36.Ladavas E, Umilta C, Ziani P, Brogi A, Minarini M. The role of right side objects in left side neglect: a dissociation between perceptual and directional motor neglect. Neuropsychologia. 1993;31:761–3. doi: 10.1016/0028-3932(93)90127-l. [DOI] [PubMed] [Google Scholar]
- 37.Cocchini G, Beschin N, Jehkonen M. The fluff test: a simple task to assess body representation neglect. Neuropsychol Rehabil. 2001;11:17–31. [Google Scholar]
- 38.Azouvi P, Barolomea P, Beis JM, Perennou D, Pradat-Diehl P, Rousseaux M. A battery of tests for the quantitative assessment of unilateral neglect. Restor Neurol Neurosci. 2006;24:273–85. [PubMed] [Google Scholar]
- 39.Zou KH, O'Malley J, Mauri L. Receiver-operating characteristic analysis for evaluating diagnostic tests and predictive models. Circulation. 2007;115:654–7. doi: 10.1161/CIRCULATIONAHA.105.594929. [DOI] [PubMed] [Google Scholar]
- 40.Efron B, Tibshirani R. An Introduction to the Bootstrap. New York: Chapman & Hall/CRC; 1994. [Google Scholar]
- 41.Halligan PW, Marshall JC, Wade DT. Visuospatial neglect: underlying factors and test sensitivity. Lancet. 1989;2:908–11. doi: 10.1016/s0140-6736(89)91561-4. [DOI] [PubMed] [Google Scholar]
- 42.Azouvi P, Samuel C, Louis-Dreyfus A, et al. Sensitivity of clinical and behavioral tests of spatial neglect after right hemispheric stroke. J Neurol Neurosurg Psychiatry. 2002;73:160–6. doi: 10.1136/jnnp.73.2.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Azouvi P, Olivier S, de Montety G, Samuel C, Louis-Dreyfus A, Tesio L. Behavioral assessment of unilateral neglect: Study of the psychometric properties of the Catherine Bergego Scale. Arch Phys Med Rehabil. 2003;84:51–7. doi: 10.1053/apmr.2003.50062. [DOI] [PubMed] [Google Scholar]


